Back to Journals » Drug Design, Development and Therapy » Volume 14
In vitro Antifungal Effects of Berberine Against Candida spp. In Planktonic and Biofilm Conditions
Received 12 September 2019
Accepted for publication 20 December 2019
Published 9 January 2020 Volume 2020:14 Pages 87—101
DOI https://doi.org/10.2147/DDDT.S230857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Tuo Deng
Yufei Xie, Xiaosong Liu, Peiru Zhou
Department of Oral Medicine, Peking University School and Hospital of Stomatology, Beijing, People’s Republic of China
Correspondence: Peiru Zhou
Department of Oral Medicine, Peking University School and Hospital of Stomatology, Beijing 100081, People’s Republic of China
Tel +86 10 8219 5349
Fax +86 10 6217 3402
Email [email protected]
Purpose: Antifungal resistance associated with the extensive use of antifungals and biofilm formation presents major clinical challenges. Thus, new therapeutic strategies for fungal infections are urgently required. This study aimed to evaluate the in vitro antifungal effects of the natural bioactive alkaloid berberine against Candida spp. in planktonic and biofilm conditions.
Methods: Using the CLSI M27-A3 reference method for broth dilution antifungal susceptibility testing of yeasts, the MICs for five standard strains comprised of Candida albicans (ATCC 10231, ATCC 90028), Candida krusei (ATCC 6258), Candida glabrata (ATCC 90030), Candida dubliniensis (MYA 646), and six clinical isolates (CLC1–CLC6) were tested. The 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay was used to evaluate the inhibitory effects of berberine against Candida biofilms. The optical density value at 490 nm was measured and illustrated using concentration-absorbance curves. Finally, the effects were quantified by confocal laser scanning microscopy (CLSM), and 3-dimensional reconstruction was performed. The viability inhibition rates, biofilm formation, and thickness decrease rates were tested and analyzed using independent-samples t-test. The differences among the five Candida strains were analyzed using one way ANOVA.
Results: The MICs for the five standard strains described above were 80, 160, 10, 20, and 40 μg/mL, respectively, which was similar to that of the clinical isolates, suggesting the stable, broad-spectrum antifungal activity of berberine. Berberine exerted concentration-dependent inhibitory effects against Candida biofilms, which were enhanced with the maturation of Candida biofilms. Berberine decreased the viability of Candida biofilms, with inhibition rates by CLSM ranging from 19.89 ± 0.57% to 96.93 ± 1.37%. Following 3-dimensional reconstruction, the biofilms of the berberine-treated group displayed a poorly developed architecture, and the biofilm thickness decrease rates ranged from 15.49 ± 8.45% to 30.30 ± 15.48%.
Conclusion: Berberine exhibited significant antifungal activity in Candida spp. The results provide a useful reference for multiple Candida infections and biofilm infections associated with antifungal resistance. Therefore, berberine might have novel therapeutic potential as an antifungal agent or a major active component of antifungal drugs.
Keywords: berberine, antifungal effect, Candida spp, biofilm
Introduction
Candida spp. can cause opportunistic infections, including superficial Candida infections and invasive candidiasis in immunocompromised individuals, such as patients with AIDS and transplant and cancer patients receiving cytotoxic drugs, thus threatening their quality of life and life span, especially in elderly patients.1,2 Superficial Candida infections, such as mucocutaneous candidiasis have been shown to increase the risk of oral cancer by 3.2 times.3,4 With the extensive use of implanted devices, including heart stents, catheters, and prostheses, the risk of invasive candidiasis caused by biofilms is increasing remarkably, resulting in high mortality rates of 40 to 60%.5–9 Previous studies have shown that Candida albicans is the predominant species causing Candida infections. However, an increasing proportion of non-albicans Candida spp., such as Candida krusei and Candida glabrata in superficial Candida infections, and Candida dubliniensis in invasive candidiasis have been widely reported in the last decade.5,8,10−14
The global rise in antifungal resistance makes fungal infections harder to treat.15 Due to the broad-spectrum and long-term application of antifungal agents, such as azoles and echinocandins, C. albicans has developed antifungal drug resistance, while some non-albicans Candida spp., such as C. krusei are even found to be intrinsically resistant to azoles. Moreover, biofilm formation has been shown to be significantly associated with antifungal resistance, and the ability of Candida spp. to form drug-resistant biofilms is an important factor in their contribution to human disease. Therefore, novel agents with higher antifungal activity and better biofilm inhibitory effects are urgently needed.
