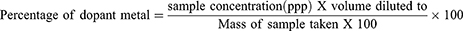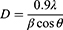Back to Journals » Journal of Experimental Pharmacology » Volume 15
In vitro Evaluation of Antibacterial Activity of Synthetic Zeolite Supported AgZno Nanoparticle Against a Selected Group of Bacteria
Received 19 November 2022
Accepted for publication 9 March 2023
Published 14 March 2023 Volume 2023:15 Pages 139—147
DOI https://doi.org/10.2147/JEP.S396118
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Berhanu Wakweya,1 Wakuma Wakene Jifar2
1Department of Chemistry, College of Natural and Computational Sciences, Mattu University, Mettu, Oromia, Ethiopia; 2Department of Pharmacy, College of Health Sciences, Mattu University, Mettu, Oromia, Ethiopia
Correspondence: Wakuma Wakene Jifar, Department of Pharmacy, College of Health Sciences, Mattu University, P.O. Box 318, Mettu, Oromia, Ethiopia, Tel +251963421238, Email [email protected]
Background: The development of novel and intriguing nanoparticle (NP)-based materials with antibacterial activity has recently received attention due to the problem of bacterial resistance to conventional antibiotics becoming more and more frequent. Thus, this study aimed to investigate the antibacterial effectiveness of a synthetic zeolite-supported AgZnO nanoparticle against selected bacteria in vitro.
Methods: Using the disc diffusion method, the antibacterial activity of synthetic zeolite-supported AgZnO nanoparticles was assessed against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). Zinc oxide (ZnO) and Ag/ZnO nanoparticles were used to create the zeolite-supported Ag/ZnO composite. Chloramphenicol was used as a standard drug. The nanoparticles and composites were characterized using powder X-ray diffraction (XRD), Fourier transform infrared (FTIR), and atomic absorption spectroscopy (AAS).
Results: Synthetic zeolite-supported AgZnO nanoparticles showed promising antibacterial properties with the largest zone of inhibition against S. aureus bacteria in comparison to E. coli. The synthetic zeolite-supported AgZnO nanoparticle displayed a zone of inhibition against S. aureus and E. coli without a remarkable difference compared to the respective standard drug (Chloramphenicol). Zinc peaks were visible in the X-ray diffractograms, which supported the theory that the characteristic hexagonal wurtzite structure of zinc oxide was present.
Conclusion: All types of ZnO, AgZnO, and AgZnO-Zeolite showed wide-spectrum activity with better effect against gram-positive bacteria, while the Zeolite-Ag/ZnO composite showed even better antibacterial activity. The findings suggest a potential bactericide that needs further evaluation in future studies was observed in synthetic zeolite-supported Ag/ZnO nanoparticles.
Keywords: nanoparticles, zeolite, Ag/ZnO nanoparticles, antibacterial activity
Introduction
Chronic infections and deaths are frequently seen across the globe because of infections caused by pathogens. Even though currently available drugs for treatment of bacterial illness is less expensive and effective, different literature reported as bacterial developed resistance.1 Nowadays bacterial developed resistance to antibiotics in three major mechanisms. Those are as follows: those who act on DNA replication, translation, and cell wall synthesis.2 Nanoparticles (NPs) have a mode of action that involves direct contact with the bacterial cell wall rather than cell penetration, rendering the majority of antibiotic resistance mechanisms irrelevant. This raises the possibility that NPs will be less likely to cause bacterial resistance than antibiotics.3 For instance, two frequent and significant causes of resistance to conventional antibiotics are increased efflux and decreased absorption of drugs in bacterial cells (such as S. aureus and E. coli).4 The ability of numerous NPs to circumvent these drug resistance-inhibiting pathways has been demonstrated by researchers. For instance, dendrimers can prevent P-glycoprotein-mediated efflux in the gastrointestinal system.5 A renewed effort is needed to find antibacterial agents that are effective against pathogenic bacteria resistant to present antibacterials because of this resistance issue.6 In order to create next-generation approaches to managing microbial diseases, it is crucial to uncover novel antimicrobial agents from natural and inorganic compounds.7 Gram-positive and gram-negative bacteria S. aureus and E. coli are examples of are capable of attaching to, colonizing, and creating biofilms on surfaces.8 These bacteria serve as industry-standard markers of food contamination, and their management shows that the surfaces and manufacturing materials used in food processing are kept clean and hygienic.9
