Back to Journals » International Journal of Nanomedicine » Volume 16
In vitro Anticancer Effects of Vernonia amygdalina Leaf Extract and Green-Synthesised Silver Nanoparticles
Authors Joseph J, Khor KZ , Moses EJ, Lim V , Aziz MY, Abdul Samad N
Received 2 February 2021
Accepted for publication 13 April 2021
Published 25 May 2021 Volume 2021:16 Pages 3599—3612
DOI https://doi.org/10.2147/IJN.S303921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Julia Joseph, 1 Kang Zi Khor, 1 Emmanuel Jairaj Moses, 2 Vuanghao Lim, 1 Mohd Yusmaidie Aziz, 1 Nozlena Abdul Samad 1
1Integrative Medicine Cluster, Advanced Medical and Dental Institute (AMDI), Sains@BERTAM, Universiti Sains Malaysia, Kepala Batas, 13200, Pulau Pinang, Malaysia; 2Regenerative Medicine Cluster, Advanced Medical and Dental Institute (AMDI), Sains@BERTAM, Universiti Sains Malaysia, Kepala Batas, 13200, Pulau Pinang, Malaysia
Correspondence: Nozlena Abdul Samad
Integrative Medicine Cluster, Advanced Medical and Dental Institute (AMDI), Sains@BERTAM, Universiti Sains Malaysia, Kepala Batas, 13200, Pulau Pinang, Malaysia
Tel +604-5622051
Fax +604 - 562 2349
Email [email protected]
Purpose: Vernonia amygdalina (VA) is a traditional African herbal medicine that has been reported to possess anticancer properties. However, the anticancer properties of VA silver nanoparticles have not been studied. The aim of the study was to examine and evaluate the anticancer activities of VA leaf extracts and VA silver nanoparticles on the human breast cancer cell line, MCF-7.
Methods: VA leaves were extracted using sequential extraction assisted with ultrasound using three different solvents: ethanol, 50% ethanol, and deionized water. The silver nanoparticles were synthesised with VA aqueous extract.
Results: The ethanol extract and VA silver nanoparticles inhibit MCF-7 cell proliferation with an average half-maximal inhibitory concentration (IC 50) value of 67μg/mL and 6.11μg/mL, respectively, after 72 hours of treatment. The ethanol extract and VA silver nanoparticles also caused G1 phase cell cycle arrest, induced apoptosis and nuclear fragmentation in MCF-7 cells.
Conclusion: VA ethanol extracts and VA silver nanoparticles decreased the cell viability in MCF-7 cells in a time and dose-dependent manner by inducing apoptosis and causing DNA damage. Further research is needed to elucidate the mechanism of action of VA leaf extracts and VA silver nanoparticles. This study is the first to report on the anticancer activity of VA silver nanoparticles in MCF-7 cells.
Keywords: Vernonia amygdalina, MCF-7, apoptosis, cytotoxicity, silver nanoparticles
Corrigendum for this paper has been published
Introduction
Traditional medicines such as herbal plants have been shown to possess antioxidant, anti-inflammatory and anti-carcinogenic activities that can potentially be developed for use in cancer therapeutics.1 One particular plant, Vernonia amygdalina could be a new source of anticancer drugs, as it has been reported to have both a myriad of therapeutic effects and anticancer properties.2 Vernonia amygdalina (VA) or as it’s commonly known, ‘bitter leaf” or “Pokok Bismillah”, is known for its crucial role in traditional African medicine. Extracts of VA have been found to possess antioxidant, anti-malarial, anti-bacterial, anti-inflammatory, anti- helminths and anticancer activities.1 It is native to Nigeria and can also be found in parts of tropical Asia like Malaysia and Singapore.3 VA is also rich in biologically active compounds such as antioxidants and polyphenols which are known to be protective against tumours.4
The green synthesis of nanoparticles is a newly developed method that is widely used in both the scientific and industrial fields.5 Green synthesis nanoparticles have sizes between 1 to 100 nm and their synthesis process is environmentally friendly and non-toxic when compared to chemical or physical methods of nanoparticle synthesis.6 They are commonly used in extension of food shelf life, medical, biomedical and cosmetic industries and are also known to exhibit antibacterial properties.7 Furthermore, nanoparticles can act as an efficient drug delivery system due to their small size. This reduces side effects and toxicity as it provides targeted delivery with an optimal dose.8 Various inorganic metallic substances such as gold, zinc, platinum and nickel, can be used in the green synthesis of nanoparticles.6 In this study, silver will be used due to its low cost and accessibility. Plant extracts can be used as capping material for the stabilisation and reduction of silver ion to form silver nanoparticles.9 Currently, there is no research on the anticancer effects of VA synthesised silver nanoparticles. Results from this study will determine whether VA silver nanoparticles show anticancer activity and if so, whether the silver nanoparticles enhance the efficiency of VA anticancer activity. This study aims to examine and evaluate the anticancer activities of VA leaf extracts and VA silver nanoparticles on the human breast cancer cell line, MCF-7.
