Back to Journals » Drug Design, Development and Therapy » Volume 13
In-vitro blood-brain barrier models for drug screening and permeation studies: an overview
Authors Bagchi S, Chhibber T, Lahooti B, Verma A, Borse V , Jayant RD
Received 21 June 2019
Accepted for publication 12 August 2019
Published 18 October 2019 Volume 2019:13 Pages 3591—3605
DOI https://doi.org/10.2147/DDDT.S218708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Yan Zhu
Sounak Bagchi,1 Tanya Chhibber,1 Behnaz Lahooti,1 Angela Verma,1 Vivek Borse,2 Rahul Dev Jayant1
1Department of Pharmaceutical Sciences, School of Pharmacy, Texas Tech University Health Sciences Center, Amarillo, TX 79106, USA; 2Centre for Nanotechnology, Indian Institute of Technology Guwahati, Guwahati, Assam 781039, India
Correspondence: Rahul Dev Jayant
Department of Pharmaceutical Sciences, School of Pharmacy, Texas Tech University Health Sciences Center, 1406 S. Coulter Street, Suite 1104, Amarillo, TX 79106, USA
Tel +1 806 414 9154
Fax +1 806 356 4034
Email [email protected]
Abstract: The blood-brain barrier (BBB) is comprised of brain microvascular endothelial central nervous system (CNS) cells, which communicate with other CNS cells (astrocytes, pericytes) and behave according to the state of the CNS, by responding against pathological environments and modulating disease progression. The BBB plays a crucial role in maintaining homeostasis in the CNS by maintaining restricted transport of toxic or harmful molecules, transport of nutrients, and removal of metabolites from the brain. Neurological disorders, such as NeuroHIV, cerebral stroke, brain tumors, and other neurodegenerative diseases increase the permeability of the BBB. While on the other hand, semipermeable nature of BBB restricts the movement of bigger molecules i.e. drugs or proteins (>500 kDa) across it, leading to minimal bioavailability of drugs in the CNS. This poses the most significant shortcoming in the development of therapeutics for CNS neurodegenerative disorders. Although the complexity of the BBB (dynamic and adaptable barrier) affects approaches of CNS drug delivery and promotes disease progression, understanding the composition and functions of BBB provides a platform for novel innovative approaches towards drug delivery to CNS. The methodical and scientific interests in the physiology and pathology of the BBB led to the development and the advancement of numerous in vitro models of the BBB. This review discusses the fundamentals of BBB structure, permeation mechanisms, an overview of all the different in-vitro BBB models with their advantages and disadvantages, and rationale of selecting penetration prediction methods towards the critical role in the development of the CNS therapeutics.
Keywords: blood-brain barrier, BBB, brain microvascular endothelial cells, BMECs, tight junctions, TJs, proteins, central nervous system, CNS, induced pluripotent cells, iPSCs, in-silico prediction methods
Introduction
The first evidence for the existence of a barrier between the central nervous system (CNS) and the systemic circulation was reported by Paul Ehrlich in 1885 and Edwin Goldmann in 1913 and the term “Blood-brain barrier” (BBB) was coined by Stern and Gaultier in 1922.1,2 The nature of the BBB is semipermeable as it restricts the movement of detrimental molecules and cells from the blood and allows uptake of selective nutrients and hormones. The major cells comprising the BBB are brain microvascular endothelial cells (BMECs), which are supported by astrocytes, and pericytes.3 We briefly introduce the biological properties and functions of individual BBB components to discuss in vitro BBB models.1,4,5 The assurance of the access of nutrients to the brain is due to the large surface area, and the diminutive diffusion distance from the BBB capillaries to the neurons in the CNS and the chemicals/molecules penetrate the BBB by utilizing intra- and intercellular routes. Tight junctions (TJs) to facilitate the passage of molecules based on their lipophilicity, ionization, polarity and other physicochemical properties regulate the intracellular route whereas, the intercellular route [(transport of molecules from the luminal (apical) to the abluminal (basolateral) side of endothelial cells] is controlled by passive diffusion, endocytosis, and the ratio of influx and efflux transporters.6
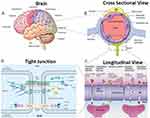 |
Figure 1 Structure and functionality of the Blood-Brain Barrier (BBB): (A) Brain structure- The brain has several barriers, including the BBB, the outer blood-cerebrospinal fluid (CSF)–brain barrier, and the blood–CSF barrier; (B) BBB structure- The BBB is formed by endothelial cells (ECs) that are in close association with astrocyte end feet and pericytes, forming a physical barrier; (C) BBB transport- Routes for molecular traffic across the BBB are shown. Some transporters are energy-dependent (P-glycoprotein, P-gp) and act as efflux transporters; (D) Tight junctions- Tight junctions are typically located on the apical region of ECs. The tight junctions form complex networks that result in multiple barriers that restrict the penetration of polar drugs into the brain.14,7 |
 |
Figure 2 Schematic representation of mechanisms available for drugs transport across the BBB: Schematic shows the main mechanism behind the drugs or small molecule transport across the BBB ie receptor-mediated transcytosis; adsorptive transcytosis (passive transport), diffusion or active transport. Notes: Reprinted from Adv Drug Deliv Rev, 103, Nair M, Jayant RD, Kaushik A, Sagar V., Getting into the brain: potential of nanotechnology in the management of NeuroAIDS, 202–217, Copyright 2016, with permission from Elsevier.14 |
The inefficacy of drugs to reach across the BBB is mainly due to poor physico-chemical or pharmacokinetic properties, i.e. inefficient absorption, distribution, metabolism, and excretion (ADME). In addition to ADME, the toxicity of CNS drugs is also one amongst the significant shortcomings.8 Drug exposure is controlled by plasma pharmacokinetic properties of the drug, which are different from the brain pharmacokinetics of the drug. Studying drug pharmacokinetics for CNS specifically involves understanding the correlation of physicochemical properties of drug compound and physiological function of the BBB.1,6,8 Therefore, in this review, we have discussed the fundamentals of BBB focusing on the permeation mechanisms, penetration measurement, prediction methods and diseases patterns that have been changing in recent times. In the case of infectious diseases of CNS or ageing disorders, the most significant factor is the incapability of BBB to maintain its integrity and open temporarily allowing the access of the drugs into the CNS. Unable to maintain the brain homeostasis and allowing minimal bioavailability of the drug into the CNS, the BBB directly contributes towards the progression of the pathological conditions. This makes BBB a potential target for designing the drugs9 that can cross the BBB to treat CNS disorders. The progress of new chemical entity into clinically effective drugs is hindered by numerous drawbacks intrinsic to modelling the CNS in vitro and in vivo for instance, the post-mitotic nature of neurons (limited access to primary cultures), presence of an intricate tissue cyto-architecture, anatomical and molecular inter-species diversities, and constrained access to the human brain. Therefore, in order to study the drug transmigration across the BBB, simplified in vitro BBB models have been developed, including the monolayer models, co-culture models, dynamic models, stem cell-based and microfluidic models, to understand the dynamics and role of the BBB. The in vitro BBB models come with the shortcoming of not being able to be replicated in vivo conditions, therefore understanding the limitations of the in vitro BBB models would be critical for experimental design and data interpretation.1
