Back to Journals » Journal of Experimental Pharmacology » Volume 11
In vitro anticoagulant effect of Crassocephalum crepidioides leaf methanol extract and fractions on human blood
Authors Ayodele OO , Onajobi FD , Osoniyi O
Received 5 June 2019
Accepted for publication 30 July 2019
Published 4 September 2019 Volume 2019:11 Pages 99—107
DOI https://doi.org/10.2147/JEP.S218261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Opeyemi Oluwayemisi Ayodele,1 Funmilayo Dorcas Onajobi,1 Omolaja Osoniyi1,2
1Department of Biochemistry, College of Health and Medical Science, Benjamin Carson (Snr.) School of Medicine, Babcock University, Ibadan, Nigeria; 2Department of Biochemistry and Molecular Biology, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence: Opeyemi Oluwayemisi Ayodele
Department of Biochemistry, Benjamin Carson (Snr.) School of Medicine, Babcock University, PMB 4003, Ilishan-Remo, Ogun State, Nigeria
Tel +234 806 620 0610
Email [email protected]
Background: Blood coagulation is a rapid and efficient process that produces clot formation which requires regulation. A derangement of blood coagulation is a feature of many disease conditions. This study investigated the in vitro effects of Crassocephalum crepidioides Benth S. Moore leaf methanol (crude) extract and its partitioned solvent fractions on blood coagulation of Healthy human volunteers.
Methods: The secondary metabolites from dried and ground C. crepidioides leaves were extracted with 70% methanol, and the concentrated crude extract was subsequently subjected to solvent partitioning with Hexane, Ethyl acetate, and Butanol. Varying concentrations (5–20 mg/mL) of the extract and fractions were tested in vitro on blood coagulation profile; clotting time (CT), prothrombin time (PT), and activated partial thromboplastin time (aPTT) of apparently healthy human volunteers, while phytochemical characterization of the Hexane fraction was done by gas chromatography-mass spectrometry (GC-MS).
Results: C. crepidioides leaf methanol extract and fractions significantly (P<0.05) prolonged the clotting time, prothrombin and activated partial thromboplastin times in the blood obtained from the volunteers. The highest prolongation effect was recorded with the Hexane fraction at concentration of 10mg/mL. GC-MS analysis of the Hexane fraction indicated the presence of phytochemicals such as unsaturated fatty acids and esters, phenolic compounds, flavonoids, and coumarin-related compounds known to exhibit antiaggregant, antiplatelet and antimicrobial activities.
Conclusion: These results showed that C. crepidioides possesses bioactive components with anticoagulant properties which may be exploited in the treatment of blood coagulation disorders.
Keywords: blood coagulation, clotting time, prothrombin time, solvent partitioning, Crassocephalum crepidioides
Introduction
Hemostasis is an actively changing process in which coagulation of blood starts and ends in a swift and highly controlled manner. This process involves three fundamental components; the vascular wall, the platelets, and the coagulation cascade (waterfall reactions). These components are essential for normal hemostasis to achieve two major purposes: to maintain blood in a liquid, clot-free state, and to induce a swift and localized blood clot at the site of vesicular injury.1 The reactions of hemostasis include spontaneous vasoconstriction, aggregation of platelets, blood clotting, and fibrinolysis (clot dissolution).
Blood clotting is an intricate cascade of reactions that involves many proteins (factors) which must act in an exact sequence to produce clot formation. The process is rapid and efficient and requires regulation because, if not controlled, excessive clotting can lead to thrombosis. A shift in the balance between blood coagulation and inhibition of coagulation to favor either pro- or anticoagulation may result in life-threatening thromboembolism or hemorrhage.2 Control of this process under many clinical situations requires drug interventions that aim at preventing tissue damage caused by reduced blood flow that occurs when coagulation process blocks the blood supply to a tissue area or an organ.3
Anticoagulants are chemical agents (compounds) that interact with the body’s natural blood coagulation system to treat and prevent abnormal blood clots like Deep vein thrombosis (DVT) and Pulmonary embolism (PE) which are types of Venous thromboembolism (VTE), and Atrial fibrillation.4 Anticoagulant drugs are widely used to control blood coagulation in both healthy and diseased conditions such as cardiovascular disease, diabetes mellitus, and cancer. Although a number of these drugs have been developed over the decades, most are usually accompanied by undesirable side effects such as bleeding which can be mild or severe to the extent of bleeding in and around the brain, while the activity of some (eg warfarin) are affected by food and drug interaction.4,5 Therefore, there is increased research interest in the discovery of natural anticoagulant drugs with less toxicity and fewer side effects.