Berberine (Figure 1) is a quaternary ammonium salt of isoquinoline alkaloid16 and can be extracted from several herbal plants, such as Coptis chinensis and Mahonia aquifolium.17–19 Berberine and its derivatives exhibit various pharmacological effects, including anti-inflammatory, antibacterial, and antifungal effects,19 with no apparent toxic or side effects reported so far, except for mild gastrointestinal reactions.20 As a traditional Chinese medicine, berberine has been commonly used to treat diarrhea for thousands of years, and is still an important component of patent medicines used for the treatment of bacterial infectious diseases. The glucose- and lipid-lowering actions of berberine in both animal models and humans are gaining increasing attention, thereby supporting its role in the management and prevention of type 2 diabetes, atherosclerosis, and obesity.16,21
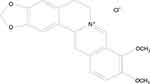 |
Figure 1 Chemical structure of berberine. |
Previous studies were mainly focused on the synergistic antifungal effect of berberine combined with fluconazole, miconazole, and amphotericin B against C. albicans, C. glabrata, and Candida tropicalis.22–26 However, due to the renal toxicity of amphotericin B and its drug resistance to azoles, there is an urgent need to explore the antifungal activity of berberine alone, especially to evaluate its effects against non-albicans Candida strains and Candida biofilms. Moreover, the antifungal activity of berberine in clinical isolates requires further validation. Hence, in this study, the in vitro antifungal effects of berberine against standard and clinical strains of Candida spp. under planktonic and biofilm conditions were thoroughly investigated with an aim to develop new therapeutic strategies for fungal diseases.
Materials and Methods
Candida Strains and Culture Conditions
Eleven Candida strains, including five standard strains and six clinical isolates, were used for this study. Five standard strains comprised of two C. albicans strains (ATCC 10231, ATCC 90028), C. krusei (ATCC 6258), C. glabrata (ATCC 90030), and C. dubliniensis (MYA 646) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). These strains were stored in 20% glycerol and frozen at −80°C. The six clinical isolates comprised of C. albicans (CLC1, CLC2), C. krusei (CLC3, CLCL4), and C. glabrata (CLC5, CLC6) were isolated from the saliva of immunocompromised outpatients at the Department of Oral Medicine, Peking University School and Hospital of Stomatology (Beijing, China), and identified using CHROMagar Candida (BioMérieux Industry Co. Ltd., Shanghai, China). Prior to the experiment, the strains were cultured on Sabouraud dextrose agar (SDA) plates (BioMérieux Industry Co. Ltd.) at 37°C for 48 h, and then stored at 4°C.
Drug and Medium Preparation
Berberine and fluconazole were purchased from the National Institutes for Food and Drug Control (Beijing, China) and their purity levels were 86.7 and 99.7%, respectively. Prior to use, berberine and fluconazole were dissolved in dimethyl sulfoxide (Sigma-Aldrich Co., St. Louis, MI, USA) to achieve final concentrations of 1.28 × 105 and 1.28 × 104 μg/mL, respectively.
Roswell Park Memorial Institute (RPMI) 1640 medium containing L-glutamine (Life Technologies Co., Madison, WI, USA) was buffered to pH 7.0 using 0.165 M 3-morpholinopropane-1-sulfonic acid (Sigma-Aldrich Co.). Yeast nitrogen base medium (YNB) - 50 G and YNB - 100 G were prepared using YNB medium (Beijing Solarbio Science and Technology Co. Ltd., Beijing, China) supplemented with 50 and 100 mM glucose, respectively.
Antifungal Susceptibility Testing
The antifungal activity of berberine and fluconazole against planktonic yeast cells was evaluated in 96-well microtiter plates using the broth dilution testing reference method M27-A3, as recommended by the Clinical and Laboratory Standards Institute (CLSI).27 The MIC was defined as the lowest drug concentration that prevented over 95% discernible growth of planktonic yeasts on visual inspection.