Fundamentals and composition of BBB
The BBB consists of a monolayer of BMECs connected by much tighter junctions than peripheral vessels and forms a cellular membrane, which is known as the physical backbone of the BBB.8,10 The key characteristics of the BBB are its uniform thickness, absence of fenestrae, least pinocytotic activity, and negative surface charge. In the BBB composition, the capillary basement membrane, pericytes, astrocytes, and microglial cells forming the neurovascular unit support BMECs. The basement membrane is made of collagen and elastin structural proteins, fibronectin and laminin, which are specialized proteins and finally proteoglycans, which gives the structural specificity and membrane stability. Additionally, pericytes are the cellular constituents of microvessels, including capillaries and postcapillary venules, which cover 22–32% of the capillaries and share the same basement membrane, helping in various structural and non-structural tasks of the BBB. It synthesizes structural and signaling proteins and contributes to the BMECs proliferation, migration, and differentiation processes.10 The astrocytes are other important types of cells, contributing as a part of the neurovascular unit of CNS. Additionally, the presence of immunocompetent brain microglial cells is crucial that checks the local microenvironment with its motile extensions and are capable of changing phenotype according to the homeostatic disturbance in the CNS.11 The interactions of the BMECs with the basement membrane, glial cells (microglia and astrocytes), neurons, and perivascular pericytes lead to specific brain microvascular biology. Presence of matrix adhesion receptors and signaling proteins form an extensive and complex matrix that is essential for the maintenance of the BBB (Figure 1).10,12
Molecular properties of BBB
The molecular constituents of TJs, adherence junctions, and signaling pathways regulate the BMECs assembly. TJs are highly dynamic structures that are responsible for the selective permeability property of the BBB as the apical region of the endothelial cells are sealed together by TJs allowing limited paracellular permeability. Structurally, TJs are formed by the interaction of integral transmembrane proteins with the adjacent plasma membrane.13 Among these proteins, junction adhesion molecules (JAM), claudins and occludins (intermembrane) bind to cytoplasmic proteins (e.g. zonula occludens, cinguline), and are well-studied for their role in the TJs constituting the BBB.14,7 Along with contribution to the physical restriction property of the BBB, TJs also provide functions such as control of gene expression, cell proliferation, and differentiation. Below the TJs, actin filaments (including cadherins and catenins) link together and form a belt of adherence junctions.6 These adherence junctions contribute to the barrier property along with additional roles such as promoting BMECs adhesions, contact inhibition throughout vascular growth, cell polarity and controlling paracellular permeability regulations. Dynamic interactions between TJs and adherence junctions, through signaling pathways, regulate the BBB permeability. These signaling pathways include mitogen-activated protein kinases, endothelial nitric oxide synthases, and G-proteins and interaction between these pathways regulate the paracellular route. The signal transduction includes signals from the interior of the cells to the TJs (facilitating the assembly and regulating the permeability) and signals from the TJs to the interior of the cell for modulating gene expression, proliferation and differentiation.8,13 In addition to the proteins with enzymatic activities, there are other specific proteins (i.e. drug efflux transporters (ATP-binding cassette (ABC),15 P-glycoprotein-Pgp, multidrug resistance proteins- MRPs, organic anion transporting polypeptides (OATPs), organic anion transporting polypeptides)7,15 that work as the BBB transporters, responsible for the rapid efflux of xenobiotic/drugs from the CNS, and for the delivery of the essential nutrients and transmitters into the brain, resulting in the specific barrier functions of the BBB, important for protecting CNS against harmful xenobiotics.16–20 BBB is complex, and transmigration of small/drugs molecules or drug-loaded nanoparticles still is a big challenge.21 The main drug transport mechanism for small/drug molecules for BBB permeation is attributed to passive diffusion, active transport, carrier-mediated/receptor-mediated and for larger peptide or proteins trans-endocytosis is the main transport mechanism (Figure 2).22,23 Other than physiological condition of basal membrane, physicochemical parameters of drugs (i.e. size, shape, charge molecular weight or lipophilicity) also effects the drug transport and need to be considered while designing the newer therapeutics. More details about the effect of these physicochemical parameters on BBB transport is covered in the review article by Teleanu et al (2018)23 and research article by Surnar et al (2018).21
In vitro BBB permeation measurement methods
To expedite the process of brain research, and the development of novel drugs for numerous neurological diseases, different types of in vitro BBB models have been established. However, as none of these in vitro models entirely reproduces the in vivo conditions, there is no perfect in vitro BBB model. Therefore, it is important to carefully choose the in vitro BBB model according to the requirement of the study and interpretation of the data, efficiently.24–26 Here, we have summarized the commonly used in vitro BBB models, including the recently developed microfluidic BBB models, along with their advantages and disadvantages. Based on the simulation of shear stress, in vitro BBB models are classified into the static and dynamic BBB models.
Static BBB models
Static BBB models are commonly used, but they do not imitate the shear stress, which is
usually generated in vivo due to the blood flow. Static BBB models are further divided into monolayer and co-culture models, based on type of cells involved in the BBB design.
