Back to Journals » International Journal of Nanomedicine » Volume 15
In vitro and in vivo Evaluation of Succinic Acid-Substituted Mesoporous Silica for Ammonia Adsorption: Potential Application in the Management of Hepatic Encephalopathy
Authors Mohammadi H , Heidari R , Niknezhad SV, Jamshidzadeh A, Farjadian F
Received 12 July 2020
Accepted for publication 20 November 2020
Published 14 December 2020 Volume 2020:15 Pages 10085—10098
DOI https://doi.org/10.2147/IJN.S271883
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Hamidreza Mohammadi,1,2 Reza Heidari,1 Seyyed Vahid Niknezhad,1 Akram Jamshidzadeh,1,2 Fatemeh Farjadian1
1Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran; 2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
Correspondence: Fatemeh Farjadian
Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, P. O. Box 158371345, Karafarin Ave., Shiraz, Fars, Iran
Tel + 987132424127 302
Fax + 987132424126
Email [email protected]
Akram Jamshidzadeh
Department of Pharmacology and Toxicology, Faculty of Pharmacy, Shiraz University of Medical Sciences, P. O. Box 158371345, Karafarin Ave., Shiraz, Fars, Iran
Email [email protected]
Purpose: Hepatic encephalopathy (HE) is a critical situation in which liver failure affects brain function. HE could result in a state of coma and death. The liver is the main organ for ammonium ion (NH4+) metabolism. Hence, acute and/or chronic liver failure could lead to hyperammonemia. NH4+ is the most suspected neurotoxic agent in HE. Thus, finding new therapeutic options to decrease plasma and brain NH4+ levels has a significant clinical value. Mesoporous silica (MS) particles have revolutionized many aspects of pharmaceutical sciences, including drug delivery systems. Moreover, recently, MS has been applied as agents for the detoxification of chemicals (eg, drugs and poisons).
Methods: First, MS particles containing amine groups (MS-NH2) were synthesized in co-condensation processes. Then, the structure was modified by succinic anhydride to have MS-SA. The MS-SA was characterized (FT-IR, XRD, X-ray photoelectron spectroscopy (XPS), DLS-Zeta FESEM-EDX, and HRTEM). Then, the potential of MS-NH2 and MS-SA particles in adsorption of NH4+ was investigated in vitro and in vivo. MS-NH2 and MS-SA were incubated with increasing concentrations (0.1– 10 mM) of NH4+, and the scavenging capacity of the investigated particles was evaluated. On the other hand, different doses (1 and 5 mg/kg per day) of nanoparticles were administered to a hyperammonemia animal model.
Results: It was figured out that both MS-NH2 and MS-SA significantly scavenged NH4+ in the in vitro model. However, the NH4+ scavenging capability of MS-SA was more significant. Administration of MS-NH2 and MS-SA also considerably decreased the level of ammonium in plasma and brain and improved cognitive and locomotor activity in hyperammonemic animals. The effects of MS-SA were more significant than MS-NH2 in the HE animal model.
Conclusion: Collectively, our data suggest that MS particles, especially succinic acid-functionalized MS, could act as special ancillary treatment in HE as a critical clinical complication.
Keywords: acute liver failure, ammonia, hyperammonemia, nanomedicine
Introduction
Nanotechnology is a new technology that deals with studies of materials at the atomic, molecular, and macromolecular in nanoscales. The biomedical application of nanoparticles is one of the major areas of interest in nanotechnology science.1 In recent years, nanoparticles have been widely utilized in various fields of studies, specifically in nanomedicine. Nanopharmaceuticals are known as the emerging reformulation of available therapeutic agents, which lessened the side effects, and acquired more safety to deliver the cargo to the designated part.2 Several types of structures are made recognition for their fruitful applications in the field of nan medicine, including natural,3 carbon,4 and silica5 -based nanoparticles. Among them, silica-based Nano-materials have gained prominent attention due to the approving their safety by the Food and Drug Administration as “Generally regarded as Safe” agents.6 These nanoparticles have become more applicable by introducing porosity to their structure with the well-known type, Mesoporous silica (MS).7 Mesoporous silica particles are well-ordered structures with pore sizes ranging from 2–10 nm. The porosity is controlled by the utilization of templating agents (“soft” or “hard”) in synthetic strategies. Through the utilization of a soft templating agent, a surfactant type structure, reach to critical micelle concentration, and in a sol-gel process, the silane coupling agents form networks. Finally, the surfactant was removed, and a well-defined porous structure was created.5