Materials and Methods
Cell Line
The human breast cancer cell line, MCF-7, and the immortalized breast fibroblast, MCF10A were purchased from American Type Culture Collection (ATCC Manassas, VA, USA). MCF-7 cells were maintained as monolayers in RPMI 1640 medium (Millipore, Merck) supplemented with 10% fetal bovine serum (Sigma), 100U/mL penicillin, and 100μg/mL streptomycin (Gibco) at 37°C in a humidified incubator with 5% CO2. MCF10A cells were maintained as monolayers in DMEM medium supplemented with 5% horse serum, 0.2% EGF (20mg/mL), hydrocortisone (0.5mg/mL), insulin (10 μg/mL), 100U/mL penicillin, and 100μg/mL streptomycin at 37°C in a humidified incubator with 5% CO2.
Preparation of Vernonia Amygdalina Leaf Extract
A voucher sample of the VA leaves used were authenticated at the School of Biological Sciences Herbarium, Universiti Sains Malaysia (Herbarium No.: 1178). VA leaves were extracted using sequential extraction methods as follows: 25g of dried VA leaves obtained from HERBagus Sdn. Bhd, Kepala Batas, Malaysia were ground into a fine powder (Ultra-Centrifugal Mill ZM 200) and dissolved in 250mL of absolute ethanol. The mixture was sonicated (Mujigae SD-120H Ultrasonic Cleaner) for 30 minutes and centrifuged for 15 minutes at 6000rpm (MIKRO 220 Hettich Micro Centrifuge). The supernatant was collected and filtered using vacuum filtration. The ethanol left in the filtrate was then removed using a rotary evaporator (Buchi Rotavapor). The sticky dark green residue left was the ethanol extract. The residue was then weighed and diluted in DMSO to a concentration of 40mg/mL for use in experiments. The remaining pellet from the previous centrifugation was dried in a fume hood for approximately 48 hours and was reused for the next sequential extractions. The extraction method above was repeated with 50% ethanol (125mL of ethanol + 125mL of deionized water). The supernatant was collected and filtered. The 50% ethanol used was removed by using the rotary vapour and subsequently, lyophilized (Alpha 1–2 LDplus). The residual brownish-green powder left was the 50% ethanol extract. The powder was then weighed and diluted in DMSO to a concentration of 40mg/mL for use in experiments. The remaining pellet was then dried once more and extracted as before with 250mL of deionized water. The supernatant was filtered and lyophilized. The dried light brown powder left was the aqueous extract. The powder was then weighed and diluted in deionised water to a concentration of 40mg/mL for use in further experiments.
Silver Nanoparticle Synthesis
25g of dried VA leaves were ground into a fine powder and dissolved in 250mL of deionized water (1:10 w/v). The mixture was then simultaneously heated and stirred at a temperature of not more than 100°C for approximately 30 minutes. The extract was then vacuum filtered and kept at 4°C for further use. 10mL of the filtrate was added to 100mL of 20mM AgNO3 and heated in a water bath (Memmert) with agitation at 70°C until a colour change is observed. The resulting solution was centrifuged for 30 minutes at 6000rpm. The supernatant was discarded. The pellet was lyophilised and stored at −18°C until further use. Before use, the nanoparticles were weighed and diluted in deionised water to a concentration of 40mg/mL. The VA silver nanoparticles were characterized with Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Energy Dispersive X-Ray (EDX) and Fourier-transform infrared spectroscopy (FTIR).