Prior to the experiment, yeast cells were subcultured from the stock solution on SDA plates at 37°C for 24 h and then harvested in RPMI 1640 medium. The initial concentration of the yeast suspension was adjusted to 1 × 103 cells/mL. The berberine and fluconazole solutions were two-fold diluted to achieve final concentrations ranging from 2.5 to 1280 and 0.25 to 128 μg/mL, respectively. Next, 100 μL of yeast suspension was added to 100 μL of berberine or fluconazole solution in each well, and incubated for 48 h at 37°C. The suspension without any drug was regarded as the drug-free control. Every experiment was repeated at least three times.
Candida Biofilm Formation Assay
The in vitro biofilm formation assay was performed following the standard method, with slight modifications.28 In brief, yeast cells were subcultured at 37°C for 18 h, followed by incubation in YNB - 50 G medium overnight at 200 rpm. Yeast cells were then harvested and adjusted to 1 × 107 cells/mL in YNB - 100 G medium. Following this, 100 μL-aliquots of the vortexed yeast suspension were added into the wells of a 96-well microtiter plate and incubated for 2 h at 37°C. Next, the yeast suspension was aspirated and the berberine solution was added to achieve final concentrations of 40–20,480 μg/mL, and incubated for 6, 12, 24, and 48 h at 37°C. The wells without drug supplementation were considered the drug-free controls.
XTT Reduction Assay
To quantify biofilm metabolic activity, the reduction of 2.3-Bis-(2-methoxy-4-nitro5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) was measured. Every 2 mL of 1 mg/mL XTT sodium salt (Sigma-Aldrich Co.) solution, 100 μL of 0.04 mol/L menadione (Sigma-Aldrich Co.) solution, and 7.9 mL of sterilized PBS were mixed to obtain 10 mL of XTT-menadione-PBS reagent. Aliquots (200 μL) of this reagent were added to each well and incubated at 37°C for 3 h in the dark. Following this, 100 μL of the suspension in each well was transferred to a new 96-well microtiter plate. The optical density (OD) value at 490 nm was measured using a microplate reader (BioTek Instruments, Inc., Waltham, MA, USA). Each experiment was repeated three times, and the mean OD value was calculated.
CLSM Image Analysis
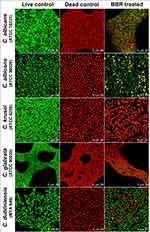
Confocal laser scanning microscopy (CLSM) was performed to further confirm the inhibitory effect of berberine against mature Candida biofilms (48 h) by assessing cell viability and biofilm structures. Before staining, Candida biofilm formation was performed in glass-bottom cell culture dishes (NEST Biotechnology Co., Ltd., Wuxi, China) using the method described above. The biofilms incubated without berberine were regarded as the live controls, and those incubated with isopropyl alcohol for 30 to 60 min were regarded as the dead controls. The berberine solution was diluted to achieve final concentrations of 40, 5120, 320, 40 and 1280 μg/mL for C. albicans (ATCC 10231), C. albicans (ATCC 90028), C. krusei (ATCC 6258), C. glabrata (ATCC 90030), and C. dubliniensis (MYA 646), respectively.
Rapid immunofluorescence staining was performed using the LIVE/DEAD FungaLight Yeast Viability Kit (Molecular Probes, Inc., Eugene, OR, USA). Live yeasts with intact cell membranes were stained fluorescent green by SYTO 9, whereas dead yeasts with damaged membranes were stained fluorescent red, indicating the penetration of propidium iodide. According to the kit manufacturer’s protocol, 500 μL of stain solution was added to each biofilm dish and incubated in the dark at room temperature for 15 to 30 min. The biofilms were observed under a confocal laser scanning microscope (Carl Zeiss, Inc., Oberkochen, Germany) and images were captured to determine the biofilm viability inhibition rates. Then, 3-dimensional (3-D) reconstruction was carried out using Leica Application Suite X (LAS X) software to reveal the changes in biofilm structure and thickness.
In the CLSM images, the live and dead cells in the berberine-treated group were counted using ImageJ software. The viability inhibition rates were calculated using the following formula: dead cell count/(live cell count + dead cell count). In addition, the cells of the biofilm per dish were collected and inoculated with an automatic spiral plater (Interscience, Inc., Troy, NY, USA) on SDA plates and cultured at 37°C for 48 h. The number of colony forming units (CFU) was counted. The biofilm viability inhibition rates were calculated using the following formula: 1 – berberine-treated group CFU/live control group CFU. The biofilm formation rates of the berberine-treated group were calculated using the following formula: biofilm thickness of the berberine-treated group/biofilm thickness of the live control group. The biofilm thickness decrease rates of the berberine-treated and dead control groups were calculated using the following formula: 1 – biofilm thickness of the berberine-treated (or dead control) group/biofilm thickness of the live control group. Each experiment was repeated three times.