Currently, drugs that derived from Nano biotechnology solutions were also shown to play a detrimental role in healing chronic disease complication like diabetes foot ulcer due to their involvement in wound healing.10 A growing number of researchers investigating the potential antibacterial processes of NPs have been conducted as a result of the expanding usage of NPs in medicine. Metal nanoparticles, for instance, can alter the metabolic activity of bacteria.11 The ability of NPs to enter biofilms also offers a practical method to inhibit biofilm formation based on the Ag-inhibited expression of genes because biofilm is a barrier that bacteria use to resist antibiotics. This capability represents a significant advantage in terms of eliminating bacteria to treat diseases.12 Silver has been shown to be a promising candidate for antibacterial activity against a wide variety of pathogenic bacteria, and silver nanoparticles (Ag-NPs) are widely employed in pharmaceutical products to make lotions and ointments that fight infections that are related to burns and open wounds.13 Applications for antibacterial activity of the other nanoparticles, such as ZnO, TiO2, WO3, SnO2, CuO, and MgO, are also of interest.14 By combining zinc oxide nanoparticles with silver, a substance with powerful antibacterial properties against both gram-negative and gram-positive microbes would result15 and in addition, AgZnO was supplemented by zeolite in order to generate a synergistic effect, which will allow for a wider range of uses for materials’ antibacterial capabilities.16,17
Using a support with larger dimensions and a porous matrix is one of the new techniques for introducing metal oxide nanoparticles into polymeric materials with the goal of decreasing their aggregation and leaching from the surface or inside of industrial chemicals or dental products. Incorporating metal oxide nanoparticles in a porous matrix of zeolites is one of the synthesis techniques that has been used to increase the antibacterial properties of these materials.18 Zeolites are three-dimensional porous aluminosilicate nanostructures made of SiO4 and AlO4 tetrahedrons joined by oxygen atoms to form a crystal structure with atomic-scale cavities and channels. Because of their special structure, cations can interact directly with microorganisms when they come into contact with zeolites.19 The antibacterial efficacy of zeolites combined with these Ag/ZnO in vitro systems against bacteria like S. aureus and E. coli has not been established, to the authors’ knowledge. As a result, the primary goals of this study were to illustrate the synthesis and characterization of AgZnO nanocomposite materials supported by zeolite. Second, an in vitro investigation was conducted to determine the antibacterial activity of a synthetic zeolite-supported AgZnO nanoparticle against a particular bacterial species.
Methods and Materials
Site for Experimental Work
Nanoscale ZnO, Ag-doped ZnO, and zeolite-Ag-doped ZnO composites were synthesized and the characterizations of the synthesized nanomaterials by AAS instruments have been carried out at the Haramaya University research laboratory. X-ray diffraction (XRD) and Fourier transform infrared (FTIR) measurements were taken at the Geological Survey of Ethiopia (GSE) and Finfinne (the largest and capital of Oromia Regional State, which is also the capital of Ethiopia).
Apparatus and Instruments
XRD, AAS, FTIR, an oven, an analytical balance, a hot plate, a furnace, a ceramic crucible, an agate mortar, a water bath, a thermometer, volumetric flasks, pipettes, graduated cylinders, desiccators, a magnetic stirrer, test tubes, and beakers were included in the studies.
Chemicals and Reagents
Chemicals used include zinc nitrate hexahydrate [Zn(NO3)2.6H2O] sodium carbonate Na2CO silver nitrate AgNO3, ethanol CH3CH2OH, zeolite (ZSM-S), HCl g/mol, HNO3, and H2O2.