Cell Viability Analysis
The effects of VA on the cell viability of MCF-7 cells and MCF10A were examined using the thiazolyl blue tetrazolium bromide (MTT) assay. The cells were seeded in 96-well plates at a density of 1×104 cells per well and allowed to recover for 24h before being treated with 0, 12.5, 25, 50, 100, 200 and 400μg/mL of VA extracts and synthesized VA silver nanoparticles with exposure times of 24, 48 and 72h for MCF-7 cells and 72h for MCF10A cells. Solvents used for extract preparation such as DMSO and deionized water were used as negative controls. 5mg/mL of MTT solution was prepared by dissolving MTT powder in sterile PBS. After exposure times, the media was aspirated and replaced with fresh media containing 10% of MTT solution. After a 4h incubation period, the MTT solution was aspirated and the purple formazan crystals that had formed were dissolved in 100μL of DMSO. The optical density was measured at 570nm with a reference wavelength of 620 nm (ELx808 Absorbance Reader, Biotek). The viability of the cells after treatment was calculated using the following formula:
Cell Viability (%) = [(A570−620 of treated cells)/(A570−620 of negative control)] x 100
The IC50 values of each treatment were examined by referencing the dose of treatment needed to reduce cell viability to 50%.
Morphological Analysis
The effects of VA on MCF-7 cell morphology was observed using an inverted microscope (CKX41, Olympus). The cells were treated with the respective IC50 values of VA ethanol and VA silver nanoparticles for 24, 48, and 72h. The cells were observed at each time point for any difference in morphology when compared with the control.
Cell Cycle Analysis Using Flow Cytometry
The effects of VA on cell cycle phases were examined using flow cytometric analysis. The cells were seeded at a density of 1×106 cells per T-25 flask in serum-free media for 24h before treatment, where they were treated with the IC50 values of VA ethanol and VA silver nanoparticles for 12h. The cells were detached via trypsinisation, washed once with PBS and fixed in 5mL of 70% ethanol added dropwise. The fixed cells were stored at 4°C until further use.
Before the flow cytometry analysis, the fixed cells were washed once with 1mL of PBS and resuspended in 1mL of solution which consisted of 1mL of PBS, 0.1μL of Triton X and 0.5μL of RNase A. After a 20 minute incubation at room temperature, 25μL of propidium iodide (1 mg/mL) was added and incubated in the dark for 20 minutes at room temperature. The DNA content in cells was analysed using flow cytometry (BD FACSCalibur).
Annexin V-FITC/PI Apoptosis Assay Using Flow Cytometry
The cells that had undergone apoptosis or necrosis were identified using the Annexin V-FITC Apoptosis Detection kit according to the manufacturer’s protocol (Merck KGaA, Darmstadt, Germany). The cells were seeded at a density of 1×106 cells per T-75 flask for 24h before being treated with VA ethanol and VA silver nanoparticles for 72h. The cells were then collected by trypsinisation, adjusted to 5×105 cells and washed in 500μL of cold PBS. Next, the cells were resuspended in 500μL of cold 1X binding buffer. 1.25μL of annexin V-FITC was added and incubated for 10 minutes at room temperature, followed by the addition of 10μL of propidium iodide. The cells were placed on ice, and kept in the dark before the flow cytometry analysis (BD FACSCalibur).
Nuclear Fragmentation Assay
Nuclear fragmentation of MCF-7 cells upon treatment was detected with acridine orange (AO) staining and subsequently observed under a fluorescence microscope. Briefly, the cells were seeded at a density of 1×106 cells per T-25 flask for 24h before being treated with VA ethanol and VA silver nanoparticles for 24h. The cells were harvested via trypsinisation and stained with 10μL of AO (10μg/mL) at room temperature and observed under a fluorescence microscope (ZEISS, Observer D1).