Statistical Analysis
The data were analyzed using parametric statistical tests. The independent-samples t-test was used to analyze the viability inhibition rates between the CLSM and CFU groups, the biofilm formation rates between the berberine-treated and live control groups, and the biofilm thickness decrease rates between the berberine-treated and dead control groups. One-way analysis of variance (ANOVA) was used to analyze differences between the five Candida strains. Statistical Product and Service Solutions (SPSS) 18.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. P < 0.05 was considered statistically significant.
Results
Berberine Had Inhibitory Effects on Standard Strains and Clinical Isolates of Candida spp. Under Planktonic Condition
The antifungal activity of berberine against Candida strains under planktonic conditions was tested according to the CLSI M27-A3 reference method.27 The MICs of berberine and fluconazole for five standard strains and six clinical isolates of Candida spp. are shown in Table 1. Of the tested standard strains (Figure 2A), C. krusei was the most susceptible to berberine, with an MIC of 10 μg/mL, followed by C. glabrata and C. dubliniensis, with MICs of 20 and 40 μg/mL, respectively. By contrast, the MICs of berberine for the two standard C. albicans strains (ATCC 10231 and ATCC 90028) were found to be higher (80 and 160 μg/mL, respectively). These results indicated that the antifungal activity of berberine against non-albicans Candida strains, especially C. krusei, was higher than that against C. albicans.
 |
Table 1 MICs of Berberine and Fluconazole for Standard Strains and Clinical Isolates of Candida Spp. In Planktonic Condition |
Of the tested clinical isolates of Candida spp. (Figure 2B), the C. krusei isolates, CLC3 and CLC4, were the most susceptible to berberine, with MICs of 10 and 20 μg/mL, respectively. The MICs for the C. albicans isolates, CLC1 and CLC2, were 80 and 160 μg/mL, respectively. These results were consistent with those of the standard strains. The MICs for the C. glabrata isolates, CLC5 and CLC6, were 80 and 160 μg/mL, respectively, which were slightly higher than those for C. glabrata standard strains. Hence, these results suggested a stable broad-spectrum antifungal activity of berberine.
Berberine Exerted Concentration-Dependent Inhibitory Effects Against Candida Biofilms
Based on the main growth phases of Candida biofilms, four time-points were chosen to evaluate the inhibitory effects of berberine on the metabolic activity of Candida biofilms by XTT reduction assay. The absorbance (optical density; OD) values represent the metabolic activity of the Candida biofilms. The results of the XTT reduction assay were presented as a concentration-absorbance curve (Figure 3). The declining trend of the concentration-absorbance curve at the four time-points demonstrated that the metabolic activity of the Candida biofilms decreased with increasing concentrations of berberine.
Berberine Inhibited the Overall Metabolic Activity of Candida Biofilms
The overall metabolic activity of Candida biofilms at each time-point was further determined by calculating the mean OD value at all berberine concentrations (Figure 4). At 6 h after berberine treatment, the overall metabolic activity of all Candida biofilms was relatively high, as indicated by their mean OD values (C. albicans ATCC 10231, 0.88; C. albicans ATCC 90028, 0.90; C. krusei, 0.93; C. glabrata, 0.79; and C. dubliniensis, 0.90). However, at 12 h after berberine treatment, their metabolic activity was reduced (mean OD values: C. albicans ATCC 10231, 0.65; C. albicans ATCC 90028, 0.55; C. glabrata, 0.56; C. dubliniensis, 0.71), with the exception of C. krusei (mean OD value: 1.18). With an increase of treatment time to 24 h, the overall metabolic activity of C. krusei (mean OD value: 1.03) and C. glabrata biofilms (mean OD value: 0.54) further reduced, reaching the lowest values at 48 h (mean OD values: C. krusei, 0.58; C. glabrata, 0.36). The overall biofilm metabolic activity of the two C. albicans strains and C. dubliniensis increased slightly at 24 h post-treatment and remained constant up to 48 h (mean OD values: C. albicans ATCC 10231, 0.78; C. albicans ATCC 90028, 0.51; C. dubliniensis, 0.80), but was still markedly lower than that at 6 h. These results indicated that with the prolongation of drug action time, the inhibitory effects of berberine on overall biofilm metabolic activity were enhanced, especially for C. krusei and C. glabrata.