A unique type of MS material is Mobil-composition of matter/No.41 (MCM-41), which is synthesized in the presence of cetyltrimethylammonium bromide (CTAB).8 MCM-41 provides excellent textual properties, such as equality of hexagonal cylindrical pores having good surface area, connectivity with different functional groups, temperature stability, high surface area, biocompatibility, uniform pore distribution. These types of particles have found vast arrays of applications recent finding as an anti-fibrillating agent of insulin.9 In past years, various investigations have been carried out on the adsorption of heavy metals by mesoporous silica.10,11 These studies resulted in the development of a novel application for MS materials in sensors,12 catalysts,13 drug delivery carrier,14 gene delivery,15 an adsorbent16,17 and also as an antidote agent.13 In this regard, Farjadian et al reported that EDTA modified MS could be potent antidote agents while assessed in vitro and in vivo in adsorption of iron18 and copper.19
HE is a severe complication in which the ability of the liver for ammonium ion detoxification is impaired.20 NH4+ is the most suspected toxic agent in HE. NH4+ is mainly derived from the gut because of the metabolic activity of gut bacteria,21 while this molecule could also be produced by the catabolism of proteins in the body.22 A high plasma level of NH4+ could affect the brain tissue.23 Increased brain level of ammonium ion leads to impaired cognitive dysfunction, locomotor disturbances, coma, or even patient death.24 Hence, finding drugs to ador plasma ammonia could significantly improve HE-associated complications.
Conventional treatments for HE could be mentioned as the administration of non-absorbable disaccharides (lactulose), antibiotics (Rifaximin and Neomycin),25 decreasing dietary protein intake,26 Zinc supplementation,27 L-ornithine and L-aspartate treatment,28 and branched-chain amino acids administration.29 On the other hand, some investigations such as Cristina et al, 2011 used nanoparticles (AST-120; Spherical Carbon Adsorbent) to remove ammonia in patients with HE.30 Therefore, finding new therapeutic strategies could significantly decrease HE-associated complications.
The current study was designed to synthesize, characterize, and in vitro and in vivo evaluation of succinic acid-substituted mesoporous silica for ammonia adsorption. The results might lead to the development of new therapeutic strategies against hyperammonemia and HE as a severe clinical complication.
Methods and Materials
Materials
The sources of materials for each step are categorized here. For MS synthesis, tetraethoxysilane (TEOS), (3-aminopropyl) triethoxysilane (APTES), CTAB, and dimethylformamide (DMF) were purchased from Merck (German). Acetone, ammonia solution (25%), ethanol (98%), and Hydrochloric acid (HCl) were bought by the company Kimia-Mavad® (Iran). For in vivo, ammonium chloride (NH4Cl), sodium hypochlorite, and succinic anhydride (SA) were purchased from Alvand-Chem® (Iran). Potassium hydroxide, phenol and sodium citrate, sodium nitroprusside, and thioacetamide were purchased from Merck (Darmstadt, Germany).
Synthesis of Amino-Functionalized Mesoporous Silica (MS-NH2)
The MS-NH2 preparation method is summarized here.19 Firstly, 4.8 g of CTAB (13.2 mmol) as a surfactant template was dissolved in 100 mL of deionized water. In the next step, 26 mL of ammonia was added to the above solution. Then 6.17 mL (27.9 mmol) of TEOS was slowly added, and subsequently, 0.74 mL (3.1 mmol) of APTES was added simultaneously to the stirring solution. The mixture was stirred for two hours to generate a white suspension. The suspension was filtered and washed (3x) with DI water/ethanol and acetone, and then it was transferred into an oven at 60 °C for 24h. Finally, the CTAB was removed from white powder by refluxing in an acidic ethanol solution (6 mL HCl/400 mL ethanol) for 48 h at 90 °C.
Synthesis of Succinic Acid-Functionalized Mesoporous Silica (MS-SA)
For synthesis MS-SA, first, 1 g of MS-NH2 was dissolved in 20 mL of dimethylformamide and sonicated for 2 min, then 0.5 g (5 mmol) of succinic anhydride and 10 mL of dimethylformamide was slowly added and stirred at 50 °C for 24 h. Subsequently, the mixture was filtered and washed with ethanol (3 times). Finally, the resulting white powder was dried in an oven at 50 °C for two days.