Statistical Analysis
Results are represented as the mean ± standard deviation of values obtained in triplicate from three different experiments. Differences between groups were analysed by either Student’s t-test or one-way analysis of variance (ANOVA). A P-value of <0.05 was considered statistically significant.
Results
VA Ethanol Extract and VA Silver Nanoparticles Decrease Cell Viability in MCF-7 Cells
The VA ethanol, 50% ethanol, aqueous, absolute aqueous extracts and VA silver nanoparticles were examined for cytotoxic activity. As shown in Figure 1, only the VA ethanol extract shows a significant decrease in cell viability. It decreased MCF-7 cell viability in a time and dose-dependent manner. Although there is a slight increase in cell viability after treatment with 25 μg/mL of ethanol extract, the cell viability steadily decreases with increasing concentrations of the ethanol extract. At higher concentrations, the cell viability appears to be the same at different time points. This indicates that the effect of VA ethanol extract on cell viability is time-independent only at higher concentrations.
VA synthesized silver nanoparticles also decreased MCF-7 cell viability significantly (Figure 2). The silver nanoparticles have a time-independent effect on the cell viability, as cell viability decreased at the same rate despite different exposure times. However, the silver nanoparticles reduced cell viability in a dose-dependent manner. VA silver nanoparticles did not show potent cytotoxic activity in normal human breast cells, MCF-10A cells. It decreased cell viability with IC50 of 128 μg/mL. (Figure 2D). The half-maximal inhibitory concentration (IC50) values were derived from the dose-response curves and compiled as shown in Table 1. The ethanol extract and VA silver nanoparticles synthesised with the absolute aqueous extract showed cytotoxic activity in MCF-7 cells as it decreased cell viability significantly. The treatments were able to achieve IC50 values of less than 100 µg/mL which indicates cytotoxicity. Thus, further analysis of the effects of the ethanol extract and VA silver nanoparticles on the MCF-7 cells were done.
 |
Table 1 Compilation of Half-Maximal Inhibitory Concentration (IC50) Values of MCF-7 Cells When Treated with VA Extracts and Silver Nanoparticles at 24, 48 and 72h |
VA Ethanol Extract and VA Silver Nanoparticles Cause Morphological Changes in MCF-7 Cells
VA ethanol extract and silver nanoparticles caused significant morphological changes in MCF-7 cells when treated with their respective IC50 doses for 24, 48 and 72h (Figure 3). Cell death increases with increasing exposure times as more rounded cells and/or debris is observed. As exposure time increases, more debris, membrane blebbing, and apoptotic bodies are observed in both treated samples when compared to the control.
VA Ethanol Extract and VA Silver Nanoparticles Induce G1 Cell Cycle Arrest in MCF-7 Cells
VA ethanol extract and VA silver nanoparticles induced cell cycle arrest in MCF-7 when treated with their respective IC50 doses. Interestingly, even after 12 hours of treatment, both the ethanol and silver nanoparticle treatments caused a growth arrest in the G1 phase of the cell cycle, with 70% and 60% of the cells in the G1 phase respectively. The treatment also caused a significant decrease in the percentage of cells undergoing the S phase when compared to the control (Figure 4).
VA Ethanol Extract and VA Silver Nanoparticles Induce Apoptosis in MCF-7 Cells
VA ethanol extract and silver nanoparticles induced apoptosis in MCF-7 when treated with their respective IC50 values for 72h (Figure 5). The lower left quadrant shows cells that are negative for propidium iodide (PI) and annexin V-FITC (healthy cells), the upper left quadrant shows cells positive for PI and negative for annexin (necrotic cells), the lower right quadrant shows cells positive for annexin and negative for PI (early apoptotic cells), and the upper right quadrant shows cells positive for both PI and annexin (late apoptotic cells).10
There is a shift towards the late apoptotic stage when the cells are treated with the ethanol extract with 54.56% of the cells in the upper right quadrant. When treated with VA silver nanoparticles, the cells shift towards the early apoptosis stage with 49.80% of the cells in the lower right quadrant.
