Back to Journals » Journal of Experimental Pharmacology » Volume 12
In vitro Antioxidant and in vivo Wound Healing Activities of the 80% Methanol Extract and Solvent Fractions of Seeds of Brassica carinata A. Braun (Brassicaceae) in Mice
Authors Alemu BK , Ayalew Getahun K , Kahaliw W
Received 26 August 2020
Accepted for publication 17 October 2020
Published 6 November 2020 Volume 2020:12 Pages 463—474
DOI https://doi.org/10.2147/JEP.S278622
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William G North
Belete Kassa Alemu,1 Kefyalew Ayalew Getahun,2 Wubayehu Kahaliw2
1Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, Dessie 1145, Ethiopia; 2Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Belete Kassa Alemu
Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, P O Box; 1145, Dessie, Ethiopia
Tel +251 918330307
Fax +251 333115052
Email [email protected]
Background: Brassica carinata is one of the traditional medicinal plants used in Ethiopia for the treatment of wounds and other diseases. However, the plant has not been scientifically validated, and thus the present study evaluated the in vitro anti-oxidant and the in vivo wound healing activity of the crude extract and solvent fractions of B. carinata seeds in mice.
Materials and Methods: The crude extract was prepared by maceration using 80% methanol and formulated as 5% and 10% w/w ointments for topical application. The acute dermal toxicity was performed in female albino rats based on Organization for Economic Cooperation and Development (OECD) guideline number 434. Excision and incision wound healing models were used to evaluate the wound healing activities of crude extract and solvent fractions ointments in mice. Wound healing parameters such as wound area contraction and the period of epithelialization were determined in an excision model, whereas tensile strength was determined in an incision model. Moreover, the crude extract and solvent fractions were evaluated for the free radical scavenging activities in DPPH assay.
Results: The acute dermal toxicity test showed that a limit dose of 2,000 mg/kg of 10% w/w crude extract ointment did not cause dermal toxicity in mice. In the excision wound model, the data revealed that 10% w/w ointment exhibited a significant wound contraction (from day 6 to 16, P< 0.001) effect with a significant decrease in epithelization period (at day 14, P< 0.001). In addition, 5% ointment of the crude extract showed a significant effect in wound contraction (from day 8 onwards, P< 0.01) and epithelization period (at day 16, P< 0.01). Despite all fractions being shown to promote wound healing, 10% w/w aqueous and ethyl acetate fractions showed a significant wound contraction (P< 0.001) effect starting from the 4th day onwards. Besides, the maximum antioxidant activity was seen in the aqueous fraction with an IC50 value of 3.45± 0.12 mg/mL.
Conclusion: The present study demonstrated that the 80% methanol extract and solvent fractions of the seeds of B.carinata possess potential wound healing and anti-oxidant effects, supporting the traditional use of the plant for wound management.
Keywords: wound, epithelialization, tensile strength, incision, excision, B. carinata, antioxidant
Introduction
In developing countries, the majority of people almost exclusively use traditional medicines in treating all sorts of diseases including wounds. Traditional medicinal plants have been used for many years for the treatment of a variety of ailments, including skin disorders such as wounds, burns, and cuts.1
A review of medicinal plants for wound healing activity demonstrated that bioactive constituents contributing to antimicrobial, anti-inflammatory, antioxidant, and mitogenic activities are the main effects towards wound healing.2–4 In addition to this, the pathophysiology of many diseases such as cancer, wound, diabetes, cardiovascular, and neurodegenerative diseases are related with oxidative stress.5–7 Therefore, due to the free radical scavenging properties, utilization of fruits and vegetables rich in anti-oxidants (example Brassica plants) is connected with the reduction of those diseases resulting from free radicals accumulation.8,9 These benefits are primarily due to the presence of bioactive constituents such as polyphenols, flavonoids, carotenoids, and vitamin E and C.8,10
Brassicaceae is the mustards or the cabbage family of flowering plants. The family contains 372 genera and 4,060 species, of which the Brassica vegetables are widely studied.11
