Back to Journals » Journal of Pain Research » Volume 11
In vitro and in vivo quantification of chloroprocaine release from an implantable device in a piglet postoperative pain model
Authors De Gregori S , De Gregori M, Bloise N , Bugada D , Molinaro M , Filisetti C , Allegri M , Schatman ME , Cobianchi L
Received 13 July 2018
Accepted for publication 2 October 2018
Published 8 November 2018 Volume 2018:11 Pages 2837—2846
DOI https://doi.org/10.2147/JPR.S180163
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Simona De Gregori,1 Manuela De Gregori,1–4 Nora Bloise,5,6 Dario Bugada,3,4,7 Mariadelfina Molinaro,1 Claudia Filisetti,8 Massimo Allegri,3,9 Michael E Schatman,3,10,11 Lorenzo Cobianchi12,13
1Clinical and Experimental Pharmacokinetics Unit, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 2Pain Therapy Service, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 3Study in Multidisciplinary Pain Research Group, Parma, Italy; 4Young Against Pain Group, Parma, Italy; 5Department of Molecular Medicine, Centre for Health Technologies, INSTM UdR of Pavia, University of Pavia, Pavia, Italy; 6Department of Occupational Medicine, Toxicology and Environmental Risks, Istituti Clinici Scientifici Maugeri, IRCCS, Lab of Nanotechnology, Pavia, Italy; 7Emergency and Intensive Care Department – ASST Papa Giovanni XXIII, Bergamo, Italy; 8“V. Buzzi” Children Hospital, Pediatric Surgery, Milan, Italy; 9Anesthesia and Intensive Care Service, IRCCS MultiMedica Hospital, Sesto San Giovanni, Milano, Italy; 10Research and Network Development, Boston Pain Care, Waltham, MA, USA; 11Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, MA, USA; 12General Surgery Department, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 13Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy
Background: The pharmacokinetic properties and clinical advantages of the local anesthetic chloroprocaine are well known. Here, we studied the pharmacokinetic profile of a new hydrogel device loaded with chloroprocaine to investigate the potential advantages of this new strategy for postoperative pain (POP) relief.
Materials and methods: We performed both in vitro and in vivo analyses by considering plasma samples of four piglets receiving slow-release chloroprocaine. To quantify chloroprocaine and its inactive metabolite 4-amino-2-chlorobenzoic acid (ACBA), a HPLC–tandem mass spectrometry (HPLC-MS/MS) analytical method was used. Serial blood samples were collected over 108 hours, according to the exposure time to the device.
Results: Chloroprocaine was consistently found to be below the lower limit of quantification, even though a well-defined peak was observed in every chromatogram at an unexpected retention time. Concerning ACBA, we found detectable plasma concentrations between T0 and T12h, with a maximum plasma concentration (Cmax) observed 3 hours after the device application. In the in vitro analyses, the nanogel remained in contact with plasma at 37°C for 90 minutes, 3 hours, 1 day, and 7 days. Chloroprocaine Cmax was identified 1 day following exposure and Cmin after 7 days, respectively. Additionally, ACBA reached the Cmax following 7 days of exposure.
Conclusion: A thorough review of the literature indicates that this is the first study analyzing both in vivo and in vitro pharmacokinetic profiles of a chloroprocaine hydrogel device and is considered as a pilot study on the feasibility of including this approach to the management of POP.