VA Ethanol Extract and VA Silver Nanoparticles Cause Nuclear Fragmentation in MCF-7 Cells
Acridine orange (AO) staining was done to observe whether VA ethanol and VA silver nanoparticles cause DNA damage in MCF-7 cells (Figure 6). AO is a cell-permeable nuclear binding dye that emits green fluorescence when bound to dsDNA and red fluorescence when bound to ssDNA or RNA and lysosomes.11 Both the ethanol extract and silver nanoparticles treated cells display red fluorescence. This indicates the presence of ssDNA, which is a sign of nuclear fragmentation.
VA Silver Nanoparticle Characterization
The surface morphology and diameter of the synthesized silver nanoparticles were obtained using scanning electron microscopy (SEM) and transmission electron microscopy (TEM), respectively. VA silver nanoparticles appear to be spherical with an average size of 41.555 ± 2.488 nm (Figure 8). Energy Dispersive X-ray (Figure 7) shows the analysis of elements present in VA silver nanoparticles. Carbon, nitrogen, oxygen, magnesium, sulphur, chlorine and silver are present in the nanoparticles, with silver and chlorine being the most abundant elements with trace amounts of magnesium and sulphur. The FTIR analysis was done to characterize the functional groups present (Figure 9). The FTIR-KBr analysis of VA silver nanoparticles and VA absolute aqueous extract shows similar peaks at approximately 3500cm−1 and 1630cm−1 which is characteristic of the hydroxyl group (-OH stretching) of alcohol and phenol and aromatic groups (C=C-C) respectively.12,13 There were additional smaller peaks for VA absolute aqueous extract at 1384cm−1 and 1097cm−1 which is characteristic of (C-H bending) and (C-O stretching) vibration of esters, which are free fatty acids of alcohol.14,15
 |
Figure 7 Energy dispersive X-ray (EDX) analysis of VA silver nanoparticles. |
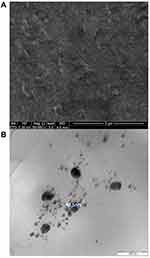 |
Figure 8 Characterisation of surface morphology. (A) SEM and (B) TEM image of VA silver nanoparticles. |
Discussion
This study aimed to evaluate the anticancer activities of VA leaf extracts and the novel VA silver nanoparticles on MCF-7 cells. The cell viability of the cells after treatment was examined using an MTT colorimetric assay. Since only viable cells will be able to reduce MTT to formazan due to their functioning mitochondria, the formation of formazan was measured in treated and untreated cells, and the cell viability was calculated as described in the methodology.4 VA ethanol extract was found to have retarded cell proliferation in a time and dose-dependent manner. The ethanol extract was the only crude extract in this study to show significant anticancer activities. This suggests that most of the anticancer compounds present in VA were already eluded out with ethanol which was the first solvent in the sequential extraction of the VA. The VA ethanol extract had achieved a half-maximal inhibitory concentration (IC50) value of 75, 60, and 67 ug/mL for 24h, 48h, and 72h respectively. A lower IC50 value indicates a higher potency of a compound as a lower concentration is needed to reduce cell viability by 50%.2 However, the decrease in cell viability can also be attributed to the PenStrep supplemented in the medium. Ryu et al and Llobet et al have shown that antibiotics commonly used in cell culture causes changes in cell differentiation and gene expression which might have an effect on cell viability as well.16,17 The slight increase in cell viability at low concentrations of the ethanol treatment might be due to the presence of mitogens in the extract which are known to increase cancer cell proliferation.18
However, the absolute aqueous extract used in this study did not achieve an IC50 value or show any significant anticancer activity. This is inconsistent with other findings. For example, Izevbigie and colleagues found that the absolute aqueous extract of VA leaves achieved an IC50 value of 5.68 ± 0.2μg/mL, which is far more potent than even the ethanol extract in this study.19 This difference could be due to the different source of VA plants used. The amount, quality and activities of compounds present in VA could vary between batches. Other contributing factors are location, seasonal conditions, age at harvest and storage conditions.20 Thus, the anticancer activity and potency may vary between source as well.
