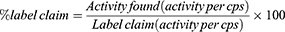Back to Journals » Drug Design, Development and Therapy » Volume 15
In vitro Comparison of Pancreatic Enzyme Preparations Available in the Indian Market
Authors Shrikhande SV, Prasad VGM, Domínguez-Muñoz JE, Weigl KE, Sarda KD
Received 12 May 2021
Accepted for publication 28 August 2021
Published 7 September 2021 Volume 2021:15 Pages 3835—3843
DOI https://doi.org/10.2147/DDDT.S319949
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Shailesh V Shrikhande,1 VG Mohan Prasad,2 J Enrique Domínguez-Muñoz,3 Kevin E Weigl,4 Kushal D Sarda5
1Division of Cancer Surgery and Gastrointestinal and Hepato-Pancreato-Biliary Service, Tata Memorial Hospital, Mumbai, Maharashtra, India; 2Department of Gastroenterology, Dr. M.G.R. Medical University and VGM Hospital, Coimbatore, Tamil Nadu, India; 3Department of Gastroenterology and Hepatology, University Hospital of Santiago de Compostela, Santiago de Compostela, Spain; 4Department of Gastroenterology, Abbott Laboratories GmbH, Hannover, Germany; 5Established Pharmaceuticals Division – Medical Affairs, Abbott India Ltd, Mumbai, Maharashtra, India
Correspondence: Kushal D Sarda
Abbott India Ltd, Floor 16, Godrej BKC, Plot No. C – 68, Bandra-Kurla Complex, Near MCA Club, Bandra (E), Mumbai, 400051, Maharashtra, India
Tel +91-22-38160938
Fax +91 22 38162400
Email [email protected]
Purpose: Pancreatic enzyme replacement therapy (PERT) involves exogenous enzyme supplementation and is used in the treatment of pancreatic exocrine insufficiency. Clinical efficacy of PERT preparations is a function of physical properties and release kinetics that vary between commercially available products. In this study, we evaluated the physical properties, in vitro dissolution, and release kinetics of commercially available pancreatic enzyme preparations available in the Indian market.
Methods: Physical properties such as particle size distribution and water content of the capsules were measured by dynamic light scattering and Karl–Fischer titration method, respectively. An analytical procedure based on the European pharmacopoeia (EP) method was used to determine lipase activity, and a modified United States pharmacopoeia (USP)–based method was used for dissolution studies. Enzyme release was ascertained under gastroduodenal conditions in buffered media.
Results: Considerable variations in physical properties such as particle size and water content were observed between pancreatic enzyme preparations. Some preparations failed to meet the labeled lipase content as per USP standards (> 90% label claim) and showed inconsistent release behavior (> 5% relative standard deviation).
Conclusion: Differences exist between pancreatic enzyme preparations in terms of physical properties, dissolution, and release behavior that can affect their clinical efficacy. The present study suggests, therefore, that these preparations should not be used interchangeably.