The genus Brassica under the mustard family (Brassicaceae) contains important species such as B.carinata, B.juncea, B.oleracea, B.napus, B.nigra, and B. rapa, which have been well known for their pharmacological and agricultural uses. Among mustards, B.juncea and B.oleracea have been extensively studied for their proven wound healing effects. Different secondary metabolites are isolated from mustard leaves and seeds contributing to promotion of wound healing. For instance, a wound healing lipid called lysophosphatidic acid was identified from consumption of cabbage leaves.12 In addition, the herbal balsam Debridan® prepared from Brassica oleracea active ingredients was used for treatment of cutaneous wounds.13,14 These Brassica plants have also been studied for their anti-oxidant activities. Polar compounds like vitamin C and phenolic compounds, as well as non-polar chemicals like vitamin E and carotenoids, are some of the natural anti-oxidants present in Brassica vegetables.15 The substantial anti-oxidant effects of these plants were evaluated in terms of various assays such as metal reducing, metal chelating, lipid reducing, and free radical scavenging activities. They also produced a positive effect on antioxidant enzymes such as glutathione peroxidase, superoxide dismutase, catalase, and ascorbate peroxidase.16 Not only for their free radical scavenging activities, they do produce significant anti-inflammatory and antipyretic activities from previous reports.17
The studied plant, Brassica carinata (locally called “Gomenzer”), is an erect, annual herb growing from 30–200 cm tall.18 It originated through hybridization between Brassica nigra and Brassica oleracea in the highlands of Ethiopia.19 Traditionally, B. carinata seeds were widely used to treat wounds in different parts of Ethiopia.20,21 Discovery of new treatment options for wound management is highly encouraged with the aim to promote wound healing in the shortest time possible with minimal pain, discomfort, and scarring to the patient. Hence, this study evaluated the in vitro anti-oxidant and the in vivo wound healing activities of 80% methanol crude extract and solvent fractions of B. carinata seeds in mice.
Materials and Methods
Plant Material
Matured seed pods of B. carinata were collected from the local farm land in West Gojjam, North Western Ethiopia. The plant material was identified and authenticated by a botanist (Mr. Abyu Enyew), and a specimen number (BK001) was deposited at the National Herbarium, College of Natural and Computational Sciences, University of Gondar for future reference.
Experimental Animals, Grouping, and Treatment
Healthy adult Swiss albino mice of either sex (25–30 g, and 6–8 weeks of age) or female adult albino rats (Rattus albus) (200–300 g, 8–12 weeks of age) were obtained from the animal house of the Department of Pharmacology, University of Gondar. Female adult rats were used since female rats are more susceptible to chemical toxicity. The animals were placed in cages under standard conditions with 12 hours light and dark cycles. They were provided with a standard pellet diet and water ad libitum throughout the experiment and acclimatized to laboratory conditions for 1 week before the experiment. The internationally accepted laboratory animal use, care, and guidelines were applied in all experiments.22
To evaluate the wound healing activities of extract ointments, mice were grouped into four treatment groups each consisting of six mice in each model. We used a power calculation to estimate the sample size and number of groups.23 Then, group one was treated with simple ointment (negative control), the second and third groups were treated with 5% and 10% of crude extract as well as fractions ointments, respectively, and the fourth group was treated with 0.2% nitrofurazone skin ointment (positive control). 0.2% nitrofurazone topical ointment was used as a positive control because it is effective for the treatment of bacterial skin infections, wounds, burns, and cutaneous ulcers, particularly for animal use, and it has been proven to promote the wound healing process.24 (Nitrofurazone is a bright lemon yellow or pale yellow ointment, containing 60 mg nitrofurazone in a 30 g water-soluble base, supplied by Shanghai General Pharmaceuticals Co, Ltd, no. 889, China).
Extraction and Fractionation
Seeds of the plant were ground to coarse powder using wooden mortar and pestle. The coarse powder was weighed (2 kg) and macerated in Erlenmeyer flasks with 80% methanol (6.250 L) for 72 hours with occasional shaking at room temperature. Then, the extract was filtered by gauze (muslin) and re-filtered using Whatman No 1 filter paper. The marc was re-macerated twice in the same manner. Then, the filtrates were combined and evaporated on a rotary evaporator (Buchi Rota Vapor R-200) under reduced pressure and dried in an oven at 40°C (Gallenkamp, UK). The extract was put in a refrigerator until used for further activities.
Then, the hydromethanolic extract was successively fractionated using different solvents having varied polarity index (chloroform, ethyl acetate, and distilled water). The crude extract (60 g) was mixed with 200 mL distilled water in a separatory funnel and an equivalent amount of chloroform was added and shaken. After a distinct layer was formed in a mixture, the chloroform fraction was separated. This procedure was repeated three times to find the chloroform fraction. Then the aqueous residue was mixed with equivalent volume of ethyl acetate and separated. The chloroform and ethyl acetate fractions were evaporated under reduced pressure using a rotary evaporator (Buchi Rota Vapor R-200) and dried in an oven under 40°C, while the aqueous fraction was dried in a freeze drier. The dried fractions were placed in a tight container at −4°C until used for the actual experiment.