Medicinal plants have been found to be relevant sources of novel therapeutic agents. Crassocephalum crepidioides Benth S. Moore, commonly called fireweed or Redflower ragleaf, is an annual edible plant that is widespread in tropical and sub-tropical regions.6,7 It is eaten by humans in many countries of Africa. The succulent leaves and stems are used as vegetable in soup and stews especially in the West and Central Africa, and it is locally used in the treatment of wounds, boils, burns, indigestion, and stomach ulcer.8–12 C. crepidioides preparations have been cited in the scientific literature as having anti-inflammatory, antioxidant, antibiotic, anti-helminthic, cytoprotective, hepatoprotective, and antidiabetic activities.12–18
C. crepidioides was demonstrated to have protective activity against oxidative damage of hepatic cells, exert cancer chemopreventive and antitumor actions.19,20 Investigations have also shown the plant to be a useful protein source in both human and animal diet which quality can be enhanced through supplementation, and a good source of nutraceuticals in prevention and treatment of diseases.8,21
In spite of the ethnomedicinal reports of C. crepidioides being used in the treatment of wounds, boils and skin-related conditions in Africa and some other parts of the world, there are limited, or no scientific investigation published on its effects on blood coagulation. Therefore, it is essential to investigate this claim and explore the potentials of C. crepidioides as a medicinal plant in the treatment of blood coagulation defects.
Materials and methods
Drugs and chemicals
All the reagents used were of analytical grade.
Reagent kits for Prothrombin Time (PT) and Activated partial thromboplastin time (aPTT) were purchased from Diagen Diagnostic Reagents Ltd., Thame, Oxon, UK.
Plant materials
Crasssocephalum crepidioides was locally obtained from farms in Ilisan-remo, Ogun State, South-Western Nigeria. The plant sample was identified at the IFE herbarium, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria. A voucher specimen was deposited with the voucher specimen registration No: IFE 17,634.
Preparation of plant sample
C. crepidioides leaves were oven-dried at 40 °C and ground into powder using an electric blender and stored in the refrigerator. The ground sample was soaked with 70% methanol using a ratio 1:8 (w/v) for 48hr at room temperature accompanied by intermittent shaking. The suspension was then filtered through a fine muslin cloth followed by Whatman No 1 filter paper.22 The crude extract was evaporated under reduced pressure using a rotary evaporator, then dried to completion in hot air- oven at 40 °C and stored in the refrigerator at 4 °C until further use. The dried extract was reconstituted with water and subjected to solvent partitioning using Hexane, Ethyl acetate and Butanol sequentially.23
Blood sample collection and preparation of plasma
Blood samples were collected from 15 healthy adult volunteers of both sexes (ages 18–35 years old), with no medication history for at least one week before blood sample collection. The volunteers were duly informed about the research, and their willingness to participate in the research was documented by the signing of written informed consent.
Blood (10ml) was drawn by venipuncture from ante-cubital part of the arm; 5ml of the whole blood was directly used for the clotting time measurement, while the remaining 5ml was transferred into centrifuge tubes containing 3.2% trisodium citrate solution (1 part of trisodium citrate solution: 9 parts of blood). This was immediately centrifuged at 2500g for 10mins to obtain pure platelet plasma (PPP). The plasma was separated and stored in the refrigerator at −4 °C until use.
Blood clotting time measurement
In vitro Clotting time measurement was carried out using a modified method of Lee and White as reported by Osoniyi and Onajobi.24 Clotting tubes containing 0.5ml each of crude extract and fractions of C. crepidioides suspended in Phosphate Buffered Saline (PBS) at varying concentrations of 5–20 mg/mL, PBS (control), and Acid citrate dextrose (anticoagulant) were incubated in a water bath at 37 °C. Freshly drawn blood (0.5ml) was carefully transferred by running it down the side of the tube into the contents of each of the incubated tubes, while simultaneously starting a stopwatch. At 30s interval, the tubes were gently slanted to an angle of 45° to check for blood clot formation. The time for the first observation of clot was recorded, and the slanting at interval continued until the tubes could be inverted without blood flowing. The stopwatch was stopped instantly, and the time was recorded as the final clotting time.