Keywords: pancreatic enzyme, physical properties, in vitro dissolution, lipase activity, release kinetics
Introduction
Pancreatic exocrine insufficiency (PEI) is defined as inadequate intraluminal pancreatic enzyme activity, due to insufficient enzyme production, insufficient enzyme activation, or early enzyme degradation leading to maldigestion and malabsorption of nutrients. PEI can be classified as primary or secondary. Primary PEI is due to lack of exocrine pancreatic tissue or disturbances in the postprandial stimulation of pancreatic secretion, whereas inappropriate enzyme activation or inadequate mixing of enzymes with the chyme after surgery causes secondary PEI.1 PEI is a condition that is commonly associated with several pancreatic diseases such as chronic pancreatitis, acute necrotizing pancreatitis, ductal obstruction of the pancreas of any etiology, cystic fibrosis, pancreatic and periampullary cancer, or surgical procedures such as pancreatectomy, gastrectomy, or gastrointestinal bypass surgery.2 Insufficient secretion of pancreatic enzymes (lipases, proteases, and amylases) into the duodenum results in nutrient malabsorption, which may cause weight loss, gastrointestinal symptoms such as diarrhea, abdominal distension, abdominal cramps, flatulence and bloating, and nutritional deficiencies of proteins, essential amino acids, fatty acids, micronutrients, and fat-soluble vitamins.2–4
Pancreatic enzyme replacement therapy (PERT) is the cornerstone of PEI management and involves oral replacement of pancreatic enzymes. Exogenous enzyme supplementation minimizes the malabsorption of fat, carbohydrate, and protein from the diet and improves symptoms of PEI.5,6 Pancreatic enzyme preparations differ in the content of their lipase, amylase, and protease enzymes and are conventionally labeled depending upon their lipase activity.7
An effective PERT preparation should intersperse well with chyme, resist inactivation by gastric juices, empty from the stomach simultaneously with nutrients, and release enzymes rapidly in the proximal small intestine.7,8 Modern pancreatic enzyme preparations are pH-sensitive, enteric-coated pellets that protect the enzymes from gastric acidity. They provide a large surface area and their small size allows the preparation to pass through the pylorus and facilitates ready dispersal in chyme.7–10 Enteric-coated microspheres of <2 mm in size are the preparations of choice for PEI.9
The United States Food and Drug Administration (US FDA) decided to change the status of pancreatic enzyme products from over-the-counter to prescription-only status due to their non-comparability and further advocated treatment monitoring by a physician. These differences were attributed to the manufacturing process used for enteric coating formulations.2,11 In order to justify their interchangeable use, variability in the lipase content and release of enzymes from different formulations make it important to investigate commercially available pancreatin preparations (branded and generic) regarding key performance metrics that can ultimately affect availability of the enzyme and clinical response in vivo. Previous studies have indicated variation in pancreatic enzyme products available in Western Europe and Russia in terms of actual enzyme content and in vitro release; however, no such studies were performed on pancreatic enzyme products marketed in India.2,7,8
The objective of this study was to compare the physical properties, formulation characteristics, and in vitro dissolution and release kinetics of various pancreatin preparations available in the Indian market.
Materials and Methods
Materials
In this study, 10 batches of pancreatic enzyme capsule preparations (Creon® 10,000, Creon® 25,000, Creon® 40,000, Agna™ 10,000, Agna™ 25,000, Enzar™ 10,000, Enzar™ 25,000 or Enzar™ HS, Enzar™ 40,000, Panlipase® 10,000, and Panlipase® 25,000) were analyzed. All preparations were encapsulated enteric-coated pellets, which were either color-coded or labeled according to their strength. The labeling on the products corresponded to lipase content in European Pharmacopoeia (EP) or United States Pharmacopoeia (USP) units.12,13 Table 1 contains an overview of the products that were analyzed in the study.
 |
Table 1 Overview of Pancreatic Enzyme Products Investigated |
Particle Size Distribution
Particle size analysis was performed using a Qicpic dynamic imaging system (Sympatec GmbH) with a gravity disperser GRADIS with 4-mm split and the feeding system VIBRI.8 Each batch was analyzed in triplicate. The start of each measurement was triggered by reaching an optical concentration of ≥0.2%. The sample was automatically dry dispersed and analyzed with the Qicpic. The M7 (10–3410 µm) measuring range was used for particle size determination. Feret max X50 was used to describe the size of the particles. Particle sizes were represented with the aid of the percentile range X50, which represents the particle size at which 50% of the material is smaller than this range.
Water Content
Water content was determined using a method based on the Karl–Fischer coulometric titration method. Water was evaporated by heating (130°C) the sample in a Metrohm KF-Oven Sample Processor 774 and transferred by a nitrogen stream (80 mL/min) into the Metrohm Coulometer 831 with 774 SC Controller measuring cell. Water in the measuring cells reacts with iodine and sulfur dioxide (contained in the Karl–Fischer reagent) by releasing iodine ions and protons. The amount of water thus correlates to the protons, which are quantitated by titration using the Metrohm Dosino 800 Dosing System and Metrohm 846 Dosing Interface. The absolute water amount of a sample is calculated by the measured value of total water in the sample minus the water determined for the blank. The water content of a sample in % (w/w) is calculated relative to the sample weighting by the system. The contents of 20 capsules were combined and then samples of 100–200 mg were analyzed (n = 2 per product).