Preparation of ZnO Nanoparticles
In order to create pure ZnO nanoparticles, equimolar solutions of Zn (NO3)2.6H2O and Na2CO3 were produced separately on doubly deionized H2O. Drop by drop, [Zn(NO3)2.6H2O] solution was added to Na2CO3 solution while constantly stirring for 2 hrs. The reaction’s precipitate was allowed to settle for 24 hr before being filtered through a 0.2-m membrane filter by Whatman and thoroughly rinsed three times with both deionized water and ethanol. The precursor for ZnO was created by drying the filtered precipitate at 100°C. The precursor thus produced was dried and then subjected to a 24-hr calcination process at 300°C in a programmable furnace to produce ZnO nanoparticles.20
Preparation of Ag ZnO Nanoparticles
Precipitation was used to prepare Ag-doped ZnO.21 In a typical synthesis, 5 g of calcined zinc oxide was mixed with 10 mL of AgNO3 (0.18 M) (Zc). The sample was stirred and cooked for 30 min at 110°C. After being calcined at 400°C for 2 hours, the powders were cooled to room temperature and ground in an agate mortar. The obtained goods were labeled “silver-doped zinc oxide” (AZ).22
Synthesized Nanoparticles with the Zeolite
Ag/ZnO NPs and synthetic CBV5524G were formed in a solid state to create the resultant supported nanoparticle zeolite (ZSM). The solid was dried and crushed to generate a homogenous solid-state combination after the solvent had evaporated. This mixture was then calcined in air for an hour at 300 degrees Celsius. Zeolite Ag/ZnO-NPs synthesised and mixed with zeolite were agitated for 1 hour at 80°C.23
Methods of Characterization of Synthesized Nanoparticles
XRD and FTIR spectrophotometer were used to characterize the synthesized ZnO, Ag/ZnO NPs, and zeolite-supported Ag/ZnO nanocomposites. The amount of zinc and silver was determined by AAS.
Powdered X-Ray Diffraction Analysis (PXRD)
The crystalline phase formation and size of ZnO, Ag/ZnO NPs, and zeolite-supported Ag/ZnO nanocomposite samples were analyzed using X-ray diffraction (XRD) (D in nm) according to the Scherer’s equation.
Fourier Transform Infrared Spectroscopy (FTIR) Analysis
FTIR spectrum is a feature of a particular compound that gives information about its functional groups, molecular geometry, and inter/intermolecular interactions. Therefore, FTIR studies were used to indicate the presence of interaction between ZnO, Ag/ZnO NPs, and the molecular chain of zeolite in the Ag/ZnO NPs.
Atomic Absorption Spectrophotometer (AAS)
AAS was used to assess the metal content of the produced nanoparticles ZnO and Ag/ZnO. Using an acid digestion tube, 0.5 g of the nanoparticle powder was digested with 4 mL of HNO3, 4 mL of HCl, and 2 mL of H2O2 until a clear solution was produced. In order to plot the standard metal solutions for each metal, the stock solutions (standard solutions) of Zn (NO3)26H2O and AgNO3 were created by dissolving 0.5 g of the corresponding Zn (NO3)26H2O and AgNO3 in deionized water, respectively, in volumetric flasks. The following are the experimental percentages of Zn and Ag-metal in the synthesized nanoparticle:
Bioassay Test
Studies on antibacterial agents were undertaken on E. coli and S. aureus, two significant microorganisms. A mixture of chloramphenicol was used as a reference in bactericidal studies. Correlations of structures with antimicrobial activity of nanoparticles were critically examined using inhibition zone data.24
Preparation of Media
Bacteria from the culture were transferred to a Mueller Hinton agar (MHA) plate, streaked on it, and then incubated for 24 hr at 37°C. The bacteria were then added to autoclaved MHA, which had been vigorously swirled while cooling at around 45°C in a water bath. The Petri dishes were cleaned by adding the medium to them. Finally, sterilized plates were filled with the media containing the spore suspension, which then solidified and was used for the bioassay.
Preparation of Sample Solutions
The 10 mL and 20 mL aliquots were utilized for the biological test after the 10 mg samples of the nanoparticles were dissolved in 10 mL of distilled water to make solutions.