Prothrombin time (PT)
Prothrombin Time was determined according to the method of Brown, as reported by Osoniyi and Onajobi.24,25 Diagen Calcium Thromboplastin Reagent kit was used. Calcium Thromboplastin reagent was reconstituted with distilled water based on manufacturer’s instruction and pre-warmed in a water bath at 37 °C for about 10 mins. Plasma (0.1 mL) was pipetted into clotting tubes and incubated in a water bath for 2–3mins at 37°C. Then, 0.1 mL of PBS (for control), and 5, 10, and 20 mg/mL of plant fractions for the test were added. Calcium thromboplastin reagent (0.1 mL) was swiftly added to the mixture, while simultaneously starting a stopwatch. The tubes were gently tilted (angle of 45°) at regular intervals until a clot was formed. The stopwatch was instantly stopped, and the time was recorded.
Activated partial thromboplastin time (aPTT)
aPTT was determined according to the method used by Brown, as reported by Osoniyi and Onajobi using a Diagen Kaolin reagent kit.24,25 The partial thromboplastin reagent (Kaolin Platelet substitute mixture), which is a mixture of Kaolin and Phospholipid was reconstituted according to the manufacturer’s instruction. The resulting suspension and 0.02 M calcium chloride were separately pre-warmed at 37 °C in a water bath. Plasma (0.1 mL) was pipetted into clotting tubes and incubated in a water bath for 2–3 mins at 37 °C, after which 0.1 mL partial thromboplastin reagent was added to the tubes and the contents rapidly mixed. The mixture was further incubated for 3 mins, after which 0.1 mL of PBS (for control), and extract solution (5, 10, and 20 mg/mL) of plant fractions for the test were added, followed by 0.1ml pre-warmed calcium chloride, while simultaneously starting a stopwatch. The tubes were gently tilted at regular intervals until a clot is formed. The stopwatch was instantly stopped, and the time for clot formation was recorded.
Phytochemical characterization
The phytochemical characterization of the Hexane fraction was done using Gas Chromatography-Mass Spectroscopy (GC-MS).
Statistical analysis
The results were expressed as the Arithmetic mean plus or minus Standard error of the mean (SEM). Data were statistically analyzed by both one- and two-way Analysis of Variance (ANOVA) followed by Tukey’s Multiple comparisons (one-way ANOVA) and Sidak’s posthoc (two-way ANOVA) using GraphPad Prism 7.0. P-values less than 0.05 (P<0.05) were considered statistically significant.
Results
C. crepidioides leaf methanol extract and fractions prolonged the Clotting time in healthy human blood
Recorded clotting times with C. crepidioides leaf methanol extract showed that clotting time was increased with increased concentration, reaching a peak at 12.5 mg/mL after which there was a decline with further increase in concentration (Figure 1). No clot formation was observed in blood treated with Acid Citrate Dextrose solution, even after 24 hrs. This result suggests the presence of anticoagulant(s) in C. crepidioides which functions optimally within a narrow concentrations range.
All partitioned fractions of C. crepidioides caused significant prolongation of clotting time at tested concentrations compared with the Control (whole blood with PBS) and Normal (whole blood alone). Highest clotting times were observed with the Hexane and Aqueous fractions (Figure 2). Clotting time was significantly (P<0.05) longer at concentration of 10 mg/mL compared to 20 mg/mL with the Methanol (crude) extract as well as Hexane and Ethyl acetate fractions (Figure 3).
C. crepidioides leaf methanol extract and fractions prolonged the Prothrombin time (PT) in normal human plasma
The methanol extract and all fractions of C. crepidioides at 10mg/mL significantly (P<0.0001) prolonged the PT compared with the control (Figure 4). There was no significant difference in Prothrombin times recorded with the Hexane fraction at concentration of 10 mg/mL compared with 20 mg/mL, while significantly (P<0.05) shorter PTs were recorded at 20 mg/mL compared with 10 mg/mL with other fractions.