Lipase Activity
Lipase enzymatic assay was performed using a Metrohm 907 Titrando and an analytical procedure based on the EP method.12 The amount of olive oil hydrolysis was compared to a reference standard with known activity (pancreas powder BRP) to determine the lipase activity of the sample.12 Since the lipase activity of Panlipase® products are listed in USP units, a USP method was additionally used to determine their lipase activity and check the validity of the label claim. The contents of 20 capsules of each product were separated from their capsule shells and combined and milled for the assay (n = 2 determinations per product). The % label claim for lipase activity was calculated as follows:
Enteric Coating Dissolution
A modified USP dissolution method as described for pancrelipase delayed-release capsules was performed using a Sotax ATR smart dissolution tester.13 Sample preparation involved removal and mixing of the pellets from 10 capsules of each product. Enough sample to be in the lipase activity measurement range per vessel was tested (n = 6 determinations per product). Briefly, the pellets were incubated in 800 mL simulated gastric fluid (pH 1) for 2 h in a rotating basket (100 rpm) apparatus (EP/USP basket apparatus). In the second part, the pellets were transferred to 800 mL of phosphate buffer (pH 6) and stirred for 30 min in an EP/USP paddle dissolution apparatus at 100 rpm. For Agna™ 10,000 and Agna™ 25,000, the pellets were exposed to 800 mL phosphate buffer (pH 6) in a rotating basket (100 rpm) for 30 min in EP/USP basket apparatus. Samples were taken in the second part at time points 10, 20, and 30 min, and the lipase activity determined. Two vessels containing a reference standard in phosphate buffer were treated under the same conditions as the samples. Actual lipase activity was determined using the procedure described for lipase activity. One sampling and titration were performed per sampling point. Reportable values were mean values per run (n = 6) and at different time points.
Enzyme Release
Enzyme release was analyzed by agitating the pellets in an Erweka Zt 304 disintegration apparatus that mimicked the conditions at the gastroduodenal transit.2 The contents of the capsules were separated from their shells and placed in a beaker containing 600 mL phosphate buffer pH 5 at 37°C. The pellets were agitated for 60 min using a basket rack with a mesh bottom after which the pH was adjusted to 6 and the incubation was continued for another 60 min. The pH value was kept constant (± 0.1 pH units) in each part with the addition of sodium hydroxide or hydrochloric acid, 4 mL samples were withdrawn every 15 min, and lipase activity of the sample was determined. The results at each time point were reported as a percentage of the actual lipase activity.
Results
Particle Size Distribution
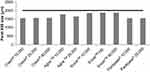
Figure 1 summarizes the particle size information for the tested products (n = 3). The continuous line at 2000 µm represents the largest particle size recommended in clinical guidelines for the treatment of PEI.9 Creon® and Panlipase® products had 50% of their pellets smaller than 1.6 mm, whereas Agna™ and Enzar™ products had 50% of their pellets <1.9 mm. Furthermore, Creon® and Agna™ products had a wider size distribution with pellets as small as 1–1.1 mm (data not shown).
Water Content
The water content of all Creon® products and Enzar™ 40,000 was below 5%, while Agna™ products, Panlipase® products, Enzar™ 10,000, and Enzar™ HS had a water content above 5% (Table 2).