Keywords: postoperative outcome, hydrogel device, chloroprocaine, ACBA, pharmacokinetics
Introduction
The mechanism of local anesthetics (LAs) in blocking the transmission of painful stimuli to the brain is well known.1 Chloroprocaine, an ester-type LA, is less cardiotoxic than most others, but it is characterized by an extremely short half-life.2 New delivery systems based on hydrogel devices may effectively and safely extend the LA’s analgesic action.3,4
Thanks to their unique properties, including biocompatibility, flexible methods of synthesis, network structure, and stability of the incorporated bioactive molecules, hydrogel devices have sparked particular interest in use in drug delivery applications.5 Currently, a wide number of natural polymers (ie, chitosan, pectin, and alginate) are employed for hydrogel preparation due to their capability of mimicking biological structures and the fact that they are well tolerated inside the human body.4,6
Postoperative pain (POP) remains a challenge.7 POP is a common feature in all surgical procedures, and it can have a profound impact on patients’ outcomes. LAs represent a cornerstone for effective postoperative analgesia8 because they block nociceptive stimulus from the periphery and provide pain relief both at rest and during movement. However, LAs have limited duration of action, and even with modern long-acting formulations, analgesic efficacy is limited to the first day following surgery. Unfortunately, POP continues much longer than the first day, and as soon as the effect of the analgesic block decreases, nociceptive and hyperalgesic mechanisms can start again.9 Since prolonged uncontrolled pain in the perioperative period can lead to immediate and long-term complications (including pain chronicization), there is strong interest in prolonging nociceptive blockage. Catheters are used to provide continuous infusion of LAs but, despite their efficacy, they require additional efforts for management by care providers and patients, and complications can occur.10 New strategies for prolonged and controlled release of LAs are required, together with the use of dedicated LAs with optimal pharmacokinetic profiles for continuous infusion (ie, low toxicity, rapid metabolism, and rapid onset/offset of analgesic effect).
Chloroprocaine has an optimal pharmacokinetic profile, and it is promising from the perspective of continuous infusion in the postoperative period.11–13 Furthermore, we have recently demonstrated that a continuous intrawound infusion of chloroprocaine can also reduce perilesional inflammation in an animal model.14
Finally, nanotechnology meanwhile offers new potential advantages in terms of controlled and sustained release of drugs over several days.
We present data on a chloroprocaine-loaded hydrogel device that might offer revolutionary advantages for postoperative analgesia through its specific pharmacokinetic profile, supported by a continuous hydrogel device administration. In this study, we analyzed both in vitro and in vivo (piglets receiving slow-release chloroprocaine) pharmacokinetic profiles of the chloroprocaine-loaded device.
Materials and methods
Materials
Alginate-based hydrogels were obtained by calcium cross-linking starting from an internal gelation mechanism.15,16 Hydrogels were produced from an alginate (alginic acid sodium salt powder, 180947, lot MKBJ0727V; Sigma-Aldrich Co., St Louis, MO, USA) solution with an initial concentration of 6.67% (w/v). For HPLC analysis, high-purity water (CHROMASOLV®, 34877; Sigma-Aldrich Co.) was used for the preparation of solutions and suspensions. The drug (chloroprocaine powder, Lot: #140376; Sintetica Company, Mendrisio, Switzerland), pre-suspended in water and mixed with a diluted solution of alginate (0.5% w/v), was added to the initial solution. The final concentrations were 36 mg/mL alginate loaded with 75 mg/mL of chloroprocaine. To prevent bacterial contamination, ethanol-washed alginate was produced by suspending 10 g alginic acid sodium salt in 50 mL of absolute ethanol. The procedure was repeated three times. In each step, alginate was suspended in ethanol for 5 minutes, left to sediment for 10 minutes, subsequent to which the supernatant was removed. Following the final wash, the remaining ethanol was allowed to evaporate for 48 hours. All the processes (including preparation of alginate solution) took place under laminar flow. Solutions and water were filtered with 0.22 µm filters. The hydrogels were produced within an ad hoc mol (14 cm×2 cm×0.4 cm) for implantation and testing, unless diversely specified.
In vitro chloroprocaine release profile
UV analysis
A chloroprocaine calibration curve was obtained for a physiological-like solution (NaMgCa medium, 145 mM NaCl, 1.5 mM MgCl2, and 2.5 mM CaCl2) measuring solution absorbance with UV-spectroscopy technique (UV/visible Spectrophotometer; Jenway, Staffordshire, UK) in a range of 200–500 nm. NaMgCa medium was developed with the aim of mimicking human plasma concentration considering not only monovalent ions (Na+) but also divalent ions (Ca2+ and Mg2+). Medium composition presents a Na+ concentration that is isotonic to human plasma and the identical concentration of Mg2+ and Ca2+ in human plasma. From a stock drug solution in NaMgCa medium with a concentration of 1.5 mg/mL, different solutions in the concentration range of 2.5–120 µg/mL were prepared by subsequent dilution. All measurements were done in 10 mm quartz cells (108-QS; Hellma Analytics, Müllheim, Germany). A calibration curve was obtained considering the absorbance peak at 291 nm.