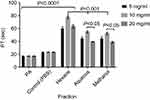
The fractions eliciting highest CT prolongation effect (Hexane and Aqueous fractions) which represent the nonpolar and polar fractions respectively and the methanol (crude) extract were tested on PT at concentrations of 5, 10 and 20 mg/mL. There was significant (P<0.001) prolongation of PT at all tested concentrations (5, 10, & 20 mg/mL) compared with the control. Prothrombin Time with Hexane fraction was significantly (P<0.05) longer than that of Aqueous fraction and methanol extract at all concentrations, while the recorded PT was significantly (P<0.05) longer at 10 mg/mL compared to other concentrations (Figure 5).
C. crepidioides leaf methanol extract and fractions prolonged Activated partial thromboplastin time (aPTT) in normal human plasma
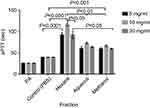
There was significant (P<0.001) prolongation of aPTT with the methanol extract and fractions at all tested concentrations compared with the control. The recorded times for Hexane and Ethyl acetate were not significantly different from one another while the aPTT for both fractions were significantly (P<0.05) longer than that of Methanol extract, Aqueous and Butanol fractions at concentration of 10 mg/mL (Figure 6). Longer aPTT times were recorded at concentration of 10 mg/mL compared with other concentrations for all tested fractions (Figure 7). Highest aPTT prolongation effects were recorded with the Hexane fraction at the tested concentrations with values of 91.90, 116.30, and 91.80 seconds at 5, 10, and 20 mg/mL respectively.
GC-MS phytochemical characterization
GC-MS analysis of the hexane fraction of C. crepidioides leaves revealed several bioactive compounds which include Eugenol (S/N 2) with antiaggregant activity; Thujone (S/N 1) with Antiplatelet activity; 1,9-octadecadiene with no known activity; coumarin-related compounds, Benzofuran and Benzofuranone (coumarin-3-one; S/N 3&4); and α-Linolenic acid (9,12,15-Octadecatrienoic acid; S/N 11) which possesses antiaggregant activity (Table 1). Other compounds and phenolic compounds with some other biological activities were tentatively identified. The reported antifungal, antiseptic, and antimicrobial activities of the phenolic compounds identified may be responsible for its ethnomedical use in the treatment of wounds.
 |
Table 1 Some GC – MS identified phytochemical components of the Hexane fraction of C. crepidioides leaf extract with possible anticoagulant activity |
Discussion
Anticoagulant and procoagulant drugs are widely used to control blood coagulation in both healthy and diseased conditions such as cardiovascular disease, diabetes mellitus, and bleeding disorders. Although a number of these drugs have been developed over the years, most are usually accompanied by undesirable side effects. Therefore, there is still an unmet need for discovery of novel anticoagulant and procoagulant drugs with fewer side effects. The present study investigated the in vitro effects of C. crepidioides leaves methanol (crude) extract and its partitioned solvent fractions on blood coagulation of Healthy human volunteers.
The results showed significant prolongation of the clotting time by the methanol extract in a concentration-dependent manner with an optimum at 12.5 mg/mL and a decline in clotting time with further increase in concentration. This suggests that clotting time prolongation effect of C. crepidioides crude leaf extract operates optimally within a narrow concentration range. Similar concentration-dependent clotting time elongation effects were observed with the partitioned solvent fractions; all the fractions significantly prolonged the clotting time beyond the control value and, in most cases, clotting time at concentrations of 5 and 20 mg per mL were lower than that observed at 10 mg per mL. A comparison of the effect of the different fractions on the clotting time showed that the hexane fraction exhibited the highest anticlotting activity. This result is comparable with the report by Ajugwo et al that Pentaclethra macrophylla seeds increased, in vitro, the clotting times of normal human blood with an optimum concentration of 100 mg/mL.31 The highest anticlotting activity observed with the hexane (non-polar) fraction compared with the polar (aqueous, butanol and methanol) fractions suggests that the major anticoagulant components of C. crepidioides leaf are non-polar. That is, hexane soluble compounds are mainly responsible for the plant anticoagulant activity.