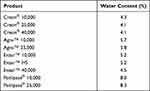 |
Table 2 Water Content of Pancreatic Enzyme Products |
Lipase Activity
The lipase activities for Creon®, Agna™, and Enzar™ products are listed in EP units while those for Panlipase® products are listed in USP units. Agna™ and Panlipase® products were found to have lipase activity significantly lower than their label claim (Table 3). The EP does not list a monograph on pancreas powder-finished products, but the USP contains a monograph on pancrelipase delayed-release capsules that provides a minimum lipase activity of 90%.13 Creon® and Enzar™ products met their label claim. In absolute terms, for drug products with a label claim of 10,000 lipase units, the rank order for lipase activity was Creon® 10,000>Panlipase® 10,000>Enzar™ 10,000>Agna™ 10,000. The rank order for lipase activity for drug products with label claim of 25,000 lipase units was Creon® 25,000>Enzar™ HS>Agna™ 25,000>Panlipase® 25,000. Finally, the lipase activities of Creon® 40,000 and Enzar™ 40,000 were similar.
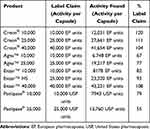 |
Table 3 Comparison of Actual Lipase Activity to Label Claim in Various Pancreatic Enzyme Products |
Dissolution
The USP monograph for pancrelipase delayed-release capsules specifies a dissolution tolerance of not less than 75% of the labeled units of lipase activity per capsule within 30 min in phosphate buffer at pH 6.13
Figure 2 shows the % dissolved (actual lipase activity) at 20 min and 30 min in phosphate buffer pH 6. After an initial incubation in simulated gastric fluid (pH 1) for 2 h, more than 90% of Creon® and Enzar™ products dissolved within 20 min in phosphate buffer at pH 6 (Figure 2). Under similar conditions, Agna™ products were slower to dissolve, but reached >80% dissolution after 30 min. Agna™ pellets were observed to be very buoyant, and thus the pellets were placed in a basket apparatus during incubation in phosphate buffer pH 6. Dissolution of both Panlipase® products was poor, with <25% of the pellets dissolving within 30 min (Figure 2).
Enzyme Release at pH 5/pH 6
To determine the stability of the gastro-resistant coating at pH 5, enzyme release from the drug products was determined using a disintegration apparatus. Ideally, the products should not release enzymes from pH 1 to pH 5 to avoid the deactivation of the enzymes in the stomach. This should be followed by disintegration of the enteric coating and enzyme release at pH 5.5, mimicking the duodenal conditions. No release of lipase activity was observed for all Creon® and Agna™ products at pH 5 followed by an immediate release at pH 6 (Figure 3A–C). In contrast, significant enzyme release was already observed for Enzar™ and Panlipase® products at pH 5. Therefore, the release of lipase activity for Enzar™ and Panlipase® products was also assessed in phosphate buffer pH 4. No release was demonstrated for all Enzar™ products; however, a significant and immediate release of lipase activity was observed for Panlipase® 10,000 and a slower, less pronounced release of lipase activity was observed for Panlipase® 25,000 (data not shown).
Discussion
This study provides an in vitro comparison of several pancreatic enzyme preparations available as capsules in the Indian market. In accordance with previous studies, the enzyme preparations differed significantly in terms of their physical properties and in vitro dissolution and release kinetics.2,7,8
Particle size and size distribution of the pellets of pancreatic enzyme preparations have been shown to have implications for their clinical efficacy.14,15 Several studies have shown that particles less than 2 mm in size allow for better dispersal with chyme and simultaneous emptying with chyme from the stomach to the duodenum.6,7,16–19 Products with a smaller particle size have been associated with higher lipolytic activity and a more rapid onset of action.14,15 In this study, the mean pellet size of Creon®, Agna™, Enzar™, and Panlipase® products was less than 2 mm. However, when considering the size distribution, Creon® and Agna™ products had pellet sizes with a lower limit of 1 mm. Agna™ pellets were found to be quite buoyant, a characteristic that could potentially affect their passage through the pyloric sphincter and delay their release into the duodenum and subsequent mixing with chyme. Kühnelt et al showed an earlier onset of intra-duodenal lipolytic activity for smaller microspheres compared to larger microspheres containing pancreatic enzymes, indicating a relationship between particle size and product efficacy.5 Enteric-coated microspheres or mini-microspheres of <2 mm size are the preparations of choice for PEI.9
The label claim for lipase activity was met by all Creon® products, Enzar™ HS, and Enzar™ 40,000 according to USP criteria (>90% of label claim).13 The lower lipase activity observed for Enzar™ 10,000, Agna™, and Panlipase® products may be associated with their high water content. Previous experience has shown that water content above 5% has a negative effect on the stability of lipase, and in our study, products with lipase activity below label claim had water content above 5%. The increased presence of water can catalyze enzymatic degradation and inactivation during product storage, resulting in a decrease in enzymatic activity. Loss of potency during storage can ultimately affect the quantity of enzymes available to support digestion and may result in a suboptimal and variable clinical response.