Monolayer BBB models
A monolayer of endothelial cells grown in the Transwell insert is used as a simple in vitro BBB model (Figure 3A-i). The insert mimics the blood (luminal) side, whereas the well in which the insert fits, mimics the parenchymal (abluminal) side. The microporous membrane support (0.2–0.4 μm) allows the exchange of small molecules and cell-secreted growth factors but prevents the migration of cells between the two compartments. To mimic the unique properties of BMECs, primary or low passage number cells are used for the BBB model preparation. This process is challenging, due to isolation and culturing the primary BMECs as well as has a considerable risk of being contaminated by the mural cells. Also, low yield of primary BMECs after isolation is a shortcoming as the brain vasculature records only for 0.1% (v/v) of the brain, therefore a large number of rodents are needed to isolate enough primary BMECs to continue with the cell culture studies.1,27 To overcome this limitation, larger species and nonhuman primates are utilized for the experiment to isolate significant amounts of BMECs, for further experiments.28 Human cells are used for the studies focusing human-specific transporters/receptors or immunological aspects, but insufficient because of ethical issues. To overcome this, many immortalized human cell lines, such as human cerebral microvascular endothelial cell line (hCMEC/D3) and immortalized human cerebral endothelial cells, have been produced, which are useful but have lower expression of some of the BBB specific transporters and enzymes, leading to decreased generation of a tight monolayer and thus, having inadequate barrier function.29 This inadequacy is counteracted by the addition of BBB modulating compounds like cAMP and glucocorticoids, which increase the endothelial monolayer tightness and stability.30,31 Monolayer models are employed in studying signaling pathways, transporter kinetics, binding affinity, and high-throughput screenings. However, the monolayer model is not ideal for BBB integrity studies, as it has only one type of cells (BMECs) and lacks to imitate the brain microenvironment in which cell to cell communication is essential between different cell CNS cell types.32,33 Therefore, for the study of BBB integrity, more vivid and complex BBB model is required, such as co-culture and dynamic models.
 |
Figure 3 Schematic representation of different in vitro BBB models: (A) Configurations for in vitro static BBB Models using brain capillary endothelial cells (BCECs) (i) Monolayer models: are constructed using BCECs on the upper side of microporous semipermeable membrane (transwell), (ii) Non-contact co-culture: Astrocytes seeded at the bottom of the culture wells with BCECs; (iii) 2D co-culture contact models: endothelial cells are grown on porous cell culture inserts and co-cultured with primary astrocytes. Reprinted from J Pharm Sci, 105(2), Tornabene E, Brodin B, Stroke and drug delivery—in vitro models of the ischemic blood-brain barrier, Page Nos.398–405, Copyright 2016, with permission from Elsevier.83 (B) Cone and Plate viscometer apparatus. (C) Dynamic in vitro blood–brain barrier (DIV-BBB) model: The endothelial cells (ECs) are cultured inside the fibronectin-coated surface of hollow fibers made up of polypropylene. This system allows co-culture because astrocytes can be cultured on the outer surface of the hollow fibers. (B) and (C) adapted from J Pharm Sci, 101(4), Naik P, Cucullo L, In vitro blood-brain barrier models: current and perspective technologies, Page Nos.1337–1354, Copyright 2012, with permission from Elsevier.50 (D) Microfluidic-based in vitro BBB models: layered PDMS channels sandwiching a polyester membrane and the organization of b.End3 endothelial cells, pericyte, and astrocytes in the co-culture model. Reprinted with permission from Wang JD, Khafagy E-S, Khanafer K, Takayama S, ElSayed MEH. Organization of endothelial cells, pericytes, and astrocytes into a 3D microfluidic in vitro model of the blood–brain barrier. Mol Pharm. 2016;13(3):895–906. Copyright © 2016 American Chemical Society.84 (E) Stem cell-derived in-vitro BBB model: Undifferentiated iPSCs were differentiated simultaneously into ECs and neural cells, and then brain like ECs were purified on a selective matrix and co-cultured with astrocytes, the ECs exhibited a high TEER and formed networks of tight junctions. Abbreviations: ACM-Astrocyte-conditioned medium; BMEC- Brain microvascular endothelial cell; and TEER- Transendothelial electric resistance; iPSCs- Induced pluripotent stem cells; UM- Unconditioned medium; E6-Essential medium; EC-Endothelial cell medium supplemented with bFGF (Basic fibroblast growth factor); RA-Retinoic acid. |
Co-culture BBB models
In order to mimic the anatomic structure of BBB in vivo, BMECs are co-cultured with other CNS cells that directly contribute to the barrier properties of BBB. Interaction between BMECs and other brain cells increases the expression of transporters, TJs in BMECs, and induces the cell polarity in BMECs, promoting a phenotype closely mimicking the BBB in vivo. In this co-culture model (BMECs with astrocytes or pericytes) fits (Figure 3A-ii).8,34–45 BMECs are seeded in the transwell insert, and astrocytes are grown either on the undersurface of the transwell insert or at the base of the well in which the insert. Since pericytes also have a key role in BBB regulation, a BMEC–astrocyte-pericyte co-culture model has also been developed, which is termed as a triple co-culture system (BMEC–pericyte–astrocyte). Addition of pericytes enhances the quality of the co-culture model compared to the monolayer model. In this model, BMECs are plated in the transwell insert with astrocytes at the bottom of the well and pericytes on the underside of the insert (Figure 3A-iii). Although it lacks the direct cell-cell to communication between BMECs, astrocytes, and pericytes, this arrangement utilizes indirect cell-to-cell communication via secreted soluble factors, which promotes BBB regulation. BMEC–pericyte–astrocyte triple co-culture model is a more reliable in vitro BBB model due to the higher transendothelial electric resistance (TEER) value and lower permeability, which generates tighter BBB, ideal for permeability studies.27,46
Dynamic BBB models
In physiological conditions, the steady blood flow generates the shear stress, which regulates transporters and TJs expression, donating towards effective barrier function. Shear stress developed by blood flow increases ZO-1 expression and reduces permeability; therefore the dynamic BBB models, with shear stress, have been developed, which are of three major types, namely the cone-plate, dynamic and microfluidic-based models.47,48
Cone-plate BBB apparatus
The cone-plate apparatus was used initially to construct shear force, in which a rotating cone produces shear force, and the angular velocity and the angle of the cone regulate the produced shear stress. The sheer stress then reaches the endothelial monolayer via the medium (Figure 3B), but it is not uniformly dispersed along the radius of the plates, and therefore the endothelial monolayer receives varying shear stress depending upon the location. This model does not include astrocytes and pericytes; therefore, it has a limited application, low reliability, and less significant to be used in the BBB studies.49,50
Dynamic in vitro BBB model
To incorporate both the components, i.e., shear stress and various cell types, microporous hollow fibers are used (Figure 3C). In this model, BMECs and astrocytes are implanted in the inner (luminal) and outer (abluminal) sides of the porous hollow fibers, respectively.1,50 The culture medium is pushed into the system through a variable-speed pump to produce shear stress equivalent to that of physiological conditions in vivo (5–23 dynes/cm2).51 To maintain the stable microenvironment, a gas-permeable tubing system was used for the exchange of O2 and CO2. This dynamic in vitro BBB model has been used to study the pathophysiology of various CNS diseases, including ischemia-reperfusion-induced injury and epilepsy.52,53 Recently, an updated model with hollow fibers with transmural microholes of 2–4 μm has been developed to facilitate transmigration/trafficking studies. However, the dynamic BBB model has many disadvantages like, i) it does not allow direct visualization of the endothelial morphology in the luminal side; ii) the cell numbers (>1×106) required to build this model are relatively high, and iii) the time required to reach steady-state TEER is longer (9–12 days) compared to co-culture models (3–4 days). These shortcomings prevent the use of dynamic in vitro BBB model in large-scale screens. This model, however, is useful in lead compound validation/optimization in new drug research and development.53