The VA silver nanoparticles were synthesised by using the VA absolute aqueous extract as a reducing agent. The phytochemicals present in VA were able to enhance the reduction of silver ions present in the silver nitrate into silver particles thus causing the formation of silver nanoparticles.6 The synthesised nanoparticles are then characterised to examine their size and morphology. The nanoparticles are shown to be less than 100nm in size which is characteristic of a nanoparticle as nanoparticles are defined as particles ranging from 1 to 100 nm in size.21
VA silver nanoparticles significant decreased cell viability of MCF-7 achieving an IC50 value of 6μg/mL independent of time which is very potent.22 This shows that the silver nanoparticles were able to enhance the potency of the VA absolute aqueous extract which did not show any cytotoxicity in this study. The enhancement of potency could be due to the ability of silver nanoparticles to cross various biological barriers and provide targeted drug delivery.8 However, to further support this, further research is needed to confirm that the cytotoxicity observed is due to the VA extract present in the nanoparticles and not due to silver. Not only that, VA silver nanoparticles displayed selectivity when treated in MCF10A cells. It achieved an IC50 of 128 μg/mL, which is not considered to toxic as it is higher than 100μg/mL.22 The selective index (SI) was calculated using the formula as described by Moran et al:
SI = IC50normal cells/IC50cancer cells
VA silver nanoparticles are highly selective with a SI of 21.3.23 The SI shows the differential activity of the nanoparticles. The higher the SI the more selective the extract. VA silver nanoparticles are more potent towards cancer cells when compared to normal cells and are not considered to possess general toxicity.24
The VA ethanol extract and VA silver nanoparticles displayed significant changes in cell viability on MCF-7 and more detailed studies were conducted to uncover how they achieve their cytotoxic effects on MCF-7 cells. The effects of VA ethanol extract and VA silver nanoparticles on the cell cycle were examined using propidium iodide and flow cytometry. Propidium iodide (PI) can bind to DNA. The cell cycle phase can be interpreted from the amount of PI in the cell which represents the amount of DNA present in the cell.25 The proportion of PI in each cell is analysed using flow cytometry.
Both the VA ethanol extract and silver nanoparticles induced G1 phase cell cycle arrest. There was a higher proportion of cells accumulating in the G1 phase when treated with VA ethanol extract and VA silver nanoparticles. Due to this accumulation, it is possible to hypothesise that the cells were unable to undergo DNA replication, divide and proceed to further phases of the cell cycle. This G1 arrest could be due to DNA damage caused by the treatment and is also one of the characteristics of pre-apoptotic cells.26 Control of cell cycle progression in cancer cells is known to be effective in the control of tumour growth.27 Thus, one of the mechanisms used by VA ethanol extract and AB20 silver nanoparticles to retard MCF-7 proliferation is by inhibiting cell cycle progression.
The cell death mode of MCF-7 cells, when treated with VA ethanolic extract and silver nanoparticles was investigated using the Annexin V-FITC/PI apoptosis assay and flow cytometry, which can distinguish alive, apoptotic and necrotic cells. Annexin V will bind to phosphatidylserine that is present in the plasma membrane of apoptotic cells, while PI will stain the DNA of cells with a compromised membrane.10 PI-positive cells will be considered to be necrotic, whereas Annexin V-positive cells will be considered to be apoptotic. Meanwhile, both Annexin V-positive and PI-positive cells are considered to be late apoptotic, as the cell membrane for late apoptotic cells will be compromised, thus allowing PI to get through and bind onto the cellular DNA. Finally, cells that are negative for both PI and Annexin V are considered healthy living cells.
Cells that were treated with VA ethanol extract and silver nanoparticles shifted towards late apoptotic and early apoptotic, respectively, when treated for 72h. This highlights a possible difference in the mechanisms of action in both treatments. Despite having similar exposure times and cell death mode through apoptosis, VA ethanol extract was able to induce apoptosis faster, causing late apoptotic cells. Neither of the treatments showed any significant necrotic activity.
