Back to Journals » Medical Devices: Evidence and Research » Volume 12
In vitro safety and performance evaluation of a seawater solution enriched with copper, hyaluronic acid, and eucalyptus for nasal lavage
Authors Huang S , Constant S , De Servi B , Meloni M , Culig J , Bertini M , Saaid A
Received 25 March 2019
Accepted for publication 20 May 2019
Published 24 September 2019 Volume 2019:12 Pages 399—410
DOI https://doi.org/10.2147/MDER.S209644
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Song Huang,1 Samuel Constant,1 Barbara De Servi,2 Marisa Meloni,2 Josip Culig,3 Marco Bertini,4 Amina Saaid5
1Epithelix, Geneva, Switzerland; 2Department of in Vitro Research, VitroScreen, Milan, 20149, Italy; 3Department of Pharmacology, University of Applied Health Sciences, Zagreb, 10000, Croatia; 4R&D Department, Laboratori Baldacci SpA, Pisa, Italy; 5Department of R&D and Innovation, Laboratoire Fumouze, Levallois-Perret, 92686, France
Correspondence: Marco Bertini
Laboratori Baldacci SpA, Via S. Michele degli Scalzi, 73, 56124 Pisa, Italy
Tel +39 5 031 3271
Fax +39 5 057 0170
Email [email protected]
Background: The common cold is a viral infectious disease with symptoms such as runny nose, sore throat, and mainly, nasal congestion. State-of-the-art therapeutic approaches focus on alleviating the symptoms of this disease by non-invasive and simple-to-use methods. Nasal irrigation is one of the most accepted approaches to ease nasal congestion which, if left untreated, has a negative impact on the quality of life of patients.
Purpose: In this study, the safety and efficacy of a novel hypertonic seawater solution for nasal lavage enriched with hyaluronic acids, eucalyptus oil, copper, and manganese salts (Stérimar Stop & Protect Cold and Flu; SSPCF) have been investigated in vitro.
Methods: An in vitro 3D reconstituted human nasal epithelium tissue model, MucilAir™, has been used in this study to investigate the safety of SSPCF on nasal epithelium by measuring transepithelial electrical resistance (TEER), lactate dehydrogenase (LDH), and interleukin-8 (IL-8) secretion. The efficacy of SSPCF was measured by mucociliary clearance (MCC), ATP release, Alcian blue and aquaporin (AQP3) stainings.
Results: SSPCF treatment respected nasal epithelium tissue integrity and enhanced barrier function without inducing a cytotoxic response. Secreted LDH and IL-8 levels were similar to untreated controls. MCC rate was increased 2.5-fold and ATP release decreased 87% upon SSPCF treatment, indicating improved decongestion activity. SSPCF treatment after hypotonic stress helped recover cellular organization, as shown by Alcian blue and AQP3 staining assays.
Conclusion: SSPCF appears as a safe and effective nasal irrigation formula that may alleviate the symptoms associated with common cold such as nasal congestion.
Keywords: common cold, hypertonic seawater, nasal irrigation, pathogens, ATP release, decongestion
Introduction
Common cold is defined as an acute rhinosinusitis disease with symptoms that last less than 10 days and affect the upper respiratory tract.1 It is transmitted by hand contact and/or inhalation of secretions from an infected person.2 Data from the US Centers for Disease Control and Prevention indicate that adults may experience 2–3 common colds per year while for children frequencies are higher. Moreover, one in three cases require medical attention, while one in four cases cause work/school absenteeism, making the common cold a significant health burden for the community.3
Available treatments for the common cold are symptomatic, ie, aim to relieve symptoms non-specifically. Moreover, treatment effectiveness is also age-specific, ie, some medications indicated for the adult population are not effective or could be potentially harmful to children. There is no conclusive evidence for or against the effectiveness of over-the-counter medicines to improve symptoms like a cough in children;4 inhaled corticosteroids or oral prednisolone for mild episodic viral wheezing also show no significant benefits in children.5,6 In addition, commonly used medications in adults including vasoconstrictors such as pseudoephedrine and phenylephrine or bronchodilators such as ipratropium, have modest effects on symptom alleviation and duration.7 Furthermore, nasally administered vasoconstrictors such as oxymetazoline, pseudoephedrine, phenylephrine are classified as prescription medications in some countries. Other strategies to reduce common cold symptoms such as herbal remedies or steam inhalations have also been shown to have no significant effect on symptom improvement.8,9 Therefore, although current strategies to ease common cold symptoms are numerous, their effects are either mild or null.