Antibacterial Activity Test
Office punches were used to make 6 mm-diameter holes in Whatman No. 1 filter paper discs, which were then sterilized for an hour at 180°C. A nanoparticle solution with two replications of 10 and 20 mL was pipetted onto the discs. The samples are then transferred using sterile forceps onto the impregnated disc paper from nutrient agar plates that have been seeded with bacteria. After that, these plates were incubated for 24 hr at 37°C.25
Result and Discussion
XRD Analysis
Figures 1: below show the XRD patterns of ZnO, Ag/ZnO, and zeolite-supported Ag/ZnO nanoparticles, respectively. The reflection from (100), (002), (101), (102), (110), and (103) crystal planes for all as-synthesized powders suggests pure hexagonal wurtzite structure of ZnO. The diffraction peaks observed at scattering angle 2θ of 31.9°, 34.6°, 36.319°, 47.8°, 56.8°, and 63° represent typical hexagonal wurtzite structure of ZnO.20 XDR of Ag-ZnO pattern likewise displayed a peak that was identical to ZnO’s peak without any doping. This suggests that the wurtzite structure is also present in the Ag/ZnO crystal structure. Moreover, an additional peak is observed at 2θ of 38.1°, evidencing the presence of Ag in the doped ZnO case. The diffraction peak patterns in ZnO and Ag-ZnO were identical in terms of position and intensity. The lack of such a shift in the recorded XRD suggests that Ag particles segregate at the grain boundaries of ZnO or that only a little amount of Ag was incorporated in the substitution Zn site. The latter alternative, however, did not appear feasible because of the silver particles’ preference for settling near the ZnO grain boundaries and the disparity in ionic radii between Ag+ (2.22 A°) and Zn+2 (0.72 A°) (Figure 1).26
 |
Figure 1 Showed the XRD patterns of ZnO (A), Ag/ZnO (B) and zeolite-supported Ag/ZnO (C) nanoparticles, respectively. |
Determination of Nanoparticle Size
Zeolite is present, as evidenced by the supported Ag-ZnO peaks with scattering angles 2 of 23.0°, 23.8°, 24.6°, and 30.0°. Additionally, the zeolite-supported Ag-ZnO composite exhibits all of the peaks noted in the Ag-ZnO instance. Using the Debye-Schires equation, the nanoparticles’ crystallite size was determined.
Where D is the average crystal size, λ is the wave length of X-ray = 1.5406 nm for copper (Cu) target Kα radiation, β is the full width at half –maximum of XRD peak and Ө is the Bragg’s diffraction angle. For ZnO, Ag/ZnO, and zeolite-Ag/ZnO composites, the strongest peaks appeared at 36.262°, 36.319°, and 36.235°, respectively. The average crystallite diameters (D) of the nanoparticles were calculated based on the strongest peaks, and the data are shown in Table 1.
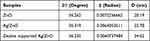 |
Table 1 Calculated Average Crystallite Size (D) of the Nanoparticles |
Atomic Absorption Spectroscopic Analysis
The metal composition of the sample served as the basis for measuring the percentages of Ag and Zn in the synthesised nanoparticles using AAS. Table 2 shows the outcomes of the AAS analysis of the metals(s) in ZnO and Ag/ZnO.