Formulation of Ointments of Extracts
The simple ointment was prepared according to the formula elaborated in the British Pharmacopoeia.25 Firstly, hard paraffin (0.50 g) was melted in a water bath. Then all ingredients of the ointment base (cetostearyl alcohol (0.50 g), white soft paraffin (8.5 g), and wool fat (0.50 g)) were added into melted hard paraffin in descending order of melting point and heated gently, with stirring until homogenous. Then the mixture was allowed to cool while stirring. Then 10 g and 20 g of the crude extract were integrated into the simple ointment base to formulate the crude extract into 5% and 10% (w/w) extract ointments, by using levigation.
Preliminary Phytochemical Analysis
Preliminary phytochemical analysis of the crude extract and solvent fractions of the seeds of B.carinata were performed following the procedures described by Trease and Evans26, and Debella.27
Acute Dermal Toxicity
The acute dermal toxicity testing was carried out based on the methods described in the OECD draft guideline number 434.28 Ten albino female rats (Rattus albus) (weight= 200–300 g, age= 8–12 weeks) were used. In considering the most appropriate sex, females are generally slightly more sensitive to acute oral toxicity as well as dermal toxicity than males.29–31 Rats were divided into two groups of five animals in each group (test and control groups). Animals that show normal skin texture were housed individually in a cage and acclimatized to the laboratory condition for 1 week prior to the test. Following acclimation, 10% of the body surface area fur was shaved 24 hours before the study on the dorsal area of the trunk of the test animals. A limit test dose of 2,000 mg/kg of the 10% w/w of the crude extract formulation was applied evenly over the shaved area for 24 hours. Initially, a sighting (pilot) study was conducted with one animal for the first 24 hours. The purpose of the sighting study is to check whether the larger dose selected is toxic or not for the main study. The remaining four rats were tested on the following day having obtained the information about safety in the sighting study. Observations for any signs of adverse skin reactions, inflammation, or edema were made for 24 hours with special attention within the first 4 hours. Regarding scoring of the skin irritation, normally we followed the Draize scoring criteria for erythema and edema. However, none of them were showing clear signs and symptoms of toxicity when the animals were followed up daily for 14 days.
Wound Healing Activities
Excision Model
Mice were anesthetized by injecting ketamine (1 mL/kg) and diazepam (1 mL/kg) subcutaneously and then the back fur of the animals was removed. Approximately 300 mm2 circular marks were prepared using a permanent marker. Then, the full thickness of this circular mark was excised at nearly the same anatomical site using forceps and sharp sterilized surgical blades to form a wound (Figure 1). The wound area was not covered with gauze.32 After 24 hours of wound creation, mice were treated as explained above. Application of the preparations was continued daily until complete wound healing was seen in the test groups. Wound healing parameters such as wound contraction and the period of epithelialization were determined. The epithelialization period is the total number of days required for the wound scabs to fall off without leaving a raw wound since the first day of wound creation.33 In addition, the progress of wound contraction was measured every other day, starting from the first day of wound creation until complete wound closure seen by using a transparency sheet and permanent marker. Then, the traced area for each mouse was calculated by using scaled paper (graph paper), each small square representing 1 mm2. The percentage of wound contraction was determined as follows.33,34
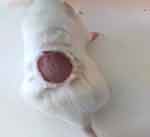 |
Figure 1 Wound created at day 0. |

where, day 0 = day of wound creation, day n = number of days, ie, second, fourth, sixth, etc., day until the wound in test groups healed.
Incision Model
The mice were anesthetized and the fur was carefully shaved in the same manner as described for the excision model. A 3 cm length, linear-paravertebral incision was made through the full thickness of the skin on either side of the vertebral column at a distance of 1 cm from the midline. The dissected skin was closed by stitching with black braided silk (no. 00) and a curved needle (no. 00) at a 1 cm interval.33,35 After 24 hours of wound creation, ointments were applied once daily for 9 days. On the 8th day, the suture was removed and the tensile strength was measured on the 10th day using diethyl ether anesthesia.