The use of saline solution is recommended as a top-line strategy for alleviating common cold symptoms.10 The largest study on the effect of saline nasal irrigation for acute upper respiratory tract infections included 401 children (6–10 years old) with common cold or flu.11,12 Results showed that isotonic nasal irrigation in combination with standard medication, improved significantly common cold symptoms compared to standard medication alone. In addition, the group that used the saline nasal wash suffered less frequently from subsequent rhinosinusitis. These observations were supported by the studies performed by Wang et al, that showed that nasal saline irrigation improved pediatric rhino-conjunctivitis and quality of life and decreased acute sinusitis symptoms.13,14 Considering the effect of saline washes on decreasing the use of prescription drugs,15 and on alleviating common cold symptoms, it is important to investigate new formulations for nasal irrigation.
Hypertonic solutions have long been considered as natural decongestants as they have a higher salt concentration than body tissues and can help, through osmosis, both drain excess water from nasal epithelial and submucosal tissues and dilute the viscous mucous that accumulates during upper respiratory tract infections including common cold. Hypertonic seawater solutions have been proven to be particularly efficient in eliminating the symptoms of nasal congestion.16
In this study, a novel formulation, SSPCF (Stérimar Stop & Protect Cold and Flu) for nasal application was investigated. SSPCF is composed of micro-filtered hypertonic seawater solution (2.3% NaCl), enriched with hyaluronic acids of different molecular weights, eucalyptus oil, as well as copper and manganese salts.
The primary objectives of this study were to evaluate the safety of SSPCF and its efficacy to enhance mucociliary clearance (MCC) rate and recovery from cellular stress in nasal epithelial cells.
Materials and methods
Biological model (test system) used for the in vitro experimental studies
The in vitro assays were performed in a 3D reconstituted human nasal epithelium model, MucilAir™ (Epithelix Sàrl, Geneva, Switzerland) for its great potential as a model to test respiratory sensitizers.17,18 For maintenance, inserts were incubated in 500 µL of MucilAir™ culture medium in a CO2 incubator (37 ºC, 5% CO2, 100% humidity, Heracell, Waltham, MA, USA).
Methods
MucilAir™ model was used to assess the safety and the efficacy of the tested solutions by transepithelial electrical resistance (TEER), Lucifer yellow (LY) permeability, lactate dehydrogenase (LDH) secretion, interleukin-8 (IL-8) secretion, MCC, ATP release quantification, aquaporin 3 (AQP3), and Alcian blue staining assays.
For TEER, LDH, IL-8 and MCC assays, tissues were treated with the solution (10 µL) twice a day within 8 hrs from the apical side for 4 days in 24-well plates. Each day, culture medium was frozen at −80ºC for further endpoint analysis. The following endpoints were assessed: TEER: Days 1 and 3; Lucifer yellow: Day 3; LDH: Days 1, 2, 3, and 4; IL-8 and MCC: Days 1 and 4.
For ATP release quantification, AQP3 and Alcian blue stainings, Mucilair™-HF tissues (Mucilair™ co-cultured with human airway fibroblasts, Epithelix Sàrl, Geneva, Switzerland) were treated with hypotonic solution (300 µL, 1 mM CaCl2 and 1 mM MgCl2 water solution) for 5 mins in order to trigger ATP release and swelling.19 Then, immediately, tissues were incubated with 100 µL saline solution (control), or SSPCF for 2 mins at room temperature. For stainings, after treatment, tissues were rinsed with saline solution and fixed in adapted fixative. Samples were embedded in paraffin blocks and 5 μm sections were obtained. All experiments in this study were performed in a double-blind manner, and details of each experiment are described below.