The Inhibitory Effects of Berberine Were Enhanced with the Maturation of Candida Biofilms
According to the concentration-absorbance curve, the biofilm metabolic activity decreased with increasing concentrations of berberine. The inflection point was defined as the minimum berberine concentration that induced a significant decrease in Candida biofilm metabolic activity, and which brought about the first apparent reduction of OD value.29 The inflection points of the five strains changed with the maturation of Candida biofilms (from 6 to 48 h; Figure 5).
From the overall trend, the inflection points of berberine for C. albicans (ATCC 10231), C. krusei, and C. glabrata gradually declined from 1280 to 40 μg/mL, 160 to 40 μg/mL, and 10,240 to 320 μg/mL, respectively, whereas those for C. albicans (ATCC 90028) and C. dubliniensis reached the highest at 48 h (5120 and 10,240 μg/mL, respectively). Most values were altered significantly during the biofilm formation phases between 6 to 24 h, and tended to be steady at the biofilm maturation phases between 24 to 48 h. These results indicated that berberine had distinct inhibitory effects on biofilm formation based on its maturation phase, and its inhibitory effect was higher in matured biofilms. Moreover, the inflection points for C. krusei remained the lowest (160 to 40 μg/mL) among all five Candida strains at each time-point, suggesting that C. krusei biofilm was the most sensitive to berberine.
Berberine Decreased the Viability Inhibition Rates for Candida Biofilms
The inhibitory effects of berberine at 48 h post-treatment were further verified by CLSM (Figure 6). The gross number of dead Candida cells in the berberine-treated groups increased significantly compared with that in live control groups, especially for C. krusei and C. glabrata. To quantify the viability changes for different Candida biofilms, the viability inhibition rates were calculated (Figure 7B) and found to be varied among the five strains. For C. krusei and C. glabrata, high viability inhibition rates of 96.93 ± 1.37% and 92.36 ± 0.32%, respectively, were obtained, followed by C. albicans ATCC 10231 (43.54 ± 1 %), C. dubliniensis (21.62 ± 0.51%), and C. albicans ATCC 90028 (19.89 ± 0.57%). The five Candida strains had significantly different viability inhibition rates (P < 0.001). In addition, the CFU of the berberine-treated groups decreased significantly compared with that of the live control group (Figure 7A), and the viability inhibition rates of the berberine-treated groups calculated by CFU were in accordance with the CLSM results. The viability inhibition rates between the CLSM groups and CFU groups were not significantly different (P > 0.05). These results suggested that 48-h berberine treatment resulted in high viability inhibition rates against mature Candida biofilms.
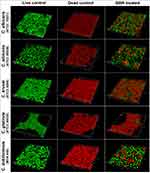
Berberine Weakened the Spatial Structures of Mature Candida Biofilms
To confirm the inhibitory effects of berberine on the spatial structures of mature Candida biofilms, 3-D reconstruction was performed (Figure 8). Compared with those of the live control groups, the 3-D images of the berberine-treated groups displayed biofilms with poorly developed architecture, lower cell densities, and loosely packed cells, especially for C. albicans (ATCC 90028) and C. dubliniensis. Next, the biofilm formation rates and biofilm thickness decrease rates were calculated. Compared with the live control groups, the biofilm formation rates of berberine-treated groups had significantly decreased biofilm formation rates (P < 0.05) (Figure 9A). For the biofilm thickness decrease rates, C. dubliniensis and C. krusei showed high rates of 30.30 ± 15.48% and 27.92 ± 3.46%, respectively, followed by C. glabrata (27.78 ± 12.22%), C. albicans ATCC 10231 (19.71 ± 8.56%), and C. albicans ATCC 90028 (15.49 ± 8.45%). There was no significant difference between the berberine-treated groups and the dead control groups (P > 0.05), except for C. albicans ATCC 10231 (P < 0.05), which may be due to the data variation in the dead control groups (Figure 9B). In addition, the thickness decrease rates among the five Candida strains were significantly different (P < 0.05). These results indicated that berberine could inhibit biofilm formation and damage the spatial structures.