Microfluidic based BBB models
Microfluidic device-based in vitro BBB models have been developed to overcome the shortcomings of dynamic BBB models.1,54,55 The microfluidic BBB (μ-BBB) comprises two perpendicularly crossing channels, allowing the dynamic flow to generate shear stress; a polycarbonate porous membrane placed over the intersection of the perpendicular channels, enabling the co-culture of BMECs and astrocytes (on the luminal and abluminal sides, respectively). The channels are 200 μm tall, 2 mm (luminal) and 5 mm (abluminal) wide. It also contains multiple built-in Ag/AgCl electrodes for facilitating the TEER measurement (Figure 3D).56 Pumps and a gas-permeable tubing system are used to generate shear stress and allow O2–CO2 exchange, respectively. This μ-BBB model is further improved by replacing the oxidation-sensitive AgCl electrodes with inert platinum ones and reducing the cross-sectional area. These adjustments offer precise measurement of TEER and reduce the number of cells required. Additionally, the microfluidic-based BBB model with the microhole, have been designed to study the BBB permeability of drugs, which is composed of two horizontally aligned chambers connected by a microhole structure, but the shortcomings of the model is that i) it lacks cell-cell contact and, ii) it fails to replicate the dimensions of microvasculature in vivo. Therefore, currently, the development of a new version of the microfluidic device, i.e., a synthetic microvasculature model of the BBB (SyMBBB) is progressing.55 The SyM-BBB model contains two microchannels separated by microfabricated pillars, which mimic the porous membrane of the μBBB model. Endothelial cells were infused to the blood chamber via ports 1 and 2, whereas astrocyte-conditioned medium or astrocytes were infused to the brain chamber from port 3. The flow velocity of medium in these chambers regulates the shear stress. This design mimics the in vivo microcirculatory system in a better way by including the diverging and converging bifurcations. In comparison to the dynamic BBB model, the microfluidic models are closer replicates of the in vivo BBB structure as they have thicker membrane (<10 μm), which allows efficacious transmigration studies conditions. With the microfluidic model, nondestructive microscopy is possible because of the transparency of the materials, it takes relatively less time (3–4 days) to achieve steady-state TEER, and require only a modest amount of cells and are less challenging in terms of technical skills.54 Although the microfluidic systems have so many advantages, they have limitations too, i.e., 1) TEER value is not high enough (250–300 Ω∙cm2), ii) only incorporate two cell types, given that the membrane and pillars (hollow fibers in the dynamic in vitro BBB model) have only two sides. With the growth of research data, these microfluidic models may be used to aid neurovascular research and new drug development in the future due to its small size, short time to reach steady state TEER, low-to-moderate technical skill requirement, and low cost.
Stem cell (iPSCs) based BBB models
Pluripotent stem cells (PSCs), like embryonic stem (ES) cells and induced pluripotent stems cells (iPSCs) exhibit excellent properties that mimic physiological in vivo conditions. Stem cell-based in vitro systems have been extensively used for drug screening and regenerative therapies via differentiating cells from patient-derived pluripotent stem cells. For the in vitro BBB development, BMECs from human ES and iPSC by co-differentiation with neural cells along with retinoic acid have been explored comprehensively comparing to other available models (Figure 3E). The differentiated cells showed similar properties comprising barrier integrity with tissue derived BMECs.57 Yamanaka’s contribution of deriving iPSCs from somatic cells and the neuronal differentiation procedure established by Zhang and co-workers on human ES, modelling neurological diseases in vitro using iPSCs had made a significant impact in the field of BBB and have been explored for the various neurological diseases like Parkinson’s disease, Huntington’s disease, Epilepsy, Down’s syndrome, and autism disorders.58 Stem cell-derived BBB model provides a human, scalable, and reproducible source of cells, which can achieve good barrier properties like in vivo. Furthermore, the use of iPSCs provides an integrated model allowing the usage of isogenic co-cultures and consequently generate models similar to the complexity observed in vivo. The use of patient-specific in vitro models of the BBB generates unique aspects to detect how genetic disorders linked with neurological indications hamper the barrier function, thus, ultimately leading the researchers to identify novel therapeutic agents. Even though the iPSCs system offers great advantages, but the cell differential procedure depends upon various random and permanent insertion of transcription factors. Another major hurdle in diseases modelling using patient-derived iPSCs is the limited source of patient-derived iPSCs lines, shortage of matched-controls (e.g., parents, siblings) and a probable characteristic genetic drifting owing to the derivation and the maintenance of iPSC lines. Furthermore, the use of a genome integrating approach in the derivation method comprises a significant hurdle in translating such outcomes into iPSC-based cell therapies. Recent advances in gene editing techniques as CRISPR/Cas 9 provides exciting opportunities in diseases modelling using iPSCs. In addition, to tackle the problems mentioned above, various approaches have been developed, e.g. non-genomes integrating re-programming techniques like the usage of episomal vectors that allow cell-reprogramming bypasses, the insertion of genomic sequences have been developed. Although, these methods still have hindrances like epigenetic reprogramming and loss of patient-specific epigenetic signature. A significant problem of in vitro modelling is the absence of 3-dimensional structures related to cellular cytoarchitecture observed in vivo. A substitute to such matter is the portrayal of brain “organoids” offering a primitive multi-layer sphere. Although, these structures have several hindrances like the absence of an extracellular matrix scaffold (in vivo), absence of neuronal projections and tracts, and the presence of multi-cellular organoids integrative of glial cells.58
Zhang et al, produced an in vitro human BBB model by differentiating the human iPSC GM25256 cell line into brain endothelial cells. The model displayed various BBB features such as tight junction proteins (ZO-1, claudin-5, and occludin), endothelial markers (von Willebrand factor and Ulex), high transendothelial electrical resistance (TEER) value and c-GTPase activity. The TEER value significantly increased when co-cultured with primary rat astrocytes. RNAseq analysis verified the expression of all the important BBB related genes in the human iPSC derived endothelial cells in comparison to primary BMECs, comprising breast cancer resistant protein (BCRP) and P-glycoprotein (Pgp). Drug transport assays were also performed and in vitro results generated from this model could predict the in vivo BBB permeability of tested drugs in a successful manner.59 In another study by Lippmann et al showed that retinoic acid enhances the expression of vascular endothelial cadherin in differentiated ECs considerably before passage/purification, and in combination with hydrocortisone, it resulted in the higher TEER value in comparison to untreated cells.59 Researchers have also demonstrated the effect of co-culture with astrocytes and pericytes on purified BMECs to achieve better barrier and higher TEER measurements (6635±315 Ω × cm2; the highest reported value of TEER till now).60 Despite substantial progress, stem cell-derived in vitro BBB models demand cell surface marker enrichment or co-differentiation and purification actions generate a pure population of specialized brain endothelial cells still a big challenge.