Drug release was performed from hydrogel samples with 15.4 mm diameter and 3 mm thickness in 1 mL of medium. Samples were put in contact with NaMgCa medium for 15 minutes, 30 minutes, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, and 24 hours, collecting the medium after each time point and replacing it with fresh medium.
All measurements were calculated in duplicate and in at least two distinct experiments.
HPLC/tandem mass spectrometry (HPLC-MS/MS) analysis
HPLC-MS/MS (TSQ Quantum Access; Thermo Fisher Scientific, Waltham, MA, USA) analytical method was used to quantify chloroprocaine and its inactive metabolite 4-amino-2-chlorobenzoic acid (ACBA) in plasma and acidified methanol, following in vivo and in vitro tests, respectively. In brief, four nanostructured devices containing chloroprocaine and four unfilled nanogel devices (used as a reference) remained in contact with plasma at 37°C for 90 minutes, 3 hours, 1 day, and 7 days (four samples) for HPLC-MS/MS analysis.
Mobile phases A (ultrapure water, Millipore Direct-QTM system) and B (CH3OH, 34860-2.5 L-R; Lot: # STBF5815V; Sigma-Aldrich Co.) were both acidified with formic acid (33015-1L; Lot: # SZBF1540V).
Chloroprocaine chlorohydrate powder (Lot: #140376; Sintetica Company) and its inactive metabolite (ACBA) were obtained from Sigma Aldrich Co. (217719-5G; Lot: # S54349V) and used to prepare the standard solutions. Lidocaine (20 mg/mL; Bioindustria L.I.M S.p.a, Fresonara, Italy) was used (following dilution) as an internal standard (IS). Stock solutions of chloroprocaine and ACBA were prepared in ultrapure water (chloroprocaine: 1 mg/mL; ACBA: 1 mg/mL).
The separation of the two analytes was performed using a Thermo Scientific Accela system, a quaternary pump coupled with an autosampler, and a Kinetex® 2.6µ C18 100 Å Column (100×4.6 mm2; Phenomenex, Bologna, Italy) with the related guard columns. The columns were also heated and maintained at 40°C; elution was carried out in the gradient mode, at a flow rate of 0.8 mL/min, with both the mobile phases A and B freshly prepared. The injection volume was 2.0 µL.
A TSQ Quantum Access triple quadrupole mass spectrometer (Thermo Fisher Scientific) with an electrospray ionization probe was used. We set up the analytical system in a multireaction monitoring mode, following the transitions m/z 271 → 154, 198; m/z 172 → 90, 154; and m/z 235 → 86.2 for chloroprocaine, ACBA, and lidocaine, respectively.
Xcalibur 2.07 and LCquan 2.5.6 software from Thermo Fisher Scientific for the HPLC–MS/MS system control, data acquisition, and data analysis were used.
The calibration curves were generated from two different weighted (1/x) linear regression curves (for chloroprocaine and ACBA quantitation). Analyte peaks were identified through a combination of retention times and the specific multiple reaction monitoring transitions. The corresponding amounts were quantified by normalizing the peak area to the IS (area analyte/area IS), and concentrations were calculated from the respective calibration curves.
The calibration curves were employed to quantitatively evaluate the drug release in plasma and to assess its relative stability in plasma. The hydrogel samples were incubated in direct contact with plasma, at 37°C, for up to 7 days.
All measurements were calculated in duplicate and in at least two distinct experiments.
Sample preparation for the detection of chloroprocaine and ACBA analysis for “in vitro tests”
The preliminary analyses showed a high concentration of chloroprocaine and its metabolite (mg/mL) following exposure of the plasma to the device loaded with the drug, as a consequence of its release.
Before proceeding with the analysis, it was necessary to dilute the samples to a ratio of 1:10,000 with acidified CH3OH (0.1% HCOOH) following processing them, according to the standardized procedures reported subsequently.
Calibrators (A–F) were prepared for quantification and quality controls (QcH, QcM, and QcL) directly in CH3OH/H+, at the concentrations specified subsequently, to compare signals from comparable matrices. The linear correlation coefficients (R2) of the calibration curves obtained from those calibrators were consistently higher than 0.99 for both chloroprocaine and ACBA.