The PT and aPTT were significantly increased at all tested concentrations of the methanol extract and fractions of the plant. Similarly, Ramya et al reported increased PT in vitro anticoagulant activity of Nelumbo nucifera leaf extracts on normal healthy blood plasma.32 Mahajan & More also reported increased PT of Human blood in Anticoagulant activity study with Aqueous and Methanol extracts of Enicostemma littorale, Acheranthus aspera, Abutilon indicum, and Tridax procumbens, while AL-Farawachi & AL-Badranii reported increased PT and aPTT in anticoagulant studies with Eminium spiculatum Aqueous leaf extract in rabbits.33,34
The PT and aPTT are coagulation parameters used in determining the mechanism of clotting. Prothrombin time (PT) is an effective assay for evaluating the activity of the factors of the extrinsic coagulation pathway, while the aPTT is used for evaluating the activity of factors of the intrinsic and common pathways. The PT is a standard test for monitoring coumarin therapy (vitamin K antagonists), while the aPTT is usually used to monitor the effectiveness of heparin treatments.35 In clinical evaluation, a long aPTT and/or PT indicates an anomaly in activities of specific clotting factors; for example, abnormally long aPTT but normal PT indicate a need to assay factors VIII, IX, and XI of the contact pathways. If both the PT and aPTT are affected, this points at factors V, X and prothrombin (factor II) of the common pathway.36 Thus, the prolonged PT and aPTT by C. crepidioides treatment suggest inhibition of factors V, X and prothrombin of the common coagulation pathway. Further research is required to elucidate the mechanism by which this is effected.
However, a possible mechanism of C. crepidioides leaf extract and fractions action may be through direct inhibition of the common pathway by decreasing thrombin (factor IIa) generation via inhibition of factor Xa and its cofactor Va, or by blocking the interaction of thrombin with substrate fibrinogen thus preventing the formation of fibrin. These suggest a possible presence of protease inhibitor in C. crepidioides leaf which may inhibit these proteases, thus preventing the conversion of the zymogens to active factors Xa, Va, and thrombin. C. crepidioides active component(s) may also activate the natural anticoagulant pathway by binding to Antithrombin III, causing a conformational change which could result in activation of protein C (PC) to activated protein C (APC), which in conjunction with its cofactor (protein S) inhibit factors Va and VIIIa (cofactors for activation of factors Xa and IIa). This ultimately prevents the activation of factor Xa and thrombin of the common pathway.
The common anticoagulant drugs used in the treatment of thrombotic conditions include heparin, warfarin, all-trans retinoic acid, and recently developed novel anticoagulants (NOACs). Heparin is a short-acting (fast) anticoagulant which action can be reversed, warfarin is long-acting and slow, with also reversible effects, all -trans retinoic acid is slow-acting, while, NOACs are fast and cannot be reversed.4,37 Heparin exerts its anticoagulant action by binding to a specific site of Antithrombin and causing a conformational change which exposes the site that binds and inactivates factor Xa and thrombin (IIa) thus, increasing the anticoagulant activity of antithrombin to about 1,000 folds.38 Warfarin is a vitamin K antagonist which inhibits the activation or synthesis of vitamin K-dependent proteins of the coagulation pathway. These proteins include factors X, IX, VII, and prothrombin. Vitamin K is a cofactor for the carboxylase enzyme which catalyzes the γ-carboxylation of Glutamic acid (Glu) residue of the polypeptide chain of this vitamin K dependent proteins. γ-carboxylation of the glutamic acid residues of these proteins is required for their calcium-binding ability, and thus their physiological activation.39,40 Studies have reported that all-trans retinoic acid exerts its anticoagulant action by downregulating Tissue factor and upregulating thrombomodulin expression, thus increasing the antithrombotic potential of microvascular endothelial cells.41–43 C. crepidioides leaf extract showed a narrow concentration range of activity peak which indicates fast and reversibility of action similar to heparin. This also corroborates the hypothesized mechanism of action of inhibiting the common coagulation pathway similar to that of heparin.