TEER
TEER is a method used to study epithelium permeability and tight junction integrity.20 Tissues were treated with saline solution (0.9% NaCl) (n=2) or SSPCF (n=2). Measurements were performed using a Millicell ERS voltohmmeter (Millipore, Burlington, MA, USA). Three measurements were performed per sample where the basal value was the measurement performed at Day 0. The resistance of the tissue was calculated by subtracting the blank resistance (insert with no tissue) from the read-out resistance (mean of three) and multiplying by the epithelium surface size (0.33 cm2).
Lucifer yellow
Lucifer yellow is a marker of cell integrity whose permeability is proportional to the damage levels of the tight junctions.21 After 3 days of treatment, with saline (0.9% NaCl) (n=2) or SSPCF (n=2), twice a day, 200 µL of Lucifer yellow (Sigma Aldrich, St. Louis, MO, USA) was applied to the apical compartment of the tissues. Five hundred microliters of HBSS medium was added to the basolateral compartment. Lucifer yellow activity was measured using a spectrofluorimeter (Tecan Infinite M200, Tecan Group Ltd, Männedorf, Switzerland) (428 nm/535 nm). Measurement of fluorescence (RFU) was performed at the apical and basolateral level, and flux was calculated as follows: Lucifer yellow Flux (%) = (RFU BL × Volume BL/RFU APT-0 × Volume AP) ×100.
LDH secretion
LDH measurement is a widely utilized method for assessing toxicity and cell membrane integrity.22 LDH measurement was performed in untreated (n=2), SSPCF-treated (n=3) and 1% Triton X-100 in saline solution-treated tissues (positive control, Fluka Biochemika, NJ, USA, n=2) by Cytotoxicity Detection KitPLUS (LDH) (Roche, St. Louis, MO, USA) following manufacturer’s instructions.
IL-8 secretion
IL-8 is one of the most abundant pro-inflammatory mediators released by airway epithelial cells and its elevated levels indicate an inflammatory process.23 IL-8 secretion evaluation was performed by ELISA (BD OptEIA™, BD Bioscience, Franklin Lakes, NJ, USA) in untreated (n=2), SSPCF-treated (n=3) and Cytomix-treated (positive control, n=2) tissues. Cytomix was composed of 1% FCS (Amimed, Cat 2-01F36-I, BioConcept Ltd, Allschwil, Switzerland), 0.2 mg/mL LPS (Sigma, St. Louis, MO, USA) and 500 ng/ml TNF-α (GeneTex, Irvine, CA, USA).
Mucociliary clearance
MCC represents the airway epithelial clearance mechanism that allows elimination of foreign particles, pathogens, and chemicals.24,25 To this end, 5 µm microbeads were added onto the apical surface of untreated (n=3), SSPCF-treated (n=3) and 50 µM isoproterenol-treated (positive control, n=3) tissues. For bead tracking, one-minute videos (images taken every second) were recorded using DMIRE2 microscope (Leica, Wetzlar, Germany) equipped with Ds-5 mc camera (Nikon, Tokyo, Japan). A total of 200–500 beads were tracked (Image Pro Plus, Media Cybernetics, Rockville, MD, USA).
ATP release quantification
ATP is a paracrine regulator of airway epithelia functions and is released in response to various stimuli such as hypotonic stress-induced swelling.26 ATP was quantified by CellTiter-Glo® Luminescent Cell Viability Assay kit (Promega, Madison, WI, USA). 30 μl of CellTiter-Glo® reagent were added to 30 μL of collected apical solution in three biological replicates. The plates were incubated at 37°C for 20 mins and read in Tecan Infinite M200 (bioluminescence mode setting integration time of 1,000 ms). Each sample was measured in technical duplicate.