In vivo tests
The hydrogels were tested in vivo using piglets as the animal model (pigs weighing 20 kg).
All animal procedures were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee (IACUC) – University of Pavia. The animal care and experimental procedures respected local, national, and European Union guidelines for protection of animals used for scientific purposes (Directive 2010/63/EU – revising Directive 86/609/EEC).
Two different types of tests were performed as follows:
- In each pig, two incisions were made in the abdominal region, one for the control without the hydrogel and one with the loaded hydrogel.
- Two incisions were made in the same region, and a hydrogel without the drug (control) was placed in one incision and in the other the loaded hydrogel was placed. The incision was performed by simulating a laparotomy (15 cm), cutting the skin, the abdominal fat, the abdominal muscles, and the peritoneum. Prior to the hydrogel insertion, the peritoneum was sutured. During the surgery, the pigs were anesthetized and all the materials used were sterile. The animals were routinely monitored for general appearance, activity, and healing of the implant sites and were sacrificed after 21 days. No pigs were lost during the study.
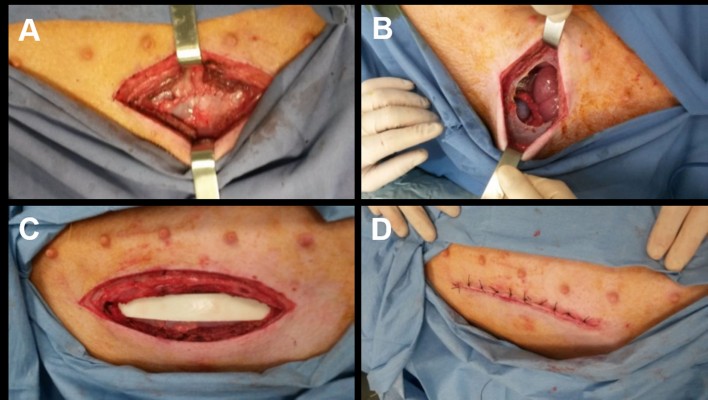
The surgery was performed simulating a laparotomy (15 cm), cutting the skin, the abdominal fat, the abdominal muscles, and the peritoneum (Figure 1A). The peritoneum was then sutured (Figure 1B), the device was inserted (Figure 1C), and finally all the other tissues were sutured (Figure 1D).
  | Figure 1 Different steps of surgery. Notes: (A) Incision of skin, abdominal fat, abdominal muscles, and peritoneum. (B) Suture of peritoneum. (C) Device insertion. (D) Suture of all other tissues. |
Working solution preparation for the detection of chloroprocaine and ACBA analysis for “in vivo tests”
Plasma samples of pigs with implanted nanostructured hydrogel devices were analyzed to evaluate chloroprocaine release.
Calibrators and the calibration curves in pigs’ plasma collected immediately prior to treatment (reference plasma) were prepared as described in the following sections.
Chloroprocaine chlorohydrate powder was weighed and dissolved in water to obtain two distinct stock solutions (M1 and M1QC: 1 mg/mL) to be used for the preparation of the six calibrators and three quality control working standard solutions – labeled A, B, C, D, E, F, and QcH, QcM, and QcL, respectively – by a serial dilution procedure.
Analogously, ACBA stock solution (1 mg/mL in CH3OH) and the related working standard solutions for the calibrators and quality control preparations were prepared.
The stock solutions M1 and M1QC (chloroprocaine and ACBA) were stored at –20°C until use, whereas the working standard solutions were prepared on a daily basis.
Preparation of calibrators, quality controls, and plasma samples
Starting with 90 µL of reference plasma and 10 µL of each working solution, six calibrators (A–F) and three quality controls (QcH, QcM, and QcL) were prepared, each containing both chloroprocaine and the inactive metabolite ACBA at the desired concentrations: F 15.65 ng/mL, E 31.25 ng/mL, D 62.5 ng/mL, C 125 ng/mL, B 250 ng/mL, A 500 ng/mL; QcH 400 ng/mL, QcM 200 ng/mL, and QcL 20 ng/mL.