Discussion
The antifungal resistance of Candida spp. to azoles and echinocandins continues to be a global problem,14,15 along with biofilm formation.30,31 The natural bioactive alkaloid berberine has been shown to exhibit significant antifungal activity,32,33 as well as inhibitory effects against biofilm formation.23,34 In this study, we investigated the antifungal activity of berberine against standard strains and clinical isolates of Candida spp. in planktonic and biofilm conditions. More importantly, we revealed the critical role of berberine in inhibiting Candida biofilms via prolongation of the drug action time, especially for non-albicans Candida biofilms. Using CLSM, we further proved that berberine achieved high inhibition rates for mature Candida biofilms and significantly weakened their spatial structures. It is noteworthy that berberine was found to have the most potent inhibitory effect against C. krusei, which is a fluconazole-resistant strain.35,36
Candida biofilms exhibit enhanced resistance to azoles, possibly due to drug efflux pump expression, alterations in membrane sterol composition, and retardation of drug diffusion in the extracellular matrix.30 Our previous research showed that the minimum fluconazole concentration required to inhibit Candida biofilms was 4–500 times higher than the MIC for planktonic cells.37 In the present study, we found that the berberine concentration that significantly reduced the biofilm activity of C. albicans, C. krusei, and C. glabrata was only 4–32 times higher than the MIC for Candida cells in planktonic condition (Table 1; Figure 3). Hence, our results, to some extent, confirmed that berberine had more potent inhibitory effects against Candida biofilms than fluconazole. Previous studies have reported that the minimum berberine concentration required to inhibit C. tropicalis biofilms is approximately 4 times higher than the MIC for planktonic cells,34 which is consistent with our findings. Furthermore, six clinical isolates of Candida spp. from the saliva of immunocompromised patients with oral candidiasis were isolated, the MICs for which were similar to those for the standard strains. These results suggest the possibility of clinical application of berberine for fungal infections.
Next, by selecting four time-points (6, 12, 24, and 48 h), we showed that berberine exerted more potent inhibitory effects against Candida biofilms with the prolongation of drug action time, especially against mature biofilms. The possible mechanism might be correlated to the different metabolic characteristics of the biofilms in different growth phases. Previous studies have shown that, in the first 8 h of biofilm formation, the metabolic activity of Candida cells is very high. However, as the biofilms mature (24–48 h), the metabolic activity reaches a plateau.38,39 Here, we showed that, in the initial stages of biofilm formation, biofilm metabolic activity was high following berberine treatment, but with the prolongation of drug action time (24–48 h), berberine exerted a potent and steady inhibitory effect against Candida biofilms. Therefore, berberine exerted the highest inhibitory effect against Candida biofilms at 48 h.
In this study, for the first time, CLSM was used to confirm the inhibitory effects of berberine by visually assessing cell viability and biofilm structures. Among the five Candida strains, berberine had most potent inhibitory effects against C. krusei and C. glabrata biofilms, with viability inhibition rates as high as 96.93 ± 1.37% and 92.36 ± 0.32%, respectively, indicating that berberine might inhibit biofilm formation primarily by killing the Candida cells. Meanwhile, compared to the other strains, the spatial structures of C. albicans and C. dubliniensis biofilms were more significantly damaged, with lower cell densities and loosely packed cells, and with biofilm thickness decrease rates ranging from 15.49 ± 8.45% to 30.30 ± 15.48%. Therefore, these findings indicate that berberine might also inhibit biofilm formation by reducing cell adhesion, promoting Candida cell dispersal, and eventually weakening the spatial structures of the biofilms.
For further exploration of the mechanism of drug action, we reviewed previous relevant researches. Park et al33 revealed that berberine could inhibit both sterol and cell wall biosyntheses by targeting the key enzymes (sterol 24-methyltransferase and chitin synthase) of C. albicans in the ergosterol and chitin biosynthesis pathways. Xu et al40 showed berberine could induce cell damage in C. albicans by increasing the production of endogenous reactive oxygen species. Moreover, da Silva et al34 suggested that berberine could disrupt plasma and mitochondrial membrane integrity and cause DNA damage in C. albicans. In addition, Shao et al25 demonstrated that berberine decreased the extracellular rhodamine 123 in C. tropicalis via inhibiting efflux transporters. Furthermore, Han et al24 examined the in vivo effects of berberine against disseminated candidiasis in mice, and showed that berberine has a synergistic effect combined with amphotericin B, as the amphotericin B dose could be reduced more than 75%. The reported possible mechanisms of berberine were different from each other, and the number of Candida spp. used was limited. Therefore, further in vitro studies investigating the underlying mechanisms and pathways using a larger number of Candida spp. and the in vivo verifications are needed.