Ideally, pancreatic enzymatic preparations should exert their effect (ie release of enzyme activity) only once the pellets enter the duodenum together with the chyme. Therefore, they should be stable at low pH values to prevent deactivation of pancreatic enzymes in the stomach. All the enzymatic products tested in this study contain an enteric coating. It was observed that the digestive enzymes in Creon®, Agna™, and Enzar™ products were protected from the simulated gastric fluid at pH 1 for 2 h, followed by an immediate release at pH 6. In contrast, the significantly reduced lipase activity of Panlipase® products is indicative of instability of its enteric coating at pH 1. Although Creon®, Agna™, and Enzar™ products complied with the dissolution specification, the release from Creon® and Enzar™ products was most consistent (relative standard deviation <4%); this may have important implications for the reproducible behavior of these products. Although the pH of pure gastric secretions is pH 1–2, the buffering capacity and volume of food ingested can cause the pH in the stomach to rise temporarily to pH 5 and subsequent premature disintegration of coating and release of enzymes can occur.7 Any further drop in pH below 4 due to gastric acid secretion will irreversibly inactivate released pancreatic enzymes. Therefore, it is important to evaluate the release kinetics of the preparations from pH 5 to pH 6. The enteric coating of Creon® and Agna™ products was stable with no release of lipase activity at pH 5, followed by immediate release at pH 6. In contrast, Enzar™ and Panlipase® products showed lipase activity at pH 5 indicating a premature release of the enzyme and a loss of activity before reaching the duodenum.
Although this study provides a comparison of the release kinetics from different pancreatin preparations under pH values that mimic gastroduodenal conditions, it is important to note that studies were carried out in buffered media. Release studies in biorelevant dissolution media or simulated gastric fluids provide a better correlation between in vitro studies and possible clinical performance and should be considered when evaluating the performance of different preparations.
Conclusion
Considerable differences found between the physical and in vitro release behavior of the pancreatic enzyme preparations available in the Indian market could potentially affect in vivo availability and clinical efficacy. The labeled lipase activity, water content, and dissolution performance specifications were not met for all products. These results confirm findings from previous studies in that various PERT products differ in their in vitro characteristics and adherence to label claim and may therefore not be equivalent in terms of therapeutic effect; thus, treating physicians should be aware of these differences and should exercise caution when recommending replacement or “switching” between products.
Data Sharing Statement
Authors agree to make their data available upon reasonable request.
Ethics Approval and Informed Consent
Not applicable as this is an in vitro study.
Acknowledgments
The authors thank PharmEDGE for providing medical writing support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by Abbott India Ltd.
Disclosure
J Enrique Domínguez-Muñoz reports personal fees from Abbott Pharmaceuticals, grants from Viatris, outside the submitted work. Kevin E. Weigl is an employee of Abbott Laboratories GmbH, Hannover, Germany. Kushal D. Sarda is an employee of Abbott India Ltd. None of the remaining authors report any conflicts of interest in this work.
References
1. Lohr JM, Oliver MR, Frulloni L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. United European Gastroenterol J. 2013;1(2):79–83. doi:10.1177/2050640613476500
2. Kuhn RJ, Eyting S, Henniges F, Potthoff A. In vitro comparison of physical parameters, enzyme activity, acid resistance, and pH dissolution characteristics of enteric-coated pancreatic enzyme preparations: implications for clinical variability and pharmacy substitution. J Pediatr Pharmacol Ther. 2007;12(2):115–128.
3. Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol. 2011;4:55–73.