AQP3 staining
AQP3 is a water channel protein that permits rapid and selective water transport across the membrane of the human airway epithelium in response to osmotic gradients.27 Three paraffin slides per condition were deparaffinized, rehydrated, and incubated at 99°C for 8 mins in citrate buffer for antigen retrieval. Tissues were then incubated overnight with primary rabbit polyclonal AQP3 antibody (1:500 in 1% BSA in PBS), 30 mins with Histofine Simple Stain AP Multi (Nichirei Biosciences Inc, Tokyo, Japan), and with New Fuchsin chromogen (Nichirei Biosciences Inc, Tokyo, Japan) and examined under the Leica DM2500 microscope (40× magnification).
Alcian blue staining
Alcian blue is the preferred stain for mucins.28 Three slides per condition were deparaffinized, rehydrated with distilled water and stained with Alcian Blue solution for 30 mins. Counterstaining was performed with nuclear fast red solution for 5 mins. Tissues were dehydrated in 95% and 100% alcohol, mounted with resinous mounting medium and analyzed under light microscopy (40× magnification).
Results
Effects of SSPCF on tissue integrity and barrier function
Treatment with SSPCF caused a statistically significant transepithelial electrical resistance decrease on Day 1 (p=0.0024) which was still above the tissue integrity limit. On Day 3, TEER values were statistically higher (p=0.0033) in SSPCF-treated tissues compared to saline control (Figure 1A and Table S1). In addition, there was no statistically significant increase in permeability after 3 days of SSPCF treatment in comparison to saline solution (p=0.062) (Figure 1B and Table S2). Together with TEER values, these data indicate that there is no increase in paracellular transport. This suggests maintenance of epithelium integrity and formation of an improved barrier in epithelium with SSPCF.
Effects of SSPCF on cytotoxicity
Tissues treated with SSPCF presented a similar LDH release profile to untreated tissues (Figure 2 and Table S3). There was no LDH secretion after treatment with SSPCF during 1, 2, 3, or 4 days, indicating no cytotoxicity, compared to tissues treated with Triton X-100 (positive control for cell lysis corresponding to 100% cytotoxicity).
Pro-inflammatory effects of SSPCF
Results in Figure 3 (Table S4) show that treatment with Cytomix induced a strong release of IL-8 both on days 1 and 4. However, treatment with SSPCF showed a similar IL-8 secretion profile to untreated tissues (p=0.1043 and p=0.2106; for Day 1 and Day 4, respectively).
These results indicate that treatment with SSPCF enhances the barrier effect of the nasal epithelial cells and is well tolerated by these cells as no cytotoxicity or inflammation activity was observed.
Effects of SSPCF on mucociliary clearance
Results in Figure 4 (Table S5) represent an average velocity of particle movement. MCC rates of cells treated with SSPCF and isoproterenol increased 2.5-fold compared to untreated cells one day after treatment (untreated vs SSPCF: p=0.0035; untreated vs isoproterenol: p=0.0181). In cells treated with SSPCF, this enhanced bead clearance rate was maintained 4 days after treatment similar to the effect observed in isoproterenol-treated tissues (untreated vs SSPCF: p=0.0061; untreated vs isoproterenol: p=0.0007).
Effects of SSPCF on tissue morphology and physiology
ATP release
Two-minute SSPCF treatment after stress decreased the stress-induced ATP release by 86.98%, compared to isotonic saline solution, which indicates the recovery from hypotonic stress (6.874 nM vs 52.789 nM, p=1.22x10−7) (Figure 5A and Table S6).