From the acridine orange staining, it is possible to interpret that both the VA ethanol extract and nanoparticles caused DNA damage in MCF-7 cells which further induced apoptosis. The increased presence of ssDNA is known to be a crucial DNA damage signal and is observed in the treated samples.28
Conclusion
VA ethanol extracts and VA silver nanoparticles have decreased the cell viability in MCF-7 in a dose-dependent manner. This is the first study to report on the anticancer activity of VA synthesized silver nanoparticles. Both the treatments were seen to have induced apoptosis, G1 cell cycle arrest and DNA damage. However, further research is needed to highlight the difference in mechanisms of action for both of these treatments. There is also a need to investigate the mechanism of action of the VA silver nanoparticles in drug delivery, as it has enhanced potency when compared to VA crude extracts. This study has shown the anticancer activity of VA ethanol extract and VA silver nanoparticles on MCF-7 cells and highlights its potential application in cancer therapeutics in the future.
Acknowledgments
This work was funded by Fundamental Research Grant Scheme, Ministry of Higher Education (MOHE), Malaysia (FRGS 203.CIPPT.6711842)
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest associated with this work.
References
1. Farombi EO, Owoeye O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int J Environ Res Public Health. 2011;8(6):2533–2555. doi:10.3390/ijerph8062533
2. Wong FC, Woo CC, Hsu A, Tan BKH. The anti-cancer activities of vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One. 2013;8(10). doi:10.1371/journal.pone.0078021
3. Moga K. Antioxidant and antiproliferative activities of plant-derived extracts against cervical and prostate cancer cell lines [Master's thesis]. Kenya: Jomo Kenyatta University of Agriculture and Technology; 2018.
4. Rohin MAK, Ridzwan N, Jumli MN, et al. Screening Of bismillah leaf (Vernonia Amygdalina) extraction for antiproliferative activies in human glioblastoma brain cancer cell lines. Res J Pharm Biol Chem Sci. 2016;7(2):1084–1089.
5. Widyaningtyas AL, Yulizar Y, Apriandanu DOB. Ag2O nanoparticles fabrication by Vernonia amygdalina Del. leaf extract: synthesis, characterization, and its photocatalytic activities. IOP Conf Ser Mater Sci Eng. 2019;509(1). doi:10.1088/1757-899X/509/1/012022
6. Aisida SO, Ugwu K, Akpa PA, et al. Biosynthesis of silver nanoparticles using bitter leave (Veronica amygdalina) for antibacterial activities. Surfaces Interfaces. 2019;17(July):100359. doi:10.1016/j.surfin.2019.100359
7. Yesilot S, Aydin C. Silver nanoparticles; a new hope in cancer therapy? East J Med. 2019;24(1):111–116. doi:10.5505/ejm.2019.66487
8. Ivanova N, Gugleva V, Dobreva M, Pehlivanov I, Stefanov S, Andonova V. Silver nanoparticles as multi-functional drug delivery systems. Nanomed IntechOpen. 2019. doi:10.5772/intechopen.80238
9. Nzekekwu A, Abosede O. Green synthesis and characterization of silver nanoparticles using leaves extracts of neem (Azadirachta indica) and bitter leaf (Vernonia amygdalina). J Appl Sci Environ Manag. 2019;23(4):695. doi:10.4314/jasem.v23i4.19
10. Yedjou CG, Izevbigie EB, Tchounwou PB. Vernonia amygdalina—induced growth arrest and apoptosis of breast cancer (MCF-7) Cells. Pharmacol Pharm. 2013;04(01):93–99. doi:10.4236/pp.2013.41013
11. Darzynkiewicz Z. Chapter 27 Differential Staining of DNA and RNA in intact cells and isolated cell nuclei with acridine orange. Methods Cell Biol. 1990;33(C):285–298. doi:10.1016/S0091-679X(08)60532-4