The breaking strength of the wound on each animal was measured by using a constant water flow method. In this method, the animals were first anesthetized in the same manner described above and positioned on a table between two metal stands. To both stands, allice forceps of equal size were suspended with strings, one of which passed on a small wheel with the stands. One of the forceps served as a support and the other forceps holed a plastic of volume 1,000 mL (plastic bottle). Then, both forceps were allowed to grip the edges of the wound 3 mm away on both sides. After this, constant water flow was allowed to enter the plastic bottle which was suspended with the string that passed on the wheel until the wound opened up (broken). The flowing water was stopped immediately as the wound breaks in each mouse. Then the volume of the water in the plastic bottle was measured by using a measuring cylinder which was taken as the wound breaking strength for the individual mouse (in grams, since the density of water=1 gm/mL). Percent tensile strength was calculated as follows.36

In vitro Antioxidant Activity in DPPH Assay
The in vitro free radical scavenging activities of the crude extract, solvent fractions, and ascorbic acid were determined by using diphenyl-2-picrylhydrazyl (DPPH; Sigma Aldrich) assays according to the methods described by Blois37 and Desmarchelier et al.38 Different concentrations of 100 mL of a methanolic solution ranging from 50–1,000 mg/mL were added to 3.9 mL of a 0.004% methanolic solution of DPPH. The absorbance was measured at 517 nm after 30 minutes, and the percentage inhibition of DPPH was calculated. The percentage of the scavenging of the DPPH free radical was calculated by the formula: (A0-A1)/A0*100, where A0 is the absorbance of the control and A1 is the absorbance of the extract/standard. All experiments were performed in triplicate measurements.
Data Analysis
The data were entered, coded, and analyzed using Statistical Package for the Social Sciences (SPSS) version 23 and presented as mean±standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey post-hoc test was performed. Finally, the results were considered significant at 95% confidence level when P-value was<0.05.
Results
Phytochemical Screening
The percentage yield value of the crude extract was 5.75% and that of chloroform, ethyl acetate, and aqueous fractions were 10.83%, 15%, and 74.17%, respectively. The phytochemical screening test revealed the presence of polyphenols, alkaloids, flavonoids, tannins, glycosides, and steroids in the crude extract and all fractions. On the other hand, the crude extract and all fractions were devoid of terpenoids, and saponins were absent in the chloroform fraction (Table 1).
 |
Table 1 Preliminary Phytochemical Analysis of 80% Methanol Extract and Solvent Fractions of B. carinata Seeds |
Acute Dermal Toxicity
Topical application of 2,000 mg/kg in 10% (w/w) ointment preparation produced no signs of skin reactions, inflammation, or edema within 24 hours of topical application. The rats were also followed up for 14 days and neither mortality nor any signs of skin toxicity were observed.
Excision Model
Wound Contraction and Period of Epithelialization
Topical applications of ointments of the 80% methanolic extracts and solvent fractions of B.carinata seeds showed a significant wound healing effect in mice. The plant extracts facilitated significant wound contraction at both dose levels from the 6th day to the 16th day as compared to the negative control group (Table 2, Figure 2). Mice treated with 10% (w/w) extract exhibited a significant (P<0.001) wound contraction effect starting from the 6th day of treatment, while 5% (w/w) extract showed a significant (P<0.01) effect at the 8th day onwards as compared to the negative control. Groups treated with 10% (w/w) and 5% (w/w) extract preparations healed completely at the 16th and 18th days of post-wounding, respectively.
 |
Table 2 Effect of Topical Application of the 80% Methanolic Crude Extracts Ointments of the Seeds of B. carinata on Wound Contraction of Excision Wound Model in Mice |
 |
Figure 2 Progress on percentage of wound area contraction of negative and positive controls, and 5% w/w and 10% w/w crude extract ointments across post-wounding days in mice in excision model. |
The 10% (w/w) extract preparation produced 96.6% (12th day), 99.1% (14th day), and 100% (16th day) of wound closures, which was comparable with the positive control (92.2%, 12th day; 98.4%, 14th day, and 100%, 16th day). On the other hand, the percentage wound closures induced by 5% (w/w) extract on the 12th, 14th, and 16th days of post-wounding were 87.6%, 96.0%, and 99.5%, respectively (Figure 3). In addition, both 10% (w/w) and 5% (w/w) extract preparations produced a significant reduction (P<0.01) in epithelialization period as compared to negative control (Figure 4).