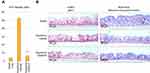
Alcian blue and AQP3 stainings
As seen in Figure 5B (left panel), in control tissues, the expression of AQP3 is dominant in the basal layer (pink staining). Upon hypotonic stress and subsequent saline treatment, the AQP3 expression increases and spreads to the whole tissue, including ciliated epithelial cells. However, SSPCF treatment after hypotonic stress partially reduces the AQP3 expression in the whole treatment, especially in the basal layer. As seen in Figure 5B (right panel, shape of the cells in pink/red, mucin production in blue), saline-only negative control presented a clear and regular organization of cuboidal mucus-producing goblet cells and ciliated epithelial cells. A two-minute isotonic solution treatment after hypotonic stress reduced mucus formation on the surface compared to saline-only negative control. Moreover, cilia were damaged, and cells were elongated. On the other hand, a 2-min treatment with SSPCF after hypotonic stress partially restored cuboidal, mucus-producing goblet cells as well as ciliated epithelial cells. In addition, mucus formation was restored.
These results indicate that SSPCF treatment helped recover the tissues from hypotonic stress and that it performed better than the isotonic solution.
Discussion
Nasal irrigation is employed for a variety of conditions including nasal post-surgery care, allergic/non-allergic rhinitis, and acute or moderate rhinosinusitis.29 There is a limited number of studies on the efficiency of nasal saline irrigations against acute upper respiratory tract infections.12 The present in vitro study aims to test safety and efficacy of a hypertonic seawater saline solution with an association of hyaluronic acids, eucalyptus oil as well as copper and manganese salts (SSPCF). Hyaluronic acid present in the formulation is a key component of the extracellular matrix and is involved in morphogenesis, tissue repair, and wound healing.30,31 It is also used to recover the integrity of the epithelial tissue thanks to its film-forming capacity and can contribute to wound healing by serving as a scaffold.32 Moreover, in acute rhinosinusitis patients, it has been shown that hyaluronic acid significantly improves disease symptoms, mucociliary transport evaluation time and reduces neutrophil count on nasal cytology.33 Eucalyptus oil has been shown to have antimicrobial effects against a wide range of bacterial pathogens that cause respiratory infections, including S. aureus.34 Also, at low concentrations, eucalyptus oil has been shown to cause an increase in ciliary beat frequency which is partially related to MCC rate.35 Copper and manganese have been demonstrated to stimulate the body’s self-defense mechanisms.36,37
To evaluate whether barrier properties are affected upon treatment with the SSPCF, changes in TEER should be considered together with the Lucifer yellow assay.21 Treatment with SSPCF increased TEER compared to saline treatment suggesting barrier effect enhancement. Transient decrease of TEER observed at Day 1 after SSPCF treatment may reflect ion channel activation as SSPCF, being hypertonic, contains high levels of Na+ and Cl− ions.38 This result is further confirmed by the Lucifer yellow assay which showed that treatment with SSPCF only marginally increased Lucifer yellow permeability compared to saline control (not statistically significant) indicating that there is no damage to tight junctions. These results suggest that SSPCF reinforces the epithelial barrier function. In addition to tissue integrity, treatment with SSPCF showed no effects on IL-8 and LDH secretion suggesting a good tolerability of the formula by nasal epithelial cells in vitro.
Given that SSPCF appears safe and well tolerated, the efficacy of SSPCF treatment was assessed by MCC rate measurements. MCC is a first line defense mechanism that aids the airway epithelia to clear foreign particles and chemicals, allowing the airways and lungs to remain healthy. It depends on cilia beating and the existence of a protective mucus layer acting to remove foreign inhaled particles and chemicals.25 During the MCC process, mucus that contains antimicrobial and antioxidant agents is secreted and microbes are trapped.39 Treatment with SSPCF caused a strong increase in the MCC rates that were maintained even 4 days after treatment compared to untreated cultures. Previous results from a clinical study had shown that administration of a 14.4% saline aerosol increased MCC in healthy and asthmatic subjects, compared to 0.9% saline aerosol, suggesting that salt concentration may have an impact on MCC.40 Since SSPCF contains a high NaCl concentration (2.3%), it is possible that the observed increase in MCC is due to the increase in salt content compared to less concentrated saline solutions.