12. Jemal K, Sandeep BV, Pola S. Synthesis, characterization, and evaluation of the antibacterial activity of allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. Journal of Nanomaterials. 2017. doi:10.1155/2017/4213275
13. Wardani M, Yulizar Y, Abdullah I, Bagus apriandanu DO. Synthesis of NiO nanoparticles via green route using Ageratum conyzoides L. leaf extract and their catalytic activity. IOP Conf Ser Mater Sci Eng. 2019;509(1):012077. doi:10.1088/1757-899X/509/1/012077
14. Ch’ng YS, Loh YC, Tan CS, et al. Vasorelaxant properties of Vernonia amygdalina ethanol extract and its possible mechanism. Pharm Biol. 2017;55(1):2083–2094. doi:10.1080/13880209.2017.1357735
15. Alara OR, Abdurahman NH, Ukaegbu CI, Kabbashi NA. Extraction and characterization of bioactive compounds in Vernonia amygdalina leaf ethanolic extract comparing Soxhlet and microwave-assisted extraction techniques. J Taibah Univ Sci. 2019;13(1):414–422. doi:10.1080/16583655.2019.1582460
16. Ryu AH, Eckalbar WL, Kreimer A, Yosef N, Ahituv N. Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-07757-w
17. Llobet L, Montoya J, López-Gallardo E, Ruiz-Pesini E. Side effects of culture media antibiotics on cell differentiation. Tissue Eng – Part C Methods. 2015;21(11):1143–1147. doi:10.1089/ten.tec.2015.0062
18. van der Burg B, Rutteman GR, Blankenstein MA, de Laat SW, van Zoelen EJJ. Mitogenic stimulation of human breast cancer cells in a growth factor‐defined medium: synergistic action of insulin and estrogen. J Cell Physiol. 1988;134(1):101–108. doi:10.1002/jcp.1041340112
19. Izevbigie EB. Discovery of water-soluble anticancer agents (Edotides) from a vegetable found in Benin City Nigeria. Exp Biol Med (Maywood). 2003;238(3):315–323. doi:10.1186/1757-1146-4-24
20. Oyugi DA, Luo X, Lee KS, Hill B, Izevbigie EB. Activity markers of the anti-breast carcinoma cell growth fractions of vernonia amygdalina extracts. Exp Biol Med. 2009;234(4):410–417. doi:10.3181/0811-RM-325
21. Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi:10.1016/j.arabjc.2017.05.011
22. Schwartsmann G, Winograd B, Pinedo HM. The development of new anticancer agents is long-term process. Radiother Oncol. 1988;12:301–313. doi:10.1016/0167-8140(88)90020-5
23. Peña-Morán OA, Villarreal ML, Álvarez-berber L, Meneses-Ascosta A, Rodríguez-López V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules. 2016;21(8):1–15. doi:10.3390/molecules21081013
24. Wardihan RM, Alam G, Lukman MMA. Selective cytotoxity evaluation in anticancer drug screening of Boehmeria virgata (Forst) guill leaves to several human cell lines: heLa, WiDr, T47D and Vero. Dhaka Univ J Pharm Sci. 2013;12(2):123–126. doi:10.3329/dujpa.v12i2.17615
25. Lifiani R, Harahap U, Anjelisa P, Hasibuan Z, Satria D. Anticancer effect of african leaves (vernonia amygdalina del). To T47D Cell Resistant. Asian Journal of Pharmaceutical and Clinical Research.2018;11(1):2–5.
26. Ruscetti FW, Bartelmez SH. Cell cycle control and check points in hematopoietic stem cells. In: Handbook of Stem Cells. Vol. 2. Elsevier Inc; 2004:115–126. doi:10.1016/B978-012436643-5/50100-0
27. Murad H, Hawat M, Ekhtiar A, et al. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016;16(1):1–11. doi:10.1186/s12935-016-0315-4
28. Bantele SCS, Lisby M, Pfander B. Quantitative sensing and signalling of single-stranded DNA during the DNA damage response. Nat Commun. 2019;10(1):1–12. doi:10.1038/s41467-019-08889-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