 |
Table 2 The Concentrations of Ag and Zn in the Synthesized ZnO, Ag/ZnO Nano Particles |
FTIR Analysis of Nanoparticles
The FTIR spectra of ZnO (A), Ag/ZnO (B), and zeolite-supported Ag/ZnO (C) nanoparticles are shown below Figure 2. All samples showed a broad band 3400–3446 cm/1, which might be caused by the OH group of nanoparticles’ vibration mode. The stretching vibration of ZnO is thought to be responsible for the absorption bands at 458.99 cm/1 and 712.75 cm/1 that can be seen in the spectra of ZnO, Ag/ZnO nanoparticles, and zeolite-supported Ag/ZnO composite, respectively.27,28 Additionally, the observed ZnO and Ag/ZnO nanoparticle maxima at 2426.73 cm−1 and 2932 cm−1, respectively, were caused by symmetric CH stretching. These extra peaks were brought on by the solvent (paraffin) used to prepare the samples. The remaining peaks, which were seen at 1469 and 1384.31, 1468.67, and 1384.31 cm−1 for ZnO and Ag/ZnO nanoparticles, respectively, were caused by the monodentate carbonate species’ symmetric OCO stretching vibration.29,30 As a result of AlOH stretching and symmetric OH, the peak at 3311.17 cm/1 was an Ag/ZnO composite supported by zeolite. The other peak, 875.87 cm/1, was caused by stretching vibrations caused by species derived from phyllosilicate species.27
 |
Figure 2 Showed the FTIR spectra of ZnO (A), Ag/ZnO (B), and zeolite-supported Ag/ZnO (C) nanoparticles. |
Antibacterial Studies
Table 3 indicated that, against gram-negative bacteria (E. coli) and gram-positive bacteria (S. aureus), the already synthesised ZnO, Ag/ZnO nanoparticles, and zeolite-supported Ag/ZnO composite activities were tested. As references antibiotics drug chloramphenicol was utilized. Finally, diameters for inhibition zone for all were determined. All synthetic nanoparticles have antibacterial properties, and the degree of bacterial growth inhibition increased with increasing nanoparticle dose. In comparison to undoped ZnO and Ag/ZnO, the antibacterial properties of Ag/ZnO supported on zeolite are more evident. ZnO and Ag/ZnO-NPs have minimally effective antibacterial effects on bacteria in both dosages. When the nanoparticles were entirely supported on zeolite, the antibacterial activity of Ag/ZnO was significantly boosted. While reference chloramphenicol has an antibacterial activity of 15.6 mm and 23.6 mm for the same antibacterial activity at the dose of 10 µl, Ag/ZnO-zeolite has an antibacterial activity of 15.5 mm and 16.2 mm for the doses of 10 µl and 20 µl, respectively. The antibacterial activity does not significantly increase as the dose is increased from 10 µl to 20 µl. When compared to the standard antibacterial drug chloramphenicol, Ag/ZnO-zeolite is more effective against the gram positive (S. aureus) bacteria, which ranges from 19.6 mm to 27.5 mm at doses of 12.5 mm and 19.5 mm for antibacterial activity, respectively. This implies that zeolite supported-Ag/ZnO NPs cover more gram-positive bacteria compared to gram-negative bacteria. The probable reason might be because of the smaller size of zeolite supported-Ag/ZnO NPs compared to other antibiotics, and they were able to easily penetrate bacteria more effectively, by rupturing their cell membrane. This improved their effectiveness as a bactericidal substance.31
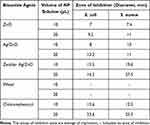 |
Table 3 Inhibition Zone of Bacterial Growth Inhibition (mm) |
Conclusion
For synthesizing Zinc oxide (ZnO) nanoparticles a precipitation method was used, by involving the reaction between [Zn(NO3)2.6H2O] and [Na2CO3] in aqueous solutions with proper concentrations of the reactants. The incipient wetness impregnation method was used to create Ag/ZnO nanoparticles by mixing synthesized ZnO nanoparticles with an aqueous solution of silver nitrate, and zeolite-supported Ag/ZnO composites were created by solidifying the mixture of synthesized ZnO and Ag/ZnO nanoparticles. The XRD data show that the entire nanoparticle composition produced has a pristine hexagonal wurtzite crystalline structure. Antibacterial tests for both gram-negative and gram-positive bacterial revealed that sterilized distilled water lacks any antibacterial capabilities; however, an inhibition was seen after dissolving synthesised (ZnO, Ag/ZnO, and zeolite supported Ag/ZnO) nanoparticles. Additionally, composites of zeolite-supported Ag/ZnO nanoparticles and Ag/ZnO were found to have superior antibacterial properties than ZnO NPs for all microorganisms. Ag/ZnO composites supported by zeolite showed the strongest antibacterial activity. These findings demonstrate that zeolite-supported Ag/ZnO nanoparticles may be altered to take on desired qualities, producing an antibacterial substance that is promising and effective to inhibit growth of S. aureus and E. coli bacterial with further studies. Evaluating the antibacterial effect of zeolite-supported Ag/ZnO nanocomposite for other bacterial and fungal strains is a future recommended studies to identify its broad spectrum. Also further characterization of the synthesized nanocomposite is encouraged to better understand its morphology and surface properties.