The IS (lidocaine) and methanol were added to each sample (100 µL of pig’s plasma) to promote protein precipitation. After mixing via vortex and centrifuge separation, the supernatant was recovered and dried under a weak stream of nitrogen. Next, the precipitate was suspended in acidified methanol and directly injected into the column.
Both the corresponding calibration curves of chloroprocaine and ACBA demonstrated an excellent linearity in the considered concentration range (15.65–500 ng/mL), characterized by a correlation coefficient (R2) consistently higher than 0.99.
Acceptance criteria of an analytical run
As reported in the “Guideline on bioanalytical method validation” (EMA/CHMP/EWP/192217/2009 Rev.1 Corr.*), the back calculated concentrations of the calibration standards should be within ±15% of the nominal value, with the exception of the lower limit of quantification (LLOQ), for which it should be within ±20%. At least 75% of the calibration standards (1 over 6), with a minimum of six, are required to fulfill this criterion. If one of the calibration standards does not meet these criteria, this calibration standard should be rejected and the calibration curve without this calibration standard should be re-evaluated, and regression analysis should be performed. The accuracy values of the Qc samples should be within ±15% of the nominal values. At minimum 67% of the Qc samples and 50% at each concentration level should comply with this criterion. If these criteria are not fulfilled, the analytical run should be rejected, and the study samples re-extracted and analyzed.
Results
In vitro evaluation of chloroprocaine-loaded hydrogel release UV spectroscopy and HPLC-MS/MS analytical method was performed for in vitro evaluation of the chloroprocaine release from the nanostructured hydrogel device.
UV analysis of chloroprocaine release
For initial quantitative evaluation of the drug in release medium, UV spectroscopy was employed to correlate the concentration of the drug with the absorbance. Chloroprocaine spectra at different concentrations in medium are displayed in Figure 2. For concentrations above 50 µg/mL, absorbance resulted in out-of-scale peaks. The spectra present characteristic peaks of different absorbance intensities.
The chloroprocaine-loaded hydrogel sample demonstrated an initial release rate of 22.5 mg/h, and in the following hours, a constant rate of ~8.0 mg/h. The release decreased after 5 hours and was prolonged until 24 hours, at which point the detection limit was reached (Figure 2B).
Evaluation of chloroprocaine and ACBA metabolite release by HPLC-MS/MS
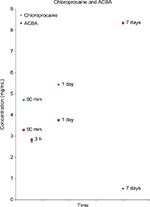
For HPLC-MS/MS detection, four nanostructured devices containing chloroprocaine and four unfilled nanogel devices (used as reference) remained in contact with plasma at 37°C for 90 minutes, 3 hours, 1 day, and 7 days (four samples). The plasma samples were analyzed via HPLC-MS/MS to assess chloroprocaine and ABCA concentrations.
The obtained concentrations for chloroprocaine and its ACBA metabolite were 0.522–5.436 and 2.84–8.348 mg/mL, respectively (Figure 3).
The analyses produced a peak of maximum plasma concentration (Cmax) of chloroprocaine after 1 day of exposure to the device (5.436 mg/mL) and a minimum (Cmin) after 7 days (0.522 mg/mL).
Otherwise, ACBA reached Cmax after 7 days (8.348 mg/mL).
Chloroprocaine plasma concentrations were undetectable in all the reference samples; in contrast, traces of ACBA were found in all the samples.
In vivo evaluation of the chloroprocaine-loaded hydrogel release
For each of the four piglets (named Gelly 2, Gelly 3, Gelly 4, and Gelly 5), 11 plasma samples were analyzed and identified as basal, T0, T3h, T6h, T12h, T24h, T48h, T72h, T96h, T102h, and T108h, according to the length of exposure (measured in hours) to the device. The basal sample was never in contact with the device (Figure 4), whereas the T0 sample remained in contact with the device for less than 3 hours.
As expected, signals of ACBA, lidocaine, and chloroprocaine were absent in the plasma of the untreated piglets.
No quantifiable amount of chloroprocaine was identified in any of the plasma samples.