iPSCs were also used to form neural progenitor-like EZ spheres, which can be differentiated into neurons and astrocytes, facilitating facile neural cell production. Canfield et al have developed an isogenic (each cell type obtained from the same patient) human BBB model containing iPSCs derived BMECs, astrocytes, and neurons. Furthermore, iPSC-derived BMECs co-culture with EZ-sphere-derived astrocytes and neurons generated in a sustained enhanced TEER compared to previously expressed models utilizing primary rat astrocytes and primary human NPCs as co-cultured neural cell sources. These models predicted that the generation of an isogenic model would help in applications for human BBB models and will serve as an excellent tool to evaluate human genetic disease on BBB, drug screening and toxicity evaluation for neurological disorders.61 The key features, advantages, and disadvantages of all the different type of in vitro BBB models are summarized in Table 1 and the comparison between different in vitro BBB models are also summarized in Table 2.
 |
Table 1 Advantages and disadvantages of different in-vitro BBB models |
 |
Table 2 Comparison of different in vitro BBB models for drug transport |
BBB permeation prediction methods (in silico methods)
The widely used in silico prediction of the BBB permeability is an inexpensive, less time consuming, and high throughput screening method for novel compounds in the drug discovery process. Although this method is based on several molecular descriptors or physicochemical characteristics of the molecule, it has its strengths and weaknesses.62–65 These computational models are typically based on the previous in vivo and in vitro experimental data. Therefore, for the predictive power of estimations, the selection of datasets is a critical component. The assumption of passive diffusion of a compound as a major route of transport through the BBB is the base for the in silico predictions that do not consider various BBB transport pathways, e.g., nanoparticle-based transport/carrier-mediated, receptor-mediated, and active efflux or influx transport methods.66 Recently, cerebrospinal fluid (CSF) penetration is also considered in in silico model while analyzing the brain penetration of the molecule.67
To improve the predictive values of these computational models, novel sophisticated approaches have been developed. Table 3 shows different computational models to predict the BBB penetration property of newly designed or synthesized compounds. For brain penetration studies, brain-to-plasma ratios is measured and in silico extrapolations are based on the available logBB data, which represents the most readily available experimental data.65,68,69 In the training set, several molecular descriptors of the compounds are calculated with known logBB values, which were experimentally determined. To derive the equation from the given relationship between logBB and the compound has computed descriptors, typically, regression methods used. Because of its physiological relevance, the permeability surface area product (PS value/logPS) would be an effective method of determining the BBB permeability for a specific molecule both in vivo and in vitro, compared with the currently more popular logBB.70,71 Unfortunately, the availability of logPS is limited due to the complicated measurement of logPS than that of logBB. Based on Lipinski’s rule, molecules which have not more than 5 H-bond donors and not more than 10 H-bond acceptors, with an MW of <500 Da and an octanol/water partition coefficient log P under 5 can be the drug candidates.72 Molecules with these physicochemical characteristics have good aqueous solubility and intestinal permeability. Approximately 90% of the orally active drug molecules, which are under Phase II clinical trials have these characteristics.73 Guidelines for the properties of new molecules that can be used as potential CNS active drug have been proposed in recent past.73–76 The relationship between the experimental data, computationally available parameters of a new compound, and its blood-brain barrier penetration has been studied for long. Among different data sets, the octanol/water partitioning coefficient (logPoct) is one of the earliest predictive factors available for the BBB permeability. For the compounds with MW <400 Da, it is possible to predict the relationship of the capillary permeability coefficient (logPC) to the logPoct.77 In 1988, when a linear correlation of antihistamines and ΔlogP between the brain-to-blood ratio was established, the computational prediction of BBB penetration for these compounds began.78 The observation of the inverse relation between the hydrogen bonding activity of the compound and BBB permeability provided a theoretical concept for designing BBB-permeable drugs.