Abbreviations
NP, Nanoparticles; ZnO, zinc oxide; XYD, X-ray diffraction; FTIR, Fourier Transform Infrared Spectroscopy; AAS, Atomic absorption Spectroscopy; GSE, Geological Survey of Ethiopia; MHA, Mueller Hinton agar; MMP-9, Matrix Metallopeptidase 9.
Data Sharing Statement
The corresponding author will provide the datasets used and/or analysed during the current work upon reasonable request.
Ethical Approval
The experiment’s protocol was approved by Haramaya University’s ethical review board.
Acknowledgment
The necessary lab equipment was provided by Haramaya University, which the authors gratefully acknowledge.
Author Contributions
All authors agreed to submit to the current journal, provided final approval of the version to be published, made significant contributions to the conception and design, collection of data, analysis, and interpretation of data, participated in writing the article or critically revised it for important intellectual content, and agreed to be responsible for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hsueh P-R. New Delhi metallo-β-lactamase-1 (NDM-1): an emerging threat among Enterobacteriaceae. J Formos Med Assoc. 2010;109(10):685–687. doi:10.1016/S0929-6646(10)60111-8
2. Knetsch ML, Koole LH. New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers. 2011;3(1):340–366. doi:10.3390/polym3010340
3. Romero D, Aguilar C, Losick R, Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci. 2010;107(5):2230–2234. doi:10.1073/pnas.0910560107
4. Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65(13–14):1803–1815. doi:10.1016/j.addr.2013.07.011
5. Mühling M, Bradford A, Readman JW, Somerfield PJ, Handy RD. An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar Environ Res. 2009;68(5):278–283. doi:10.1016/j.marenvres.2009.07.001
6. Satcher D. Emerging infections: getting ahead of the curve. Emerg Infect Dis. 1995;1(1):1. doi:10.3201/eid0101.950101
7. Lanje AS, Sharma SJ, Ningthoujam RS, Ahn J-S, Pode RB. Low temperature dielectric studies of zinc oxide (ZnO) nanoparticles prepared by precipitation method. Adv Powder Technol. 2013;24(1):331–335. doi:10.1016/j.apt.2012.08.005
8. Zhao L, Wang H, Huo K, et al. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011;32(24):5706–5716. doi:10.1016/j.biomaterials.2011.04.040
9. Zhang X, Li M, He X, et al. Antibacterial activity of single crystalline silver-doped anatase TiO2 nanowire arrays. Appl Surf Sci. 2016;372:139–144. doi:10.1016/j.apsusc.2015.12.094
10. Jifar WW, Atnafie SA, Angalaparameswari S. A review: matrix metallopeptidase-9 nanoparticles targeted for the treatment of diabetic foot ulcers. J Multidiscip Healthc. 2021;14:3321. doi:10.2147/JMDH.S343085
11. Chatzimitakos TG, Stalikas CD. Qualitative alterations of bacterial metabolome after exposure to metal nanoparticles with bactericidal properties: a comprehensive workflow based on 1H NMR, UHPLC-HRMS, and metabolic databases. J Proteome Res. 2016;15(9):3322–3330. doi:10.1021/acs.jproteome.6b00489
12. Zhao L, Ashraf M. Influence of silver-hydroxyapatite nanocomposite coating on biofilm formation of joint prosthesis and its mechanism. West Indian Med J. 2015;64(5):506. doi:10.7727/wimj.2016.179
13. Khan I, Saeed K, Khan I. Review nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(2):908–931. doi:10.1016/j.arabjc.2017.05.011
14. Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18(22):225103. doi:10.1088/0957-4484/18/22/225103