The GC-MS characterization of the hexane fraction of C. crepidioides leaf indicated the presence of several bioactive compounds with various biological activities including Benzofuranone, Benzofuran (coumarin-related compounds), Eugenol, other phenolic compounds, and flavonoids. Alpha-linolenic acid (9,12,15-Octadecatrienoic acid) has been reported to act as an antiaggregant (which decreases platelet aggregation and inhibits thrombus formation); Eugenol was reported as an anticoagulant agent in Cinnamomum cassia.29 Thujone and flavonoids have also been reported to inhibit platelet aggregation; 1,9-octadecadiene, with no reported activity, may have anticoagulant activity, while the coumarin compounds may also function as anticoagulant agents in the plant.27,44 Adjatin et al similarly reported the presence of coumarins in a qualitative phytochemical screening of C. crepidioides.8 The reported antifungal, antiseptic, and antimicrobial activities of the phenolic compounds identified in the plant may be responsible for its ethnomedical use in the treatment of wounds. Arawande et al reported that the qualitative phytochemical screening of the powdered and water extract of C. crepidioides revealed the presence of Flavonoids, Phenol, oxalate, tannin, saponin, phytate, and ascorbic acid (which also possesses antiaggregant activity).45 Bahar et al similarly reported that qualitative phytochemical profile of the methanol extract of C. crepidioides showed the presence of alkaloids, glycosides, tannins, flavonoids, phenolic compounds, saponins and reducing sugar.6,46 The findings from this study indicate that C. crepidioides leaves contain bioactive compounds which possess anticoagulant activity with great potentials in the development of novel anticoagulant drug.
Conclusion
The study showed that C. crepidioides possesses anticoagulant activity which can be exploited in the treatment of blood coagulation disorders. Further in vivo studies are required to elucidate the mechanisms by which the anticoagulant components of the plant effect their activity.
Ethics approval and informed consent
The study was approved by Babcock University Health Research Ethics Committee (BUHREC), Nigeria with the Approval No. BU/BUHREC436/17. The volunteers were given written informed consent forms which contain details about the research and contacts of investigators. Volunteers’ willingness to participate in the research was documented by the signing of the written informed consent.
Data sharing statement
All data supporting the conclusions of this article are included in the article. Plant materials used in this study was identified at the IFE herbarium where a voucher specimen was deposited.
Abbreviations
Aptt, activated partial thromboplastin time; CT, clotting time; GC-MS, gas chromatography-mass spectrometry; min, minutes; PA, plasma alone; PBS: phosphate buffered saline; PT, prothrombin time; sec: seconds; WBA, whole blood alone; mg, milligram; mL, milliliter.
Acknowledgment
The authors would like to thank Mr. Wale Adetosoye (phlebotomist) of Hematology department, Babcock University Teaching hospital for his assistance in blood sample collection.
Author contributions
All authors contributed to data analysis, drafting and revising of the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Kumar V, Abbas AK, Fausto N. Robbins and Cotran: Pathologic Basis of Disease (7th Ed.). Elsevier: Philadelphia; 2005.
2. Ovanesov MV. Study of the regulation of blood coagulation by factors VIIa and IXa. U.S food & Drug Aministration, Biologic research project, New Hampshire Avenue, Silver Spring, MD. 2015. Available from: https://www.fda.gov/BiologicsBloodVaccines/ScienceResearch/…/ucm434257.htm.
3. Karch AM. Pharmacologial review: Drugs that alter blood coagulation. Am Nurse Today. 2012;7(11):26–31.
4. Wadhera RK, Russell CE, Piazza G. Warfarin versus novel oral anticoagulants how to choose? Circulation. 2014;130:e191–e193. doi:10.1161/CIRCULATIONAHA.114.010426
5. Compton K. Blood thinners: anticoagulants & antiplatelet drugs. Drugwatch, South Orange Avenue, Orlando, FL. 2019. Available from: https://www.drugwatch.com/health/blood-thinners/.
6. Bahar E, Kazi-Marjahan A, Geum-Hwa L, et al. β-Cell protection and antidiabetic activities of Crassocephalum crepidioides (Asteraceae) Benth. S. Moore extract against alloxan-induced oxidative stress via regulation of apoptosis and reactive oxygen species (ROS). BMC Complement Altern Med. 2017;17:179. doi:10.1186/s12906-017-1697-0
7. Rajesh KJ. Terpene composition of Crassocephalum crepidioides from Western Ghats region of India. Int J Nat Prod Res. 2011;1(2):19–22.
8. Adjatin A, Dansi A, Badoussi E, et al. Phytochemical screening and toxicity studies of Crassocephalum rubens (Juss. ex Jacq.) S. Moore and Crassocephalum crepidioides (Benth) S. Moore consumed as vegetable in Benin. J Chem Pharma Res. 2013;5(6):160–167.