 |
Table 3 In-silico models and their parameters used for predicting drug penetrability |
Calculations of logPoct, -cyclohexane, and -dichloromethane systems were used to obtain the descriptors for general Linear Free Energy Response (LFER) equation.79 Calculation of physicochemical and biochemical properties of the compound would be useful to estimate the blood-to-brain distribution ratio.79 Universal quantitative scales of hydrogen-bond acidity and basicity of the solute have been standardized for the first time, and along with other descriptors, these descriptors have been used in equations to calculate, predict, analyze and correlate various solute properties. At the same time, these equations may be used for the analysis of physicochemical (LFERs) and biological properties of various compounds, such as quantitative structure-activity relationships (QSARs) set up for blood-brain distribution. Although these rule-based models can be used for the qualitative BBB permeability estimation using various physicochemical descriptors, these may not be useful to predict the active BBB transport through efflux pumps, carriers, and receptor-mediated transmigration. Overall, these in silico quantitative models as classification tools have more than 70% accuracy in predicting logBB.62,65 For the analysis of various molecular descriptors listed in Table 3, both linear and non-linear statistical methods would be used. These in silico models for predicting logBB and logPS are reliable and popular than in vitro and in vivo BBB models in the drug discovery process, as these methods are economical and faster. However, the size and quality of the training set play a key role in the accuracy of predicting the passive permeability. Similar to the recently developed model for P-glycoprotein substrate properties, if new models for the active transport mediated by carriers, receptors, and efflux pumps are developed, the predictive power of the in silico models will be tremendously increased.64,65,80
The rationale for BBB model selection
In vitro BBB models are extensively used for the initial stages of novel drug development, which includes lead identification, hit identification and target identification, optimization (Figure 4). Once a target (enzyme, receptors, etc.) is identified, high-throughput screening (HTS) is employed to identify probable drug contenders. At this phase, a large number of compounds need to be screened and thus, requires easy and fast in vitro BBB screening model. Selecting suitable in vitro models not only enables accurate interpretation of the data but also saves time and money. The main criteria in the model selection are the purpose of study, in case of monolayer or co-culture models, which generally take 3–4 days to reach steady-state TEER value and are reasonably easy to construct, can be used. Previous studies have revealed that the use of different immortalized cell lines provides the best correlation between in vitro and in vivo data for permeability assays. The co-culture models and dynamic in vitro BBB models are the best models to study the drug permeability.1,25 Many multi-culture models (2D or 3D) are commercially available now, which considerably reduces the efforts and time, but increases the cost. For trafficking/migration studies, microfluidic BBB model is the right option due to the incorporation of shear stress component and ability to mimic in vivo conditions.1 To study signaling pathways/transporter kinetics, or to quantify binding affinities, monolayer model is the best option due to its inherent simplicity. For the lead identification/optimization phase studies, validation, and structure-activity relationships (SAR) and toxicological profile, more sensitive in vitro models that replicate the majority of the in vivo conditions are required, i.e., iPSCs based model or static co-culture and dynamic models along with newly developed microfluidic-based BBB models can serve as an alternate choice. Primary human-derived cells are a better option than immortalized cell lines due to more closeness to biological properties of the BMECs in vivo. Authentication using human-derived primary cells is highly recommended for the generation of in vitro BBB model to evade species based difference that may lead to the failure of a product in the later development stages. Based on the above problems and suggestion, Figure 4 summarizes the selection of in vitro BBB models at different research and development stages for the therapeuticdrug development.28,62,68,81,82 The selection criteria of BBB models still a big puzzle for small molecule transport versus nanoparticle loaded drugs (Nanoformulation). Based on the available literature and newer development, iPSC based model (CNS organoids) may able to provide a better answer compared to traditional 2D or 3D cell-based models due to human-like pathophysiology and overcome the interspecies (rodent vs human) variability.
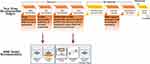 |
Figure 4 Applications of BBB models in drug discovery and development. |
Conclusion and future aspects
In vitro models of the BBB have proven exceptionally valued for investigations of endo-thelial cell properties and mechanistic studies of drug transport via brain endothelial cells. The early effort of the pioneers has been followed up by a large community of investigators and has resulted in a range of in-vitro model configurations. In general, the cell culture models of the brain endothelium are believed to reflect the properties of the healthy BBB. In vitro BBB models are essential to our understanding of the BBB functions in physiological and pathological situations and the research and development of novel drugs for different neurological conditions. Different in vitro BBB models have been established and used for a variety of permeation studies; no single model can imitate the in vivo conditions physiologically. Further, knowing the advantages and disadvantages of each of these models and rationale of selecting the appropriate model may allow the precise understanding of the data and elaborate the development of novel drugs for the treatment or management of neurological diseases. In summary, we attempt to provide an overview of regularly used, newly developed and advanced in vitro BBB models, equated their strengths and weaknesses and attempted to rationalize the model selection. Model selection parameters are critical for predicting drug transport because the disease in question may affect the barrier properties. A combinatorial approach of in vitro BBB models and in-vivo methods will be the key to the development of CNS therapeutics with improved pharmacokinetic properties and better BBB penetrability.
Acknowledgments
RDJ would like to acknowledge the financial support from NIH (R03DA044877); The Campbell Foundation (Florida, USA) and start-up funds from the Department of Pharmaceutical Sciences, School of Pharmacy, Texas Tech University Health Sciences Center (TTUHSC), TX, USA. VB would like to acknowledge the Department of Science and Technology, Ministry of Science and Technology, Government of India for the INSPIRE Faculty Award (IFA18-ENG266, DST/INSPIRE/04/2018/000991).
Disclosure
The authors report no conflicts of interest in this work.
References
1. He Y, Yao Y, Tsirka SE, Cao Y. Cell-culture models of the blood–brain barrier. Stroke. 2014;45(8):2514–2526. doi:10.1161/STROKEAHA.114.005427
2. Stern L, Gautier R
3. Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75(3):388–397. doi:10.1189/jlb.0303114
4. Cecchelli R, Berezowski V, Lundquist S, et al. Modelling of the blood–brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6(8):650–661. doi:10.1038/nrd2368
5. Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 2012;33(12):579–589. doi:10.1016/j.it.2012.07.004
6. Jouyban A, Soltani S. Blood brain barrier permeation. Toxic Drug Test. In: Bill Acree, Editor, Croatia: InTech. 2012;1:1–24.
7. Nair M, Jayant RD, Kaushik A, Sagar V. Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv Drug Deliv Rev. 2016;103:202–217. doi:10.1016/j.addr.2016.02.008
8. Abbott NJ Physiology of the blood–brain barrier and its consequences for drug transport to the brain.
9. Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi:10.3390/molecules24050892
10. Cardoso FL, Brites D, Brito MA. Looking at the blood–brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64(2):328–363. doi:10.1016/j.brainresrev.2010.05.003
11. Prinz M, Mildner A. Microglia in the CNS: immigrants from another world. Glia. 2011;59(2):177–187. doi:10.1002/glia.21104
12. Aday S, Cecchelli R, Hallier-Vanuxeem D, Dehouck M, Ferreira L. Stem cell-based human blood–brain barrier models for drug discovery and delivery. Trends Biotechnol. 2016;34(5):382–393. doi:10.1016/j.tibtech.2016.01.001
13. Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi:10.1016/j.nbd.2003.12.016
14. Helms HC, Abbott NJ, Burek M, et al. In vitro models of the blood–brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab. 2016;36(5):862–890. doi:10.1177/0271678X16630991
15. Löscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. doi:10.1602/neurorx.2.1.86
16. Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76(1):22–76. doi:10.1016/j.pneurobio.2005.04.006
17. Kaushik A, Jayant RD, Bhardwaj V, Nair M. Personalized nanomedicine for CNS diseases. Drug Discov Today. 2018;23(5):1007–1015. doi:10.1016/j.drudis.2017.11.010