The detected ACBA plasma concentrations are reported in Table 1. The LLOQ was 15 ng/mL.
  | Table 1 ACBA plasma concentrations in four piglets following application of the nanostructured drug-loaded hydrogel Abbreviations: ACBA, 4-amino-2-chlorobenzoic acid; N.C, not calculable. |
Plasma concentrations were detectable (≥LLOQ), primarily between T0 and T12h.
The T0 plasma concentration of Gelly 2 (although revalued several times) was an outlier (3,209.42 ng/mL).
The peak of maximum ACBA plasma concentration (Cmax) was observed 3 hours following the device application (Table 1).
Although the chloroprocaine plasma concentrations were consistently below the LLOQ, a well-defined peak in the chromatograms of each animal (between T3h and T6h) was detected, albeit at a different retention time from that expected.
In order to identify their well-defined retention times, some working solutions of ABCA, lidocaine, and chloroprocaine were at first added to a piglet-free drug plasma and analyzed; the related chromatographic peaks were obtained at 2.82, 2.73, and 1.90 minutes, respectively (Figure 5).
On the contrary, unexpected peaks at 2.58 minutes (differing from 1.90 minutes) were observed in the chloroprocaine mass channel during the chromatographic runs of Gelly 2, Gelly 3, Gelly 4, and Gelly 5, and they were observed between T3h and T6h.
Discussion
LAs are useful in treating acute POP,17 although severe side effects are related to their systemic usage (lidocaine and bupivacaine).18,19 Less toxic LAs (eg, chloroprocaine) have been developed, but present short half-lives.20 To prolong drug effectiveness and minimize drawbacks, LA drug delivery systems have been developed.1
Currently, hydrogels are being widely exploited in biomedical applications as drug delivery systems. An innovative aspect of the present work regards the use of alginate, commonly employed to enhance biocompatibility of PLGA microspheres or as coating to tailor drug release,21–23 as a principal material for hydrogel device development.
Alginate is an anionic naturally occurring biopolymer, typically extracted from brown algae, that has many possible applications in different biomedical applications. The favorable properties of alginate, including biocompatibility and ease of gelation, enable its use as a matrix for the entrapment and delivery of proteins, drugs, cells, wound healing, and tissue engineering.24,25
The usage of an anionic polymer could also have the advantage of creating electrostatic interactions between polymer and drug, which may reduce drug diffusion coefficients, prolonging the release time. Although alginate is inherently non-degradable in mammals as they lack the enzyme (ie, alginase) which can cleave the polymer chains, it has been reported that the degradation of alginate gels can be controlled by the release of the divalent ions involved in the gel cross-linking into the surrounding media via exchange reactions with sodium ions.25 Furthermore, as already evidenced by other studies, the partial oxidation of alginate hydrogels can produce an easy degradation in vivo,26 which constitutes further evidence supporting the clinical utilization of alginate as a device that will not need to be removed after its implantation. All these features, including its anatomical compatibility with soft tissues, make alginate suitable for the controlled release of LAs as an alternative to the use of synthetic biodegradable polyesters.
Through an UV and an HPLC-MS/MS system, we analyzed in vitro and in vivo release of an experimental chloroprocaine-loaded hydrogel sample. First, in vitro results demonstrated an initial release rate of 22.5 mg/h and in subsequent hours, a constant rate of ~8.0 mg/h. The release decreased after 5 hours and was prolonged until 24 hours. Further analysis associated the decrease in chloroprocaine concentration with an increase in ACBA concentration, indicating additionally an in vitro drug degradation.
From an in vivo analysis, we found that at the specified sampling time, LA’s concentration was never detectable. Conversely, ACBA was quantified in almost all plasma samples, with a peak of Cmax observed 3 hours following the device application.
The T0 concentration value of Gelly 2 (although revalued several times) was found not to align with that of the others. We assume that blood was incorrectly collected immediately following the nanodevice hydrogel device application rather than prior to the application.
Moreover, the presence of a molecule similar to chloroprocaine was detected through molecular weight and the same fragmentation pattern, although differed structurally. This may have resulted from specific interactions with the stationary phase of the analytical column and the mobile phases of another chloroprocaine metabolite undergoing an in-source fragmentation producing chloroprocaine.27–29
In vitro, the ACBA metabolite reached the Cmax after 7 days, whereas chloroprocaine obtained a Cmax following only 1 day of exposure to the device, and a minimum (Cmin) after 7 days. These results suggest that the drug degraded slowly over time. As was the case in the analysis of reference samples, quantifiable traces of the metabolite emerged, and it was assumed that the device released some material, initially present on the surface of contact with the plasma.