Characterization Techniques and Instrumentation
Diverse techniques performed the characterization of synthesized materials. Morphological characterization of the MS-SA was taken using HR-TEM by JEOL-2100F, 200 kV instrument (USA). To determine the size of MS-NH2 and MS-SA, FE-SEM carried out with the Zeiss Merlin PV, and EDX was done to determine the type and percentage of elements in the particles. Dynamic light scattering (DLS) was determined with a Microtrac instrument. Zeta-potential were performed in phosphate buffer (pH=7.4) with (ZETA-check®, Colloid Metrics GmbH, Germany). X-ray diffraction (XRD) was collected over the low-angle by tube voltage of 40 kV and 30 mA, to determine the crystal structure of the nanoparticle. The Bruker instrument compiled FT-IR spectra.
The N2 adsorption-desorption isotherms were applied for surface area determination were measured by the Brunauer-Emmet-Teller (BET) method with the BEL Japan Inc. instrument. The XPS analysis was performed with an X-ray/8025-BesTec system using Al Kα X-ray source (photon energy of 1486.6 eV). Bioimaging was performed with a KODAK In-Vivo Imaging System (Carestream Health, Inc).
Determination of Ammonium Ion
The level of ammonium ion level in the in vitro model, as well as plasma ammonia (in vivo), was measured according to a reported procedure.31,32 In summary, 100 µL of the samples were added to a medium containing 720 µL of deionized water, 40 µL of sodium nitroprusside (5%), and 40 µL of phenol (10%). Then, 100 µL of oxidizing reagent (4: 1 alkaline citrate and sodium hypochlorite) was added, and samples were stored at room temperature (1 h, without light). And eventually, after centrifuging the samples (15 min at 12,000 g), the color absorbance was measured at λ = 625 nm. For the determination of brain tissue ammonium level, 100 mg of rat brain tissue was weighed, homogenized, and added to 3 mL of trichloroacetic acid-cooled lysis solution (6% w/v). Then, samples were centrifuged (15 min, 12,000 g for at 4 °C); in the next step, transfer 100 µL of supernatant to a pre-prepared medium (method in vitro). Finally, brain ammonia content was measured according to the protocol mentioned in the in vitro model and plasma ammonia.
In vitro Ammonium Ion Scavenging Capability of MS-NH2 and MS-SA
Ammonium chloride solutions (0.1, 1, 5, and 10 mM) were incubated in phosphate buffer (pH =7.4) at room temperature, with different concentrations of the MS-SA and MS-NH2 (1 and 5 mg/mL) for 1, 2, and 3 hours. Ammonia concentration for each sample was measured, and the data were displayed as a percentage of adsorbed ammonia. The scavenging ability (%) was quantified by the following equation: scavenging ability (%) = 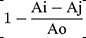 * 100. Where Ai is the sample absorbance (MS-NH2 and MS-SA complex), Aj is the background absorbance for the sample without ammonium, and Ao is the absorbance of the control.
* 100. Where Ai is the sample absorbance (MS-NH2 and MS-SA complex), Aj is the background absorbance for the sample without ammonium, and Ao is the absorbance of the control.
Preparation of Rhodamine-B-Labeled MS and Bioimaging of MS Nanoparticles in Rat
For labeling MS nanoparticles, rhodamine B was covalently conjugated to these particles as previously described (with some modifications).33 Briefly, rhodamine B (0.06 mmol) was activated in in dimethylformamide (DMF) (4 mL) solution containing N-hydroxysuccinimide (0.12 mmol) and 1-ethyl-3-(3dimethylaminopropyl) carbodiimide (EDC) (0.06 mmol) for 30 min at room temperature and then MS-NH2 was added and stirred vigorously for 24 h (25°C, in the dark). Afterward, samples were vacuum dried and washed with DMF to remove un-reacted rhodamine B. Finally; samples were filtered and dried again under vacuum. Rhodamine B-labeled MS was used for the bio-imaging. For this purpose, rhodamine-B labeled MS-NH2 particles were administered to rats (10 mg/kg, oral), and animals were imaged at times of 0, 2, 6, 12, and 24 h post particle administration (Kodak In-Vivo F Pro Imaging System).
In vivo Evaluation of Ammonium Adsorption of MS-SA
Animals
Male Sprague Dawley rats (200–250 g) were obtained from the Animal Breeding Center of Shiraz University of Medical Sciences, Shiraz, Iran. Rats were kept in polyethylene cages on wood-chip bedding in a typical environment (temperature of 24±1 °C, a cycle of light/darkness, 12/12 h, and relative humidity ≈40%). Animals had free access to a standard rodents’ food (Royan Feed®, Esfahan, Iran) and tap water. Animals were handled according to the Shiraz University of Medical Sciences guidelines for care and use of laboratory animals approved by a local ethics committee at Shiraz University of Medical Sciences, Shiraz, Iran (Certificate # 98–01-05-19,528). Brain samples were obtained from rats.