9. Sakpere AMA, Adedeji O, Folashade AT. Flowering, post-pollination development and propagation of ebolo (Crassocephalum crepidioides (Benth.) S. Moore) in Ile-Ife. Nigeria J Sci Technol. 2013;33(2):37–49.
10. Burkill HM. The useful plants of West tropical Africa. vol. 2. Britain: White Friars Press Limited. 1985:534.
11. Ajibesin KK. Ethnobotanical survey of plants used for skin diseases and related ailments in Akwa Ibom State, Nigeria. Ethnobot Res Applic. 2102;10:463–522.
12. Rajesh KJ. Study on Essential oil composition of the roots of Crassocephalum crepidioides (Benth.)S. Moore. J Chil Chem Soc. 2014;59:1.
13. Bogning ZC, Olounlade PA, Alowanou GG, et al. In vitro anthelmintic activity of aqueous extract of Crassocephalum crepidioides (Benth.) S. Moore on Haemonchus contortus. J Exp Integr Med. 2016;6(1):31–37. doi:10.5455/jeim.061215.or.144
14. Chaitanya MVNL, Dhanabal SP, Rajendran I, Rajan S. Pharmacodynamic and ethnomedicinal uses of weed species in nilgiris, Tamilnadu State, India: a review. Afr J Agric Res. 2103;8(27):3505–3527.
15. Owokotomo IA, Ekundayo O, Oladosu IA, Aboaba SA. Analysis of the essential oils of leaves and stems of Crassocephalum crepidioides growing in South Western Nigeria. Int J Chem. 2012;4(2):34–37.
16. Tomimori K, Nakama S, Kimura R, Tamaki K, Ishikawa C, Mori N. Antitumor activity and macrophage nitric oxide producing action of medicinal herb, Crassocephalum crepidioides. BMC Complement Altern Med. 2012;12(78). doi:10.1186/1472-6882-12-78
17. Odukoya OA, Inya-Agha SA, Segun FA, Sofidiya MO, Ilori OO. Antioxidant activity of selected Nigerian green leafy vegetables. Am J Food Technol. 2007;2(3):169–175. doi:10.3923/ajft.2007.169.175
18. Wijaya S, Nee TK, Jin KT, Din WM, Wiart C. Antioxidant, anti- inflammatory, cytotoxicity and cytoprotection activities of Crassocephalum crepidioides (Benth.) S. Moore. Extracts and its phytochemical composition. Eur J Sci Res. 2011;67(1):157–165.
19. Aniya Y, Koyama T, Miyagi C, et al. Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol Pharm Bull. 2005;28(1):19–23. doi:10.1248/bpb.28.19
20. Chia CH, Yi-Ping C, Jyh-Horng W, et al. Galactolipid possesses novel cancer chemo preventive effects by suppressing inflammatory mediators and mouse B16 melanoma. Cancer Res. 2007;67:6907–6915. doi:10.1158/0008-5472.CAN-07-0158
21. Dairo FAS, Adanlawo IG. Nutritional quality of Crassocephalum crepidioides and Senecio biafrae. Pak J Nutr. 2007;6(1):35–39. doi:10.3923/pjn.2007.35.39
22. Khan MRI, Islam MA, Hossain MS, et al. Antidiabetic effects of the different fractions of ethanolic extracts of Ocimum sanctum in normal and alloxan induced diabetic rats. J Sci Res. 2010;2(1):158–168. doi:10.3329/jsr.v2i1.2769
23. Otsuka H. Purification by solvent extraction using partition coefficient. In: Sarker SD, Latif Z, Gray AI, editors. Natural Products Isolation. Methods in Biotechnology. Vol. 20. Humana Press Inc., Totowa, NJ. 2006:269–273.
24. Osoniyi RO, Onajobi FD. Coagulant and anticoagulant activities in Jatropha curcas latex. J Ethnopharmacol. 2003;89:101–105.