The presented data suggest that the hydrogel device maintains the structural characteristics of drugs. Therefore, the initial rapid drug release may be effective in treating POP in the initial postoperative hours, while a reduced rate of release may be effective to prevent chronic pain in the subsequent period.
In the near future, we intend to repeat the analyses on the reference samples, starting from devices for which no release is expected, placed only in contact with serum and then water. Finally, we will validate the pharmacokinetic results by substituting the procaine for the chloroprocaine. Doing so will highlight any differences between the two drugs in terms of release and degradation processes.
By following a precision medicine approach, drug loading will be modulated depending on the type of pain to be managed. We are hopeful that such translational research will soon result in the clinical utilization of the hydrogel device.
Limitations of the study
This study reports qualitative results of a device implanted in piglets, to evaluate chloroprocaine release, for potential future management of human POP. Piglets, as larger animals, are considered the most similar pharmacokinetic model to human being in terms of experimental response,30 although the cost of piglets compared to rats necessitates a smaller sample size. This limitation, as well as the absence of previous literature data regarding analog in vitro and in vivo results, does not allow us to perform a power statistical analysis or to support any power calculation. Accordingly, the present study is to be considered a pilot, and our expectation is that future, larger investigations will allow us to move closer to the ultimate goal of human application in the clinical setting.
Conclusion
The experimental chloroprocaine-loaded hydrogel sample was developed to produce a new effective analgesic method, which will contribute to improving POP relief.
Acknowledgments
The study has been funded by a grant by the Italian Health Ministry (“New nanotechnology and biomedical approaches to improve postoperative pain treatment reducing risks related to opioids” – GR-2010-2318370; Principal Investigator Massimo Allegri, MD) and a grant by Foundation “Banca del Monte di Lombardia” (“La Nanotecnologia applicata al Trattamento del dolore”; Principal Investigator Lorenzo Cobianchi, MD, USA). The authors would like to thank Paola Petrini from Politecnico di Milano for providing the hydrogels used in this work, Livia Visai, Università di Pavia, for the in vitro tests, and Sintetica® for providing chloroprocaine for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Zorzetto L, Brambilla P, Marcello E, et al. From micro- to nanostructured implantable device for local anesthetic delivery. Int J Nanomedicine. 2016;11:2695–2709. | ||
Kuhnert BR, Kuhnert PM, Philipson EH, Syracuse CD, Kaine CJ, Yun CH. The half-life of 2-chloroprocaine. Anesth Analg. 1986;65(3):273–278. | ||
Taraballi F, Minardi S, Corradetti B, et al. Potential avoidance of adverse analgesic effects using a biologically “smart” hydrogel capable of controlled bupivacaine release. J Pharm Sci. 2014;103(11):3724–3732. | ||
Grillo R, de Melo NF, de Araújo DR, et al. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. J Drug Target. 2010;18(9):688–699. | ||
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071. | ||
Liu Z1, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60(15):1650–1662. | ||
Gordon DB, de Leon-Casasola OA, Wu CL, Sluka KA, Brennan TJ, Chou R. Research gaps in practice guidelines for acute postoperative pain management in adults: findings from a review of the evidence for an american pain society clinical practice guideline. J Pain. 2016;17(2):158–166. | ||
Golembiewski J, Dasta J. Evolving role of local anesthetics in managing postsurgical analgesia. Clin Ther. 2015;37(6):1354–1371. | ||
Rivat C, Bollag L, Richebé P. Mechanisms of regional anaesthesia protection against hyperalgesia and pain chronicization. Curr Opin Anaesthesiol. 2013;26(5):621–625. | ||