15. Siddiqi KS, Husen A. Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Res Lett. 2016;11(1):1–13. doi:10.1186/s11671-016-1695-z
16. Kim I, Viswanathan K, Kasi G, Thanakkasaranee S, Sadeghi K, Seo J. ZnO nanostructures in active antibacterial food packaging: preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Rev Int. 2022;38(4):537–565. doi:10.1080/87559129.2020.1737709
17. Krishnamoorthy R, Athinarayanan J, Periyasamy VS, et al. Antibacterial mechanisms of zinc oxide nanoparticle against bacterial food pathogens resistant to beta-lactam antibiotics. Molecules. 2022;27(8):2489. doi:10.3390/molecules27082489
18. Azizi-Lalabadi M, Alizadeh-Sani M, Divband B, Ehsani A, McClements DJ. Nanocomposite films consisting of functional nanoparticles (TiO2 and ZnO) embedded in 4A-Zeolite and mixed polymer matrices (gelatin and polyvinyl alcohol). Food Res Int. 2020;137:109716. doi:10.1016/j.foodres.2020.109716
19. Azizi-Lalabadi M, Ehsani A, Divband B, Alizadeh-Sani M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci Rep. 2019;9(1):17439. doi:10.1038/s41598-019-54025-0
20. Chen C, Liu J, Liu P, Yu B. Investigation of photocatalytic degradation of methyl Orange by using nano-sized ZnO catalysts. Adv Chem Eng Sci. 2011;1(01):9. doi:10.4236/aces.2011.11002
21. Mirkhani V, Tangestaninejad S, Moghadam M, Habibi M, Vartooni AR. Photodegradation of aromatic amines by Ag-TiO 2 photocatalyst. J Iran Chem Soc. 2009;6:800–807. doi:10.1007/BF03246172
22. Srithar A, Kannan J, Senthil T. Preparation and characterization of Ag doped ZnO nanoparticles and its antibacterial applications. J Adv Chem. 2017;13:6273–6279.
23. Petkowicz DI, Pergher SB, Da Silva CDS, Da Rocha ZN, Dos Santos JH. Catalytic photodegradation of dyes by in situ zeolite-supported titania. Chem Eng J. 2010;158(3):505–512. doi:10.1016/j.cej.2010.01.039
24. Somayeh R, Mahmood AA, Salmah BI, Pouya H. Antibacterial activity of leaf extracts of Baeckea frutescens against methicillin-resistant Staphylococcus aureus. Biomed Res Int. 2014;2014:521287.
25. Gonelimali FD, Lin J, Miao W, et al. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol. 2018;9:1639. doi:10.3389/fmicb.2018.01639
26. Tesfaye E, Animut G, Urge M, Dessie T. Moringa oleifera leaf meal as an alternative protein feed ingredient in broiler ration. Int J Poult Sci. 2013;12(5):289–297. doi:10.3923/ijps.2013.289.297
27. Pham TAT, Tran VA, Le VD, et al. Facile preparation of ZnO nanoparticles and Ag/ZnO nanocomposite and their photocatalytic activities under visible light. Int J Photoenergy. 2020;2020:1–14. doi:10.1155/2020/8897667
28. Hameed S, Khalil AT, Ali M, et al. Greener synthesis of ZnO and Ag–ZnO nanoparticles using Silybum marianum for diverse biomedical applications. Nanomedicine. 2019;14(6):655–673. doi:10.2217/nnm-2018-0279
29. Tankov I, Cassinelli W, Bueno J, Arishtirova K, Damyanova S. DRIFTS study of CO adsorption on praseodymium modified Pt/Al2O3. Appl Surf Sci. 2012;259:831–839. doi:10.1016/j.apsusc.2012.07.138
30. Cao Y, Yu M, Qi S, et al. Formation and evolution of orientation-specific CO2 chains on nonpolar ZnO (1010) surfaces. Sci Rep. 2017;7(1):1–7. doi:10.1038/s41598-016-0028-x
31. Wu S-Q, Li M-Y, Fang B-S, Tong H. Reinforcement of vulnerable historic silk fabrics with bacterial cellulose film and its light aging behavior. Carbohydr Polym. 2012;88(2):496–501. doi:10.1016/j.carbpol.2011.12.033
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.