It is assumed that hypertonic solutions, when used for nasal irrigation, naturally act as decongestants through draining water from the nasal epithelial tissues. Increased water in the nasal cavity may dilute mucus and enable clearance. In order to measure the water draining potential of SSPCF, ATP release quantification assay was used. Airway epithelial cells release ATP, a paracrine regulator, in response to stress situations such as hypotonic stress-induced swelling, loss of cell membrane integrity, etc.19,41 SSPCF treatment, after hypotonic stress, decreased the amount of ATP release by 87%, compared to treatment with an isotonic saline solution.
In addition to the effect of SSPCF treatment on recovery from hypotonic stress, the morphology of the tissues was monitored by aquaporin (AQP3) and Alcian blue staining. Aquaporins are membrane-bound water channel proteins with distinctive expression pattern in the nasal epithelium. They ensure rapid water transport across cell membranes in response to osmotic gradients.27,42 One member of aquaporins, AQP3, is expressed in the basolateral cells of the airway epithelial lining (nasal conchae, tracheal, and nasopharyngeal epithelium) which suggests that the modulation of airway surface liquid, air humidification, and generation of nasopharyngeal secretions involve a coordinated network of aquaporin water channels.27,43 On the other hand, Alcian blue stains the cell membrane and is used to visualize the cellular organization of the tissues. Using these approaches, Figure 5 shows that SSPCF treatment helped recover the tissue structure from hypotonic stress only 2 mins after application.
The limitations of the study include the low number of replicates used in the assays and the controls used in different experiments: ie, saline in TEER, LY, and ATP release assays, and untreated conditions in the rest of the experiments. Based on the positive outcomes, further experiments with higher number of samples and use of consistent inter-experiment controls should be conducted in the future.
Overall, the present study suggests that SSPCF, based on hypertonic seawater, is safe, well tolerated and effective in promoting and maintaining MCC and recovery from hypotonic stress, without affecting the cellular organization of the epithelial cells. Thus, we hypothesize that SSPCF works to alleviate symptoms of nasal congestion. In addition, since nasal saline solutions are non-prescription medicinal products, their use could limit the cost and frequency of medical visits and the irrational use of some medical solutions.
Conclusion
This study suggests the in vitro efficacy of SSPCF, a novel hypertonic seawater solution enriched with hyaluronic acids, eucalyptus oil, copper and manganese salts in MCC and recovery from hypotonic stress with good levels of tolerability. These collective findings suggest a potential for SSPCF to relieve symptoms of common cold patients by contributing to airway decongestion. Further studies are required to better understand the benefits of the SSPCF at a clinical level.
Abbreviation list
3D, three dimensional; AQP3, Aquaporin 3; ATP, adenosine 5′-triphosphate; BSA, bovine serum albumin; ELISA, enzyme-linked immunosorbent assay; HF, human fibroblast; IL-8, interleukin 8; LDH, lactate dehydrogenase; LY, Lucifer yellow; MCC, mucociliary clearance; PBS, phosphate-buffered saline; SSPCF, Stérimar Stop & Protect Cold and Flu; TEER, transepithelial electrical resistance.
Acknowledgments
The authors would like to thank Dr. Philippe Contencin and Dr. Annahita Ghassemi for their valuable contribution to the elaboration and revision of the manuscript. This study has been sponsored by Church & Dwight, Co., Inc. Poster presentation: Partial presentations of this work have been made as posters at the “VIII Rhinology Breaking Barriers to Excellence” Congress at Sao Paulo (Brazil), and at the “27th Congress of the European Rhinologic Society” at London (United Kingdom), both in April 2018.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
AS works as EU Technology & Innovation Manager at Church & Dwight, Co., Inc. which owns the Stérimar Brand. Dr. Song Huang and Dr. Samuel Constant have received funding from Epithelix, during the conduct of the study. Dr. Amina Saaid report personal fees from Laboratoires Fumouze, outside the submitted work and has patents EP3415154 A1,EP2985027 A1 and EP2985019 A1 issued. The authors report no other conflicts of interest in this work.