Animal Model of Acute Hepatic Failure and Hyperammonemia
Thioacetamide is extensively used for the induction of hepatic failure and hyperammonemia.34,35 In the current study, thioacetamide was administered by intraperitoneal injection (200 mg/kg) to rats for three consecutive days.36 After the induction of hepatic failure and HE (day 4) the treatment steps were as follows: 1) Control group (Vehicle-treated); 2) thioacetamide; 3) MS (1 mg/kg/h, three doses/day, every 2 h, oral,) + thioacetamide; 4) MS (5 mg/kg/h, three doses/day, every 2 h, oral,) + thioacetamide; 5) MS-SA (1 mg/kg/h, three doses/day, every 2 h, oral,) + thioacetamide; 6) MS-SA (5 mg/kg/h, three doses/day, every 2 h, oral) + thioacetamide. The control group received normal saline. Behavioral tests were assessed on day 5 (24 h after the last dose of MS or MS-SA). And at the end, animals were anesthetized with thiopental (80 mg/kg, i. p) (Day 5), and blood and brain samples were collected.
Motor Coordination Tests
All motor coordination and activity tests were done for each group five hours before animal anesthesia (80 mg/kg thiopental, i.p) and collected samples.
Rotarod Test
Animals underwent the rotarod test according to a previously reported protocol.3,4 Briefly, rats were trained on a rotarod apparatus (5 min, three consecutive days) to get used to the environment. On the experiment day, each animal was tested three times in the rotarod system. The rotating rod speed was 5, 10, and 15 rpm with a stop time of 5min. The time that animals stayed on the rotating rod was measured.38,39
Open Field Test
This test was used as another test for assessing the locomotor activity in the hyperammonemic animals.40,41 Briefly, the apparatus was constructed of a white wood box (100 cm long × 100 cm wide × 30 cm high, the box floor was divided into 25 squares of 20×20 cm). The field was equipped with a webcam (Gig aware®, UK), and the animals’ activities were recorded from another room. Animal’s activities were monitored for 15 minutes. Finally, the sum of the squares the animals passed through during the monitoring time was counted.42,43
Gait Test
The gait stride test is used to evaluate animals’ locomotion in diverse types of brain injury.4,5 Briefly, the animals’ hind paw was soaked with ink. Then, rats were allowed to move in a corridor covered with a paper strip (60 cm long, 10 cm wide). Eventually, the distance between the right and left hind paws were measured.38,44
Serum Biochemistry and Tissue Histopathology
After performing motor co-ordination tests to determine the biochemistry of blood, animals were anesthetized with thiopental (80 mg/kg, i.p). Then, blood was collected from the abdominal aorta, moved to standard tubes (Heparin-coated tubes), and centrifuged (3000 g, for 15 minutes, 4 °C) to prepare plasma. Standard kits (Biorexfars, Shiraz, Iran) and a Mindray BS-200® auto analyzer was used to analyze plasma biomarkers of liver damage such as aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), and bilirubin. For histopathological assessments, samples of liver were fixed in buffered formalin solution (0.4% sodium phosphate monobasic, NaH2PO4, 0.64% sodium phosphate dibasic, Na2HPO4, and 10% formaldehyde in distilled water).45 Paraffin-embedded sections of tissue (5 µm) were prepared and stained with hematoxylin and eosin (H&E) before light microscope viewing.
Statistical Analysis
Data were given as the mean ± SD. Data were compared with the one-way analysis of variance (ANOVA) and followed by Tukey’s multiple comparisons as the post hoc test. Sigma plot 12 and Prism 6 was used for visualization and analyzing the data.
Results
Characterizations and the Morphology of MS-SA
FT-IR spectrums could provide useful information about the functional groups available in the structure of materials. In this study, the spectrum of MS-NH2 and MS-SA is presented in Figure 1.
 |
Figure 1 FT-IR spectroscopy related to structures of MS-NH2 and MS-SA. Abbreviations: FT-IR, Fourier transform infrared; MS-SA, succinic acid-modified mesoporous silica. |
The peak attributed to Si-O-Si asymmetric stretching vibration appeared at 1100 cm−1, and the characteristic bands of amine and hydroxyl groups appeared as broad-spectrum around 3440 cm−1. In the MS-SA chromatograph, the peaks at 1480 and 1520 cm−1 are ascribed to the N-C=O band, and 1810 cm−1 respectively belongs to the carbonyl group of carboxylic acids.