4. Brennan GT, Saif MW. Pancreatic enzyme replacement therapy: a Concise Review. JOP. 2019;20(5):121–125.
5. NHS UHoL. Pancreatic Enzyme Replacement Therapy (PERT) UHL Guideline B10 2019. Available from: https://secure.library.leicestershospitals.nhs.uk/PAGL/Shared%20Documents/Pancreatic%20Enzyme%20Replacement%20Therapy%20(PERT)%20UHL%20Guideline.pdf. Accessed on 02 Sep 2021.
6. Shandro BM, Nagarajah R, Poullis A. Challenges in the management of pancreatic exocrine insufficiency. World J Gastrointest Pharmacol Ther. 2018;9(5):39–46. doi:10.4292/wjgpt.v9.i5.39
7. Lohr JM, Hummel FM, Pirilis KT, Steinkamp G, Korner A, Henniges F. Properties of different pancreatin preparations used in pancreatic exocrine insufficiency. Eur J Gastroenterol Hepatol. 2009;21(9):1024–1031. doi:10.1097/MEG.0b013e328328f414
8. Maev IV, Kucheryavyy YA, Gubergrits NB, et al. Differences in in vitro properties of pancreatin preparations for pancreatic exocrine insufficiency as marketed in Russia and CIS. Drugs R D. 2020;20(4):369–376. doi:10.1007/s40268-020-00326-z
9. Lohr JM, Dominguez-Munoz E, Rosendahl J, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5(2):153–199. doi:10.1177/2050640616684695
10. Dominguez-Munoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9(2):116–122. doi:10.1007/s11894-007-0005-4
11. Federal Register. Docket No. 2003N-0205, Vol. 69, No. 2. Food and Drug Administration; April 28, 2004.
12. European Pharmacopeia, Monograph 0350, Pancreas Powder; 2020. Available from: https://www.edqm.eu/sites/default/files/medias/fichiers/European_Pharmacopoeia/The_European_Pharmacopoeia/European_Pharmacopoeia_10th_Edition/Index/european_pharmacopeia_supplement_10.2_english_index.pdfhttps://crs.edqm.eu/db/4DCGI/leaflet?leaflet=Y0001632_4. Accessed on 02 Sep 2021.
13. USP43-NF38—page 3384, Current DocID: GUID-B713FD29-6584-4961-8C46-4A56F436AB85_1_en-US, Pancrelipase Delayed-Release Capsules; 2020. Available from: https://pdf.hres.ca/dpd_pm/00057600.PDF. Accessed on 02 Sep 2021
14. Layer P, Groger G, Dicke D. Enzyme pellet size and luminal nutrient digestion in pancreatic insufficiency. Digestion. 1992;52:100–101.
15. Kuhnelt P, Mundlos S, Adler G. [Effect of pellet size of a pancreas enzyme preparation on duodenal lipolytic activity]. Z Gastroenterol. 1991;29(9):417–421. German.
16. Meyer JH, Elashoff J, Porter-Fink V, Dressman J, Amidon GL. Human postprandial gastric emptying of 1-3-millimeter spheres. Gastroenterology. 1988;94(6):1315–1325. doi:10.1016/0016-5085(88)90669-5
17. Stead RJ, Skypala I, Hodson ME, Batten JC. Enteric coated microspheres of pancreatin in the treatment of cystic fibrosis: comparison with a standard enteric coated preparation. Thorax. 1987;42(7):533–537. doi:10.1136/thx.42.7.533
18. Norregaard P, Lysgaard Madsen J, Larsen S, Worning H. Gastric emptying of pancreatin granules and dietary lipids in pancreatic insufficiency. Aliment Pharmacol Ther. 1996;10(3):427–432. doi:10.1111/j.0953-0673.1996.00427.x
19. Mundlos S, Kuhnelt P, Adler G. Monitoring enzyme replacement treatment in exocrine pancreatic insufficiency using the cholesteryl octanoate breath test. Gut. 1990;31(11):1324–1328. doi:10.1136/gut.31.11.1324
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.