References
1. Passioti M, Maggina P, Megremis S, Papadopoulos NG. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413. doi:10.1007/s11882-013-0413-5
2. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. Cmaj. 2014;186(3):190–199. doi:10.1503/cmaj.121442
3. Leder K, Sinclair MI, Mitakakis TZ, Hellard ME, Forbes A. A community-based study of respiratory episodes in Melbourne, Australia. Aust N Z J Public Health. 2003;27(4):399–404.
4. Smith SM, Schroeder K, Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database Syst Rev. 2014;11:CD001831.
5. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;2:CD001107.
6. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329–338. doi:10.1056/NEJMoa0804897
7. Taverner D, Latte J. Nasal decongestants for the common cold. Cochrane Database Syst Rev. 2007;1:CD001953.
8. Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353(4):341–348. doi:10.1056/NEJMoa044441
9. Singh M. Heated, humidified air for the common cold. Cochrane Database Syst Rev. 2013;6:CD001728.
10. Bondon-Guitton E. Effets indésirables cardio-vasculaires et neurologiques des specialités à base de vasoconstricteur à visée décongestionnante. Bulletin d’informations de pharmacologie du CHU de Toulouse. 2012;19(1):1–17.
11. Slapak I, Skoupa J, Strnad P, Hornik P. Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children. Arch Otolaryngol Head Neck Surg. 2008;134(1):67–74. doi:10.1001/archoto.2007.19
12. King D, Mitchell B, Williams CP, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;4:CD006821.
13. Wang YH, Yang CP, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 2009;73(12):1696–1701. doi:10.1016/j.ijporl.2009.09.001
14. Wang YH, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in atopic children. J Microbiol Immunol Infect. 2014;47(1):63–69. doi:10.1016/j.jmii.2012.08.018
15. Heatley DG, McConnell KE, Kille TL, Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngol Head Neck Surg. 2001;125(1):44–48. doi:10.1067/mhn.2001.115909
16. Culig J, Leppee M, Vceva A, Djanic D. Efficiency of hypertonic and isotonic seawater solutions in chronic rhinosinusitis. Med Glas (Zenica). 2010;7(2):116–123.
17. Essaidi-Laziosi M, Brito F, Benaoudia S, et al. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J Allergy Clin Immunol. 2018;141(6):2074–2084. doi:10.1016/j.jaci.2017.07.018
18. Balogh Sivars K, Sivars U, Hornberg E, et al. A 3D human airway model enables prediction of respiratory toxicity of inhaled drugs in vitro. Toxicol Sci. 2018;162(1):301–308. doi:10.1093/toxsci/kfx255
19. Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281(32):22992–23002. doi:10.1074/jbc.M603019200
20. Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20(2):107–126. doi:10.1177/2211068214561025
21. Volpe DA. Application of method suitability for drug permeability classification. AAPS J. 2010;12(4):670–678. doi:10.1208/s12248-010-9227-8
22. Cho MH, Niles A, Huang R, et al. A bioluminescent cytotoxicity assay for assessment of membrane integrity using a proteolytic biomarker. Toxicol In Vitro. 2008;22(4):1099–1106. doi:10.1016/j.tiv.2008.02.013
23. Cao HB, Wang A, Martin B, et al. Down-regulation of IL-8 expression in human airway epithelial cells through helper-dependent adenoviral-mediated RNA interference. Cell Res. 2005;15(2):111–119. doi:10.1038/sj.cr.7290275
24. Antunes MB, Cohen NA. Mucociliary clearance–a critical upper airway host defense mechanism and methods of assessment. Curr Opin Allergy Clin Immunol. 2007;7(1):5–10. doi:10.1097/ACI.0b013e3280114eef
25. Delmotte P, Sanderson MJ. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am J Respir Cell Mol Biol. 2006;35(1):110–117. doi:10.1165/rcmb.2005-0417OC
26. Ohbuchi T, Takenaga F, Hohchi N, Wakasugi T, Ueta Y, Suzuki H. Possible contribution of pannexin-1 to ATP release in human upper airway epithelia. Physiol Rep. 2014;2(2):e00227. doi:10.1002/phy2.227
27. Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol. 1997;273(5 Pt 1):C1549–C1561. doi:10.1152/ajpcell.1997.273.5.C1549