18. Kaushik A, Jayant RD, Nair M. Nanomedicine for neuroHIV/AIDS Management. London, UK: Future Medicine Ltd; 2018.
19. Kaushik A, Yndart A, Atluri V, et al. Magnetically guided non-invasive CRISPR-Cas9/gRNA delivery across blood-brain barrier to eradicate latent HIV-1 infection. Sci Rep. 2019;9(1):3928. doi:10.1038/s41598-019-40222-4
20. Lee M, Jayant R. Penetration of the blood-brain barrier by peripheral neuropeptides: new approaches to enhancing transport and endogenous expression. Cell Tissue Res. 2019;375(1):287–293. doi:10.1007/s00441-018-2959-y
21. Surnar B, Basu U, Banik B, et al. Nanotechnology-mediated crossing of two impermeable membranes to modulate the stars of the neurovascular unit for neuroprotection. Proc Natl Acad Sci. 2018;115(52):E12333–E12342. doi:10.1073/pnas.1816429115
22. Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx. 2005;2(4):554–571. doi:10.1602/neurorx.2.4.554
23. Teleanu D, Chircov C, Grumezescu A, Volceanov A, Teleanu R. Blood-brain delivery methods using nanotechnology. Pharmaceutics. 2018;10(4):269. doi:10.3390/pharmaceutics10040269
24. Czupalla CJ, Liebner S, Devraj K. In vitro models of the blood–brain barrier. In: Milner R. Editor. Cerebral Angiogenesis: Methods and Protocols; NY: Humana Press. 2014;1135:415–437.
25. Garberg P, Ball M, Borg N, et al. In vitro models for the blood–brain barrier. Toxicol in Vitro. 2005;19(3):299–334. doi:10.1016/j.tiv.2004.06.011
26. Wilhelm I, Fazakas C, Krizbai IA. In vitro models of the blood-brain barrier. Acta Neurobiol Exp (Wars). 2011;71(1):113–128.
27. Nakagawa S, Deli MA, Kawaguchi H, et al. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54(3):253–263. doi:10.1016/j.neuint.2008.12.002
28. Lippmann ES, Al-Ahmad A, Palecek SP, Shusta EV. Modeling the blood–brain barrier using stem cell sources. Fluids Barriers CNS. 2013;10(1):2. doi:10.1186/2045-8118-10-2
29. Daniels BP, Cruz-Orengo L, Pasieka TJ, et al. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J Neurosci Methods. 2013;212(1):173–179. doi:10.1016/j.jneumeth.2012.10.001
30. Franke H, Galla H-J, Beuckmann CT. An improved low-permeability in vitro-model of the blood–brain barrier: transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res. 1999;818(1):65–71. doi:10.1016/s0006-8993(98)01282-7
31. Hurst R, Fritz I. Properties of an immortalised vascular endothelial/glioma cell co-culture model of the blood-brain barrier. J Cell Physiol. 1996;167(1):81–88. doi:10.1002/(SICI)1097-4652(199604)167:1<81::AID-JCP9>3.0.CO;2-8
32. Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte‐derived angiopoietin‐1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie‐2 activation in vitro. J Neurochem. 2004;89(2):503–513. doi:10.1111/j.1471-4159.2004.02343.x
33. Toimela T, Mäenpää H, Mannerström M, Tähti H. Development of an in vitro blood–brain barrier model—cytotoxicity of mercury and aluminum. Toxicol Appl Pharmacol. 2004;195(1):73–82. doi:10.1016/j.taap.2003.11.002
34. Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi:10.1038/nature09522
35. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi:10.1038/nature09513
36. Atluri VSR, Jayant RD, Pilakka-Kanthikeel S, et al. Development of TIMP1 magnetic nanoformulation for regulation of synaptic plasticity in HIV-1 infection. Int J Nanomedicine. 2016;11:4287. doi:10.2147/IJN.S108329
37. Jayant R. Layer-by-Layer (LbL) assembly of anti HIV drug for sustained release to brain using magnetic nanoparticle.
38. Jayant R, Nair M. Nanotechnology for the treatment of neuroAIDS. J Nanomed Res. 2016;3(1):00047. doi:10.15406/jnmr.2016.03.00047
39. Jayant R, Nair M. Role of biosensing technology for neuroAIDS management. J Biosens Bioelectron. 2016;7(1).
40. Jayant RD, Madhavan N Materials and methods for sustained release of active compounds. US Patent App. 15/082,611. 2016.
41. Kaushik A, Jayant RD, Nair M. Advancements in nano-enabled therapeutics for neuroHIv management. Int J Nanomedicine. 2016;11:4317. doi:10.2147/IJN.S109943
42. Tomitaka A, Arami H, Raymond A, et al. Development of magneto-plasmonic nanoparticles for multimodal image-guided therapy to the brain. Nanoscale. 2017;9(2):764–773. doi:10.1039/c6nr07520g
43. Nair M, Guduru R, Liang P, Hong J, Sagar V, Khizroev S. Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat Commun. 2013;4:1707. doi:10.1038/ncomms2717
44. Pilakka-Kanthikeel S, Atluri VSR, Sagar V, Saxena SK, Nair M. Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: an in-vitro study. PLoS One. 2013;8(4):e62241. doi:10.1371/journal.pone.0062241
45. Ding H, Sagar V, Agudelo M, et al. Enhanced blood–brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology. 2014;25(5):055101. doi:10.1088/0957-4484/25/5/055101
46. Nakagawa S, Deli MA, Nakao S, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27(6):687–694. doi:10.1007/s10571-007-9195-4
47. Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi:10.1016/j.brainres.2007.02.029
48. Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87(2):320–330. doi:10.1093/cvr/cvq146
49. Bussolari SR, Dewey CF
50. Naik P, Cucullo L. In vitro blood–brain barrier models: current and perspective technologies. J Pharm Sci. 2012;101(4):1337–1354. doi:10.1002/jps.23022