Conclusion
This study confirmed the antifungal activity of berberine against five different standard Candida strains and six clinical isolates in planktonic condition. More importantly, the critical role of berberine in inhibiting the Candida biofilms of the five standard strains, especially for the three non-albicans Candida strains, was revealed. Furthermore, CLSM was first used to confirm that berberine could induce high viability inhibition rates for Candida biofilms, inhibit their formation, and significantly weaken their spatial structures. Therefore, this research provides a useful reference for multiple Candida infections and biofilm infections associated with drug resistance. Berberine might have novel therapeutic potential as an antifungal agent or a major active component of antifungal drugs. Nevertheless, further research is required to elucidate the underlying molecular mechanisms of berberine.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (grant number 81670991).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lockhart SR, Guarner J. Emerging and reemerging fungal infections. Semin Diagn Pathol. 2019;36:177–181. doi:10.1053/j.semdp.2019.04.010
2. Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical Practice guideline for the management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):409–417. doi:10.1093/cid/civ1194
3. Patil S, Rao RS, Majumdar B, Anil S. Clinical appearance of oral Candida infection and therapeutic strategies. Front Microbiol. 2015;6:1391. doi:10.3389/fmicb.2015.01391
4. Alnuaimi AD, Wiesenfeld D, O’Brien-Simpson NM, Reynolds EC, McCullough MJ. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case-control study. Oral Oncol. 2015;51(2):139–145. doi:10.1016/j.oraloncology.2014.11.008
5. Calandra T, Roberts JA, Antonelli M, Bassetti M, Vincent JL. Diagnosis and management of invasive candidiasis in the ICU: an updated approach to an old enemy. Crit Care (London, England). 2016;20(1):125. doi:10.1186/s13054-016-1313-6
6. Colombo AL, Guimarães T, Sukienik T, et al. Prognostic factors and historical trends in the epidemiology of candidemia in 379 critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med. 2014;40:1489–1498. doi:10.1007/s00134-014-3400-y
7. Lortholary O, Renaudat C, Sitbon K, et al. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014;40:1303–1312. doi:10.1007/s00134-014-3408-3
8. Bassetti M, Merelli M, Righi E, et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol. 2013;51(12):4167–4172. doi:10.1128/JCM.01998-13
9. Leroy O, Bailly S, Gangneux JP, et al. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care. 2016;6:2. doi:10.1186/s13613-015-0103-7
10. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–50. doi:10.1093/cid/civ1194
11. Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;
12. Klingspor L, Tortorano AM, Peman J, et al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006-2008). Clin Microbiol Infect. 2015;21(1):
13. Montagna MT, Lovero G, Borghi E, et al. Candidemia in intensive care unit: a nationwide prospective observational survey (GISIA-3 study) and review of the European literature from 2000 through 2013. Eur Rev Med Pharmacol Sci. 2014;18(5):661–674. doi:10.1016/j.pnpbp.2013.11.011
14. Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019;25:792–798. doi:10.1016/j.cmi.2019.03.028
15. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383–e392. doi:10.1016/S1473-3099(17)30316-X
16. Cicero AF, Baggioni A. Berberine and its role in chronic disease. Adv Exp Med Biol. 2016;928:27–45. doi:10.1007/978-3-319-41334-1_2
17. Vollekova A, Kost’alova D, Kettmann V, Toth J. Antifungal activity of Mahonia aquifolium extract and its major protoberberine alkaloids. Phytother Res. 2003;17(7):834–837. doi:10.1002/ptr.1256
18. Wang J, Wang L, Lou GH, et al. Coptidis Rhizoma: a comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm Biol. 2019;57(1):193–225. doi:10.1080/13880209.2019.1577466