 |
Figure 3 Percentage of wound area contraction effects of crude extract ointments of seeds of B. carinata in mice in excision model. |
As shown in Table 3, all fractions produced wound healing activity at varying degrees. The 5% (w/w) and 10% (w/w) preparations of ethyl acetate and aqueous fractions produced significant wound contraction (P<0.01) beginning from the 4th day of post-wounding as compared to negative control. The 10% (w/w) and 5% (w/w) chloroform fraction showed significant wound contraction beginning from the 6th and 8th post-wounding days, respectively. Besides, all fractions produced a significant reduction (P<0.01) in epithelialization period as compared to negative control, though there were observable differences among them (Figure 4).
 |
Table 3 Effects of Topical Application of the Chloroform, Ethyl Acetate, and Aqueous Fractions of the Seeds of B. carinata on Wound Contraction of Excision Wound Model in Mice |
The higher dose (10%) of aqueous fraction exhibited a higher percentage of wound closure at day 10 (93.5%), 12 (98.99), and 14 (100%). The corresponding wound closure percentages for 10% (w/w) ethyl acetate fraction were 92.8%, 97.1%, and 99.4% at the 10th, 12th and 14th days, respectively and the percentages of wound contractions exhibited by 10% (w/w) chloroform fraction at the 12th, 14th, and 16th days were 95.5, 98.8, and 99.8, respectively (Figure 5).
 |
Figure 5 Percentage of wound area contraction effects of chloroform, ethyl acetate, and aqueous fractions of B. carinata seed extract in excision model. |
Incision Model
Wound Breaking Strength (Tensile Strength)
Groups treated with the 10% (w/w), standard drug, and 5% (w/w) showed a significant increase in tensile strength by 33.2% 3, 31.2%, and 14.5%, respectively, when compared to the negative control (P<0.05). Moreover, there was a statistically significant difference between 10% (w/w) and 5% (w/w) extracts treated groups (P<0.01) (Table 4).
 |
Table 4 Effects of Topical Application of the 80% Methanolic Crude Extracts Ointment of B. carinata Seeds on Tensile Strength of Incision Wound Model in Mice |
Antioxidant Activity of Crude Extract and Solvent Fractions
The crude extract and solvent fractions of seeds of B. carinata were tested for antioxidant activity using DPPH free radical assay. The DPPH free radical scavenging activities of the B. carinata extracts were concentration dependent with an IC50 value of 3.45±0.12, 5.85±0.37, 10.54±0.57, and 15.64±0.42 mg/mL for the aqueous fraction, crude extract, ethyl acetate fraction, and the chloroform fraction, respectively. Higher free radical scavenging activity was observed in the aqueous fraction among other fractions (Table 5).
 |
Table 5 Anti-Oxidant Activities of the Crude Extract and Solvent Fractions of Seeds of B.carinata in DPPH Assay Model |
Discussion
Traditionally, different medicinal plants have been used for many years as topical preparations to promote wound healing. The beneficial traits of plant extracts used to treat wound healing are to produce minimal pain, and reduced scarring to the patient.39 Therefore, the studied plant was evaluated for its anti-oxidant and wound healing activities.
The crude extract and fractions produced good anti-oxidant activities in this study. Higher free radical scavenging activity (89.57%) was observed in the aqueous fraction, and lowest effect (70.32%) was observed in the chloroform fraction. But the percentage of inhibition of ascorbic acid was found to be 96.07%.