25. Brown BA. Hematology: Principles and Procedures.
26. Cordier W, Steenkamp V. Herbal remedies affecting coagulation: a review. Pharm Biol. 2011;50(4):443–452..
27. Duke JA. Dr. Duke’s Phytochemical and Ethnobotanical Databases. USA: Agricultural Research Service, AGRIS; 2013.
28. Kim Y, Koo K, Koo Y. Platelet anti-aggregation activities of compounds from Cinnamomum cassia. J Med Food. 2010;13:1069–1074. doi:10.1089/jmf.2009.1365
29. Bendre RS, Rajput JD, Bagul SD, Karandikar PS. Outlooks on medicinal properties of eugenol and its synthetic derivatives. Nat Prod Chem Res. 2016;4:212. doi:10.4172/2329-6836.1000212
30. Asif M. Mini review on important biological properties of benzofuran derivatives. J Anal Pharm Res. 2016;3(2):00050. doi:10.15406/japlr.2016.03.00050
31. Ajugwo AO, Adias TC, Osuala FN, Aghatise K, Onoriode DO, Anosike AU. In vitro studies of anticoagulation activity of pentaclethra macrophylla. J Nutr Health. 2013;1(2):15–17. doi:10.12691/jnh-1-2-2
32. Ramya D, Thirunavukkarasu P, Barathi A, Asha S. In vitro anticoagulant activity of Nelumbo nucifera leaf extracts on normal healthy blood plasma. Int J Green Pharm. 2017;11(3):166–170.
33. Mahajan R, More D. Evaluation of anticoagulant activity of aqueous and ethanolic extract and their isolated phytochemicals of some medicinal plants. Int J Pharm Pharm Sci. 2012;4(4):498–500.
34. Al-Farwachi MI, Al-Badranii BA. Anticoagulant effects of eminium spiculatum aqueous leaf extract in rabbits. Basra J Vet Res. 2013;12(2):332–337. doi:10.33762/bvetr.2013.83828
35. Achneck HE, Sileshi B, Parikh A, Milano CA, Welsby IJ, Lawson JH. Pathophysiology of bleeding and clotting in the cardiac surgery patient: from vascular endothelium to circulatory assist device surface. Circulation. 2010;122:2068–2077. doi:10.1161/CIRCULATIONAHA.110.936773
36. Hood JL, Eby CS. Evaluation of a prolonged prothrombin time. Clin Chem. 2008;54(4):765–768. doi:10.1373/clinchem.2007.100818
37. Bryan J. Short acting and reversible effects made heparin a great anticoagulant. Pharm J. 2013;290:188. doi:10.1211/PJ.2013.11116881
38. Blann AD, Khoo CW. The prevention and treatment of venous thromboembolism with LMWHs and new anticoagulants. Vasc Health Risk Manag. 2009;5:693–704. doi:10.2147/VHRM.S4621
39. Truong JT, Booth SL. Emerging issues in vitamin K research. J Evid Based Complement Altern Med. 2011;16(1):73–79. doi:10.1177/1533210110392953
40. Suttie JW. Vitamin K. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern Nutrition in Health and Disease.
41. Ghaffari H, Varner JD, Petzold LR. Analysis of the role of thrombomodulin in all-trans retinoic acid treatment of coagulation disorders in cancer patients. Theor Biol Med Model. 2019;16(3):1–18. doi:10.1186/s12976-018-0097-6
42. Aoshima K, Asakura H, Yamazaki M, et al. Treatment of disseminated intravascular coagulation (DIC) with all-trans retinoic acid in an endotoxin induced rat model. Semin Thromb Hemost. 1998;24:227–231. doi:10.1055/s-2007-995846
43. Koyama T, Hirosawa S, Kawamata N, Tohda S, Aoki N. All-trans retinoic acid upregulates thrombomodulin and downregulates tissue-factor expression in acute promyelocytic leukemia cells: distinct expression of thrombomodulin and tissue factor in human leukemic cells. Blood. 1994;84(9):3001–3009.
44. Formica JV, Regelson W. Review of biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080.
45. Arawande JO, Komolafe EA, Imokhude B. Nutritional and phytochemical compositions of fireweed (Crassocephalum crepidioides). Inter J Agric Technol. 2013;9(2):439–449.
46. Bahar E, Siddika MS, Bashutosh N, Yoon H. Evaluation of in vitro antioxidant and in vivo antihyperlipidemic activities of methanol extract of aerial part of Crassocephalum crepidioides (Asteraceae) Benth S. Moore Trop J Pharma Res. 2016;15(3):481–488. doi:10.4314/tjpr.v15i3.8
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.