28. Scott JE. Alcian blue. Now you see it, now you don’t. Eur J Oral Sci. 1996;104(1):2–9.
29. Brown CL, Graham SM. Nasal irrigations: good or bad? Curr Opin Otolaryngol Head Neck Surg. 2004;12(1):9–13.
30. Prosdocimi M, Bevilacqua C. Exogenous hyaluronic acid and wound healing: an updated vision. Panminerva Med. 2012;54(2):129–135.
31. Lee JS, Lee SU, Che CY, Lee JE. Comparison of cytotoxicity and wound healing effect of carboxymethylcellulose and hyaluronic acid on human corneal epithelial cells. Int J Ophthalmol. 2015;8(2):215–221. doi:10.3980/j.issn.2222-3959.2015.02.01
32. Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12(2):79–87. doi:10.1006/scdb.2000.0244
33. Ciofalo A, Zambetti G, Altissimi G, et al. Pathological and cytological changes of the nasal mucosa in acute rhinosinusitis: the role of hyaluronic acid as supportive therapy. Eur Rev Med Pharmacol Sci. 2017;21:4411–4418.
34. Salari MH, Amine G, Shirazi MH, Hafezi R, Mohammadypour M. Antibacterial effects of Eucalyptus globulus leaf extract on pathogenic bacteria isolated from specimens of patients with respiratory tract disorders. Clin Microbiol Infect. 2006;12(2):194–196. doi:10.1111/j.1469-0691.2005.01284.x
35. Neher A, Gstöttner M, Thaurer M, et al. Influence of essential and fatty oils on ciliary beat frequency of human nasal epithelial cells. Am J Rhinol. 2008;22(2):130–134. doi:10.2500/ajr.2008.22.3137
36. Borkow G, Gabbay J. Copper as a biocidal tool. Curr Med Chem. 2005;12(18):2163–2175.
37. Brophy MB, Nolan EM. Manganese and microbial pathogenesis: sequestration by the Mammalian immune system and utilization by microorganisms. ACS Chem Biol. 2015;10(3):641–651. doi:10.1021/cb500792b
38. Hollenhorst MI, Richter K, Fronius M. Ion transport by pulmonary epithelia. J Biomed Biotechnol. 2011;2011:174306.
39. Zanin M, Baviskar P, Webster R, Webby R. The interaction between respiratory pathogens and mucus. Cell Host Microbe. 2016;19(2):159–168. doi:10.1016/j.chom.2016.01.001
40. Daviskas E, Anderson SD, Gonda I, et al. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. Eur Respir J. 1996;9(4):725–732.
41. Vidal LS. Mechanisms of ATP Release in Airway Epithelial Cells. University of North Carolina at Chapel Hill; Chapel Hill, NC, USA; 2010.
42. Ablimit A, Aoki T, Matsuzaki T, et al. Immunolocalization of water channel aquaporins in the vomeronasal organ of the rat: expression of AQP4 in neuronal sensory cells. Chem Senses. 2008;33(5):481–488. doi:10.1093/chemse/bjn015
43. Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24(3):224–234. doi:10.1165/ajrcmb.24.3.4367
Supplementary materials
Results of individual experiments
 |
Table S1 TEER |
 |
Table S2 LY |
 |
Table S3 LDH |
 |
Table S4 IL-8 |
 |
Table S5 MCC |
 |
Table S6 ATP |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.