The results of the present study indicated that the crude extract and the solvent fractions significantly increased wound healing effects with both 10% (w/w) and 5% (w/w) extract ointment treated groups at varying degrees both in the excision and incision models. The enhanced wound contraction and shorter period of epithelialization induced by the crude extract as well as fractions of B. carinata seeds might be associated with increased collagen synthesis and facilitate proliferation of epithelial cells, which might be ascribed to its potent antioxidant activity as revealed in the present study. This fact can be further supported by a study reporting that the leaves of B. carinata have antigenotoxic and deoxyribo nucleic acid (DNA) damage protective effects.40 Protection of macromolecules like DNA, proteins, and lipids can be achieved through scavenging excess free radicals released as a result of oxidative stress that can be induced by a variety of environmental and chemical factors and natural radiation.41
In the excision model, the 10% (w/w) extract ointment treated group showed significant wound area contraction from day 6 to day 16, whereas the 5% (w/w) extract ointment treated group showed significant wound area contraction beginning from the 8th day on wards. Besides, the epithelialization period was significantly reduced from 20 days (negative control) to 16 days (5% w/w extract) and 14 days (10% w/w extract). Hence, a faster rate of wound contraction and shorter period of epithelialization of the extract ointments may be due to the synergistic effects of different secondary metabolites present in the plant. This finding is strengthened by previous reports of a review on health promoting bioactive phytochemicals from Brassica genus viz B. nigra, B. juncea, B. oleracea, and B. rapa showed that high content of poly phenols and flavonoids are found in the seeds, and these phytochemicals contribute to their antioxidant and anti-inflammatory property. Particularly, sinapic acid, an isolated phenolic compound and kaempferol and quercetin, the flavonoids isolated from Brassica vegetables were reported to have anti-oxidant activity.42 Furthermore, Brassinosteroids, a group of steroidal substance, found in brassica species are plant growth hormones involved in cell growth, division, and differentiation. Topical application of brassinosteroid significantly reduced wound size and accelerated wound healing in treated animals in the mouse model of cutaneous wound healing.43 Moreover, sinigrin, a major glucosinolate isolated from the Brassica species including B.carinata, was studied for a number of activities such as anticancer, anti-inflammatory, antifungal, antioxidant, and wound healing effects.44
A better wound healing effect of the crude extract was further evidenced by the tensile strength in incision wounds. In this model, there was a significant increase in wound breaking strength (tensile strength) in 10% extract, 5% extract ointments, and the standard drug as compared to the negative control. This fact is evidenced by the antigenotoxic effects of B. carinata and its protection of DNA damage that can be caused by oxidative stress. Further, the present finding can be supported by published literature stated that Brassica oleracea var. capitata showed maturation of the extracellular matrix in skin wounds of wistar rats as evidenced by significant levels of total collagen and glycosaminoglycans found to occur starting from the 8th day compared to negative control. The tensile strength indicates how much the repaired tissue resists to breaking under tension, which is related to an increase in collagen synthesis and maturation, formation of stable intra and intermolecular cross-link, matrix deposition, and cell migration, and may indicate the quality of repaired tissue.45
In excision wound model, despite all fractions showed varying degrees of wound area contraction compared to the negative control at both dose levels, better activity was observed for 10%w/w aqueous and ethyl acetate fractions, as shown in Table 3. Among all fractions, aqueous 10% w/w fraction showed better wound healing activity. This finding may indicate the phytochemicals responsible for wound healing activity are largely polar compounds and are found in large amount in the aqueous fraction. Phenolic compounds, flavonoids, and glucosinolates that are found in Brassica species, and which were explained for their antioxidant or anti-inflammatory activity, are usually polar and highly soluble in water because the naturally occurring glycosides sugar moieties elevate their hydrophilicity.46 Therefore, these compounds may contribute to facilitate wound healing in aqueous fraction and ethyl acetate fractions as well.
On the other hand, significant wound closure of chloroform fraction was seen starting from the 8th day compared to negative control, which may account for the presence of non-polar substances such as fatty acids, as indicated in the oils of other mustard seeds. Chemical analysis of Brassica juncea (L.) in the previous study demonstrated the presence of a significant amount of unsaturated fatty acids, namely oleic acid (18.2%), linoleic acid (16.9%), erucic acid (42.0%), eicosenoic acid (8.8%), and others, which all contribute to anti-oxidant and wound healing effects.47 Besides, the current phytochemical screening of chloroform fraction revealed the presence of phenols, flavonoids, tannins, glycosides, alkaloids, and steroids, despite the type and content being unknown yet. Hence, these chemicals may produce a combined effect of wound healing.
Generally, from the findings of the present study, it is therefore plausible to assume that the 80% methanol extract and solvent fractions of B.carinata seeds constituted secondary metabolites that contribute to promote wound healing either individually or synergistically, justified by the traditional claims of the plant for use in the treatment of wounds.
Conclusion
The present study investigated that topical ointments of the 80% methanol extract at both dose levels and the solvent fractions of B. carinata seeds showed considerable wound healing activity as evidenced by the significant increase in wound contraction and tensile strength, and a shorter period of epithelialization as compared to the negative control. The study also indicated that both the crude extract and solvent fractions possess anti-oxidant effects at varying degrees. The aqueous fraction has better wound healing as well as anti-oxidant activity among other fractions. Hence, the present study highlighted the potential wound healing as well as anti-oxidant activities of the plant and justified the traditional use of the plant for the treatment of wounds.
Abbreviations
ANOVA, one way analysis of variance; DPPH, 2, 2-diphenyl-2-picrylhydrazyl hydrate; OECD, Organization for Economic Cooperation and Development; SEM, standard error of the mean; SPSS, Statistical Package for Social Sciences.