Dhanapal B, Sistla SC, Badhe AS, Ali SM, Ravichandran NT, Galidevara I. Effectiveness of continuous wound infusion of local anesthetics after abdominal surgeries. J Surg Res. 2017;212:94–100. | ||
Gebhardt V, Kiefer K, Bussen D, Weiss C, Schmittner MD. Retrospective analysis of mepivacaine, prilocaine and chloroprocaine for low-dose spinal anaesthesia in outpatient perianal procedures. Int J Colorectal Dis. 2018;33(10):1469–1477. | ||
Teunkens A, Vermeulen K, van Gerven E, Fieuws S, van de Velde M, Rex S. Comparison of 2-chloroprocaine, bupivacaine, and lidocaine for spinal anesthesia in patients undergoing knee arthroscopy in an outpatient setting: a double-blind randomized controlled trial. Reg Anesth Pain Med. 2016;41(5):576–583. | ||
Förster JG, Rosenberg PH, Harilainen A, Sandelin J, Pitkänen MT. Chloroprocaine 40 mg produces shorter spinal block than articaine 40 mg in day-case knee arthroscopy patients. Acta Anaesthesiol Scand. 2013;57(7):911–919. | ||
Allegri M, Bugada D, de Gregori M, et al. Continuous wound infusion with chloroprocaine in a pig model of surgical lesion: drug absorption and effects on inflammatory response. J Pain Res. 2017;10:2515–2524. | ||
Moreira HR, Munarin F, Gentilini R, et al. Injectable pectin hydrogels produced by internal gelation: pH dependence of gelling and rheological properties. Carbohydr Polym. 2014;103:339–347. | ||
Gentilini R, Munarin F, Bloise N, et al. Polysaccharide-based hydrogels with tunable composition as 3D cell culture systems. Int J Artif Organs. 2018;41(4):213–222. | ||
Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev. 2012;10:CD007105. | ||
Gitman M, Barrington MJ. Local anesthetic systemic toxicity: a review of recent case reports and registries. Reg Anesth Pain Med. 2018;43(2):124–130. | ||
Allegri M, Bugada D, Grossi P, et al. An incidence analysis from a prospective clinical survey. Minerva Anestesiol. 2016;82(4):392–402. | ||
Kuhnert BR, Kuhnert PM, Philipson EH, Syracuse CD, Kaine CJ, Chang-Hyon Y. The Half-Life of 2-Chloroprocaine. Anesth Analg. 1998;65:273–278. | ||
Liu DZ, Sheu MT, Chen CH, Yang YR, Ho HO. Release characteristics of lidocaine from local implant of polyanionic and polycationic hydrogels. J Control Release. 2007;118(3):333–339. | ||
Zhang H, Lu Y, Zhang G, Gao S, Sun D, Zhong Y. Bupivacaine-loaded biodegradable poly(lactic-co-glycolic) acid microspheres I. Optimization of the drug incorporation into the polymer matrix and modelling of drug release. Int J Pharm. 2008;351(1-2):244–249. | ||
Cong Z, Shi Y, Wang Y, et al. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int J Biol Macromol. 2018;107(Pt A):855–864. | ||
Sriamornsak P, Thirawong N, Korkerd K. Swelling, erosion and release behavior of alginate-based matrix tablets. Eur J Pharm Biopharm. 2007;66(3):435–450. | ||
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. | ||
Reakasame S, Boccaccini AR. Oxidized alginate-based hydrogels for tissue engineering applications: A Review. Biomacromolecules. 2018;19(1):3–21. | ||
Xu YF, Lu W, Rabinowitz JD, Joshua D. Avoiding misannotation of in-source fragmentation products as cellular metabolites in liquid chromatography-mass spectrometry-based metabolomics. Anal Chem. 2015;87(4):2273–2281. | ||
Demarque DP, Crotti AE, Vessecchi R, Lopes JL, Lopes NP. Fragmentation reactions using electrospray ionization mass spectrometry: an important tool for the structural elucidation and characterization of synthetic and natural products. Nat Prod Rep. 2016;33(3):432–455. | ||
Yan Z, Caldwell GW, Jones WJ, Masucci JA. Cone voltage induced in-source dissociation of glucuronides in electrospray and implications in biological analyses. Rapid Commun Mass Spectrom. 2003;17(13):1433–1442. | ||
Gigliuto C, de Gregori M, Malafoglia V, et al. Pain assessment in animal models: do we need further studies? J Pain Res. 2014;7:227–236. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.