19. Wang K, Feng X, Chai L, Cao S, Qiu F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab Rev. 2017;49(2):139–157. doi:10.1080/03602532.2017.1306544
20. Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. 2019;33(3):504–523. doi:10.1002/ptr.v33.3
21. Xu JH, Liu XZ, Pan W, Zou DJ. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol Med Rep. 2017;15(5):2765–2787. doi:10.3892/mmr.2017.6321
22. Iwazaki RS, Endo EH, Ueda-Nakamura T, Nakamura CV, Garcia LB, Filho BP. In vitro antifungal activity of the berberine and its synergism with fluconazole. Antonie Van Leeuwenhoek. 2010;97(2):201–205. doi:10.1007/s10482-009-9394-8
23. Wei GX, Xu X, Wu CD. In vitro synergism between berberine and miconazole against planktonic and biofilm Candida cultures. Arch Oral Biol. 2011;56(6):565–572. doi:10.1016/j.archoralbio.2010.11.021
24. Han Y, Lee JH. Berberine synergy with amphotericin B against disseminated candidiasis in mice. Biol Pharm Bull. 2005;28(3):541–544. doi:10.1248/bpb.28.541
25. Shao J, Shi G, Wang T, Wu D, Wang C. Antiproliferation of Berberine in combination with fluconazole from the perspectives of reactive oxygen species, ergosterol and drug efflux in a fluconazole-resistant Candida tropicalis isolate. Front Microbiol. 2016;7:1516. doi:10.3389/fmicb.2016.01516
26. Shi G, Shao J, Wang T, Wu D, Wang C. Mechanism of berberine-mediated fluconazole-susceptibility enhancement in clinical fluconazole-resistant Candida tropicalis isolates. Biomed Pharmacother. 2017;93:709–712. doi:10.1016/j.biopha.2017.06.106
27. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
28. Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494–1500. doi:10.1038/nprot.2008.141
29. Di Veroli GY, Fornari C, Goldlust I, et al. An automated fitting procedure and software for dose-response curves with multiphasic features. Sci Rep. 2015;5:14701. doi:10.1038/srep14701
30. Mukherjee PK, Chandra J. Candida biofilm resistance. Drug Resist Updat. 2004;7(4–5):301–309. doi:10.1016/j.drup.2004.09.002
31. Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes and Infection. 2016;18(5):310–321. doi:10.1016/j.micinf.2016.01.002
32. Nakamoto K, Sadamori S, Hamada T. Effects of crude drugs and berberine hydrochloride on the activities of fungi. J Prosthet Dent. 1990;64(6):691–694. doi:10.1016/0022-3913(90)90298-Q
33. Park KS, Kang KC, Kim JH, Adams DJ, Johng TN, Paik YK. Differential inhibitory effects of protoberberines on sterol and chitin biosyntheses in Candida albicans. J Antimicrob Chemother. 1999;43(5):667–674. doi:10.1093/jac/43.5.667
34. da Silva AR, de Andrade Neto JB, da Silva CR, et al. Berberine antifungal activity in fluconazole-resistant pathogenic yeasts: action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob Agents Chemother. 2016;60(6):3551–3557. doi:10.1128/AAC.01846-15
35. Orozco AS, Higginbotham LM, Hitchcock CA, et al. Mechanism of fluconazole resistance in Candida krusei. Antimicrob Agents Chemother. 1998;42(10):2645–2649. doi:10.1128/AAC.42.10.2645
36. Gong J, Xiao M, Wang H, et al. Genetic differentiation, diversity, and drug susceptibility of Candida krusei. Front Microbiol. 2018;9:2717. doi:10.3389/fmicb.2018.02717
37. Zhou P, Fu J, Hua H, Liu X. In vitro inhibitory activities of magnolol against Candida spp. Drug Des Devel Ther. 2017;11:2653–2661. doi:10.2147/DDDT.S146529
38. Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45(9):2475–2479. doi:10.1128/AAC.45.9.2475-2479.2001
39. Ramage G, Vandewalle K, Wickes BL, López-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001;18(4):163–170.
40. Xu Y, Wang Y, Yan L, et al. Proteomic analysis reveals a synergistic mechanism of fluconazole and berberine against fluconazole-resistant Candida albicans: endogenous ROS augmentation. J Proteome Res. 2009;8(11):5296–5304. doi:10.1021/pr9005074
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.