Data Sharing Statement
All data generated or analyzed during this study are included in the manuscript and are also available from the corresponding author upon request.
Ethics Approval and Consent to Participate
All the experiments were conducted in accordance with a guide for the care and use of laboratory animals: Eighth Edition by National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. We also followed OECD guideline 434. Ethical approval was obtained from Research and Ethical Review Board of College of Medicine and Health Sciences, University of Gondar before the initiation of this study (protocol number sop4/73/09). At the end of the experiment, the animals were killed by Phenobarbital sodium at a dose of 150 mg/kg (euthanasia, mercy killing).
Acknowledgment
The authors would like to thank University of Gondar for the financial support extended to complete the research work.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
University of Gondar covered the costs of chemicals, reagents and equipments used.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol. 2009;124(2):311–315. doi:10.1016/j.jep.2009.04.038
2. Soni H, Singhai AK. A recent update of botanicals for wound healing activity. Int Res J Pharm. 2012;3(7):1–7.
3. Ghosh PK, Gaba A. Phyto-extracts in wound healing. J Pharm Pharm Sci. 2013;16(5):760–820. doi:10.18433/J3831V
4. Kumarasamyraja D, Jeganathan N, Manavalan R. A review on medicinal plants with potential wound healing activity. Int J Pharm Sci. 2012;2:105–111.
5. Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54(2):271–284. doi:10.1124/pr.54.2.271
6. Naskar S, Mazumder UK, Pramanik G, et al. Comparative in vitro antioxidant activity of different parts of Cocos nucifera (Linn.) on reactive oxygen and nitrogen species. Int J Pharm Pharm Sci. 2011;3(Suppl 3):104–107.
7. Islam S, Nasrin S, Khan MA, et al. Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. BMC Complement Alt Med. 2013;13(1):142. doi:10.1186/1472-6882-13-142
8. Rahman MM, Islam MB, Biswas M, Alam AK. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015;8(1):621. doi:10.1186/s13104-015-1618-6
9. Hamid K, Saha MR, Urmi KF, Habib M, Rahman MM. Screening of different parts of the plant pandanus odorus for its antioxidant activity. Int J Appl Biol Pharm Technol. 2010;1364–1368.
10. Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21(2):143–152. doi:10.1016/j.jsps.2012.05.002
11. Britannica IE. Encyclopædia britannica. Encyclopaedia Britannica, Incorporated; 1957.
12. Tanaka T, Horiuchi G, Matsuoka M, et al. Formation of lysophosphatidic acid, a wound-healing lipid, during digestion of cabbage leaves. Biosci Biotechnol Biochem. 2009;0905071456.
13. Akhtar MS, Munir M. Evaluation op the gastric antiulcerogenic effects of Solanum nigrum, Brassica oleracea and Ocimum basilicum in rats. J Ethnopharmacol. 1989;27(1–2):163–176. doi:10.1016/0378-8741(89)90088-3
14. Monsalve C, Cano A. The Brassicaceae Family in Huaylas Province, Ancash. Revista Peruana de Biología. 2003;10(1):20–32.
15. Podsędek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. Food Sci Technol. 2007;40(1):1–11. doi:10.1016/j.lwt.2005.07.023
16. Nawaz H, Shad MA, Muzaffar S. Phytochemical Composition and Antioxidant Potential of Brassica. Brassica Germplasm. 2018;7.
17. Rashmi HB, Negi PS. Health Benefits of Bioactive Compounds from Vegetables. Plant-Derived Bioactives. Springer; 2020.
18. Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol. 2007;25(1):19–25. doi:10.1016/j.clindermatol.2006.12.005
19. Chuong PV, Beversdorf W. High frequency embryogenesis through isolated microspore culture in Brassica napus L. and B. carinata Braun. Plant Sci. 1985;39(3):219–226. doi:10.1016/0168-9452(85)90178-5
20. Bitew H, Gebregergs H, Tuem KB, Yeshak MY. Ethiopian medicinal plants traditionally used for wound treatment: a systematic review. Ethiop J Health Dev. 2019;33(2).
21. Birhanu T, Abera D, Ejeta E, Nekemte E. Ethnobotanical study of medicinal plants in selected Horro Gudurru Woredas, Western Ethiopia. J Biol Agric Healthc. 2015;5(1):83–93.