51. Koutsiaris AG, Tachmitzi SV, Batis N, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44(5–6):375–386.
52. Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood–brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48(3):505–516. doi:10.1111/j.1528-1167.2006.00960.x
53. Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab. 2011;31(2):767–777. doi:10.1038/jcbfm.2010.162
54. Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip. 2012;12(10):1784–1792. doi:10.1039/c2lc40094d
55. Prabhakarpandian B, Shen M-C, Nichols JB, et al. SyM-BBB: a microfluidic blood brain barrier model. Lab Chip. 2013;13(6):1093–1101. doi:10.1039/c2lc41208j
56. Booth R, Kim H A multi-layered microfluidic device for in vitro bloodbrain barrier permeability studies.
57. Kokubu Y, Yamaguchi T, Kawabata K. In vitro model of cerebral ischemia by using brain microvascular endothelial cells derived from human induced pluripotent stem cells. Biochem Biophys Res Commun. 2017;486(2):577–583. doi:10.1016/j.bbrc.2017.03.092
58. Page S, Patel R, Raut S, Al-Ahmad A. Neurological diseases at the blood-brain barrier: stemming new scientific paradigms using patient-derived induced pluripotent cells. Biochim Biophys Acta Mol Basis Dis. 2018. doi:10.1016/j.bbadis.2018.12.009
59. Li Y, Sun X, Liu H, et al. Development of human in vitro brain-blood barrier model from induced pluripotent stem cell-derived endothelial cells to predict the in vivo permeability of drugs. Neurosci Bull. 2019:1–15. doi:10.1007/s12264-019-00384-7
60. Hollmann EK, Bailey AK, Potharazu AV, Neely MD, Bowman AB, Lippmann ES. Accelerated differentiation of human induced pluripotent stem cells to blood-brain barrier endothelial cells. Fluids Barriers CNS. 2017;14(1):9. doi:10.1186/s12987-017-0059-0
61. Canfield SG, Stebbins MJ, Morales BS, et al. An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J Neurochem. 2017;140(6):874–888. doi:10.1111/jnc.13923
62. Vastag M, Keseru GM. Current in vitro and in silico models of blood-brain barrier penetration: a practical view. Curr Opin Drug Discov Devel. 2009;12(1):115–124.
63. Abbott NJ. Prediction of blood–brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov Today Technol. 2004;1(4):407–416. doi:10.1016/j.ddtec.2004.11.014
64. Goodwin JT, Clark DE. In silico predictions of blood-brain barrier penetration: considerations to “Keep in mind”. J Pharmacol Exp Ther. 2005;315(2):477–483. doi:10.1124/jpet.104.075705
65. Mensch J, Oyarzabal J, Mackie C, Augustijns P. In vivo, in vitro and in silico methods for small molecule transfer across the BBB. J Pharm Sci. 2009;98(12):4429–4468. doi:10.1002/jps.21745
66. Deli MA. Drug Transport and the Blood-brain Barrier. Solubility, Delivery, and ADME Problems of Drugs and Drug-Candidates. Washington: Bentham Science Publ Ltd; 2011:144–165.
67. Bendels S, Kansy M, Wagner B, Huwyler J. In silico prediction of brain and CSF permeation of small molecules using PLS regression models. Eur J Med Chem. 2008;43(8):1581–1592. doi:10.1016/j.ejmech.2007.11.011
68. Garg P, Verma J, Roy N. In silico modeling for blood—brain barrier permeability predictions. In: Ehrhardt C, Kim KJ, Editors. Drug Absorption Studies. Springer; 2008;VII:510–556.
69. Konovalov DA, Coomans D, Deconinck E, Vander Heyden Y. Benchmarking of QSAR models for blood-brain barrier permeation. J Chem Inf Model. 2007;47(4):1648–1656. doi:10.1021/ci700100f
70. Liu X, Tu M, Kelly RS, Chen C, Smith BJ. Development of a computational approach to predict blood-brain barrier permeability. Drug Metab Dispos. 2004;32(1):132–139. doi:10.1124/dmd.32.1.132
71. Abraham MH. The factors that influence permeation across the blood–brain barrier. Eur J Med Chem. 2004;39(3):235–240. doi:10.1016/j.ejmech.2003.12.004
72. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1–3):3–25. doi:10.1016/S0169-409X(96)00423-1
73. Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi:10.1016/j.ddtec.2004.11.007
74. Glave W, Hansch C. Relationship between lipophilic character and anesthetic activity. J Pharm Sci. 1972;61(4):589–591. doi:10.1002/jps.2600610420
75. Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2(4):541–553. doi:10.1602/neurorx.2.4.541
76. Hitchcock SA. Blood–brain barrier permeability considerations for CNS-targeted compound library design. Curr Opin Chem Biol. 2008;12(3):318–323. doi:10.1016/j.cbpa.2008.03.019
77. Levin VA, Dolginow D, Landahl HD, Yorke C, Csejtey J. Relationship of octanol/water partition coefficient and molecular weight to cellular permeability and partitioning in S49 lymphoma cells. Pharm Res. 1984;1(6):259–266. doi:10.1023/A:1016393902123
78. Young RC, Mitchell RC, Brown TH, et al. Development of a new physicochemical model for brain penetration and its application to the design of centrally acting H2 receptor histamine antagonists. J Med Chem. 1988;31(3):656–671. doi:10.1021/jm00398a028
79. Abraham MH, Takács‐Novák K, Mitchell RC. On the partition of ampholytes: application to blood–brain distribution. J Pharm Sci. 1997;86(3):310–315. doi:10.1021/js960328j
80. Liu X, Chen C, Smith BJ. Progress in brain penetration evaluation in drug discovery and development. J Pharmacol Exp Ther. 2008;325(2):349–356. doi:10.1124/jpet.107.130294
81. Sakolish CM, Esch MB, Hickman JJ, Shuler ML, Mahler GJ. Modeling barrier tissues in vitro: methods, achievements, and challenges. EBioMedicine. 2016;5:30–39. doi:10.1016/j.ebiom.2016.02.023
82. Veszelka S, Kittel Á, Deli MA. Tools of Modelling Blood–brain Barrier Penetrability. Solubility, Delivery and ADME Problems of Drugs and Drug Candidates. Washington: Bentham Science; 2011:166–188.
83. Tornabene E, Brodin B. Stroke and drug delivery—in vitro models of the ischemic blood-brain barrier. J Pharm Sci. 2016;105(2):398–405. doi:10.1016/j.xphs.2015.11.041
84. Wang JD, Khafagy E-S, Khanafer K, Takayama S, ElSayed MEH. Organization of endothelial cells, pericytes, and astrocytes into a 3D microfluidic in vitro model of the blood–brain barrier. Mol Pharm. 2016;13(3):895–906. doi:10.1021/acs.molpharmaceut.5b00805
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