22. National Research Council. Division on Earth and Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals.
23. Festing MF, Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. Inst Labor Anim Res J. 2002;43(4):244–258. doi:10.1093/ilar.43.4.244
24. Karapolat S, Karapolat B, Buran A, et al. The Effects of Nitrofurazone on Wound Healing in Thoracoabdominal Full-thickness Skin Defects. Wounds. 2020;32(5):134–141.
25. British Pharmacopoeia. Department of health and social security Scottish home and health department. Br Pharmacopoeia Commission. 1988;2:713.
26. Trease G, Evans M. Text Book of Pharmacognosy 13th Edition Bailiere Tindall, London, Toronto. Tokyo Pgs; 1989.
27. Debella A. Manual for Phytochemical Screening of Medicinal Plants. Addis Ababa, Ethiopia: Department of Drug Research, Ethiopian Nutrition and Research Institute; 2002.
28. Amcoff P. Appendix 5: organization for Economic Cooperation and Development Guidelines for the Testing of Chemicals. ALTA. 2005;33(1_suppl):223–228. doi:10.1177/026119290503301s04
29. Wilhelm K-P, Maibach HI. Organization for Economic Cooperation and Development Guidelines for Testing of Chemicals. Dermatotoxicology. 2007;303.
30. Occupational Safety and Health Administration. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). United Nations Economic Commission for Europe (UNECE); 2013.
31. Lipnick R, Cotruvo J, Hill R, et al. Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem Toxicol. 1995;33(3):223–231. doi:10.1016/0278-6915(94)00136-C
32. Morton J, Malone M. Evaluation of vulneray activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196(1):117.
33. Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement Altern Med. 2015;15(1):341. doi:10.1186/s12906-015-0878-y
34. Yesuf A, Asres K. Wound healing and antiinflammatory properties of Allophylus abyssinicus (Hochst.) Radlk. Phytopharmacology. 2013;4(2):442–453.
35. Ehrich H. Effect of cortisone and anabolic steroids on tensile strength of healing wound. Ann Surg. 1969;170(2):203–206. doi:10.1097/00000658-196908000-00007
36. Lee K. Studies on the mechanism of action of salicylates III. Effect of vitamin A on the wound healing retardation action of aspirin. J Pharm Sci. 1968;57(7):1238–1240. doi:10.1002/jps.2600570736
37. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi:10.1038/1811199a0
38. Desmarchelier C, Novoa Bermudez M, Coussio J, Ciccia G, Boveris A. Antioxidant and prooxidant activities in aqueous extracts of Argentine plants. Int J Pharmacogn. 1997;35(2):116–120. doi:10.1076/phbi.35.2.116.13282
39. Ayal G, Belay A, Kahaliw W. Evaluation of wound healing and anti-inflammatory activity of the leaves of Calpurnia aurea (Ait.) Benth (fabaceae) in mice. Wound Med. 2019;25(1):100151. doi:10.1016/j.wndm.2019.100151
40. Lozano-Baena M-D, Tasset I, Obregón-Cano S, Haro-Bailon D, Muñoz-Serrano A, Alonso-Moraga Á. Antigenotoxicity and tumor growing inhibition by leafy Brassica carinata and Sinigrin. Molecules. 2015;20(9):15748–15765. doi:10.3390/molecules200915748
41. Arunachalam KD, Subhashini S, Annamalai S. Wound healing and antigenotoxic activities of Aegle marmelos with relation to its antioxidant properties. J Pharm Res. 2012;5(3):1492–1502.
42. Kumar S, Andy A. Health promoting bioactive phytochemicals from Brassica. Int Food Res J. 2012;19(1):141–152.
43. Esposito D, Rathinasabapathy T, Schmidt B, Shakarjian MP, Komarnytsky S, Raskin I. Acceleration of cutaneous wound healing by brassinosteroids. Wound Repair Regen. 2013;21(5):688–696. doi:10.1111/wrr.12075
44. Mazumder A, Dwivedi A, Du Plessis J. Sinigrin and Its Therapeutic Benefits. Molecules. 2016;21(4):416. doi:10.3390/molecules21040416
45. Getie M, Gebre-Mariam T, Rietz R, Neubert R. Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae). Die Pharmazie. 2002;57(5):320–322.
46. Holst B, Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep. 2004;21(3):425–447. doi:10.1039/b204039p
47. Toosi AF, Bakar B, Tayyab S. Chemical Analysis of Brassica juncea (L.) Czern var. Ensabi. Vegetos. 2013;26(2):93–97. doi:10.5958/j.2229-4473.26.2.059
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