Nitrogen adsorption and desorption isotherms of the computation have shown MS-NH2 have a specific surface area of 966.53 m2 g−1 and a pore size of 1.21 nm. In contrast, a severe change is seen when compared with the MS-SA specific surface area that, to be 895.81 m2 g−1. Therefore, we concluded that the specific surface area reduction ascribed to the successful introduction of SA in MS-NH2 pores (from 966.53 to 895.81 m2 g−1).
XRD was performed at low angles MS-NH2 and MS-SA, and the presence of a peak at 0.8 ° 2theta indicates that our structure is crystalline with mesopores (Figure 2).
 |
Figure 2 XRD pattern of MS-NH2 and MS-SA. Abbreviations: XRD, x-ray diffraction; MS-SA, succinic acid-modified mesoporous silica. |
In the EDX spectrum of the MS-NH2 and MS-SA, the difference in weight percentages of elements C, Si, N, O was obtained and showed that, after loading of MS by succinic acid groups, the weight percent of C increased from 15.28% to 16.95% (Figure 3).
 |
Figure 3 EDX analysis of MS-NH2 (A) and MS-SA (B). Abbreviations: EDX, energy-dispersive x-ray spectroscopy; MS-SA, succinic acid modified mesoporous silica. |
The morphological features of MS-NH2 and MS-SA were investigated by FE-SEM and HR-TEM images (Figure 4A–C). The FE-SEM image demonstrated a spherical shape with sizes <500 nm. Besides, the HR-TEM image revealed a porous structure.
In Figure 5A, we show the dispersion and distribution of MS-SA in solution (PBS (pH = 7.4)), and the hydrodynamic particle size of MS-SA is approximately 344 nm. In Figure 5B, we illustrate the Zeta-potential of MS-NH2 in solution (PBS (pH = 7.4)) before and after the addition of succinic acid, MS-SA has a higher negative charge, so it can efficiently react with ammonia (NH4+), which has a positive charge.
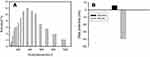 |
Figure 5 Particle size distribution of synthesized MS-SA (A). Zeta potential of synthesized MS-NH2 and MS-SA (B). Abbreviation: MS-SA, succinic acid-modified mesoporous silica. |
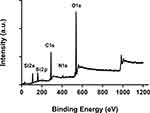
In Figure 6. We show the XPS spectrum of pure MS-SA. The binding energy (BE) of the Si 2s and Si 2p orbital in were about 108 eV and 159 eV. Furthermore, a prominent peak at 537 eV showed O 1s. Moreover, the binding energy (BE) of the C 1s peak and N1s peak respectively appears about 285 eV and 407eV. Hence, the functional group is linked to the inner surface of the MS particles.
In vitro Adsorption of Ammonia by MS-SA
The adsorption of ammonia by MS-SA was rapid, and approximately 60% of ammonium ion was adsorbed during the first hour of incubation (Figure 7A). MS-NH2 also significantly scavenged ammonium ion (Figure 7A–C). However, the scavenging capability of MS-SA was considerably higher than MS at different time intervals (Figure 7A–C). The scavenging activity of MS-NH2 and MS-SA was not time-dependent, and these nanoparticles significantly scavenged NH4+ at different times of incubation (Figure 7A–C).
Bioimaging of MS-Labelled Nanoparticles
After oral administration of rhodamine-B labeled MS-SA particles (0.5 mL of 1 mg/mL of nanoparticle solution), animals were monitored at different time intervals (Figure 8). As shown in Figure 8, the bulk of MS particles are retained in the gastrointestinal tract, and there is no significant distribution of administered MS nanoparticles (Figure 8). MS particles were excreted after 24 hours of administration (Figure 8).
 |
Figure 8 Bio-imaging of fluorescent-labeled MS-SA in the rat. Abbreviation: MS-SA, succinic acid-modified mesoporous silica. Note: Numbers indicate hour’s post-MS-SA administration. |
In vivo Capability of MS-NH2 and MS-SA for Ammonium Ion Adsorption
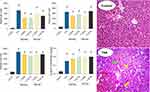
Thioacetamide treatment (200 mg/kg, i.p) caused histopathological changes, including fatty changes, tissue necrosis, and inflammation in the liver tissue. On the other hand, increased plasma levels of ALT, AST, LDH, and bilirubin were evident in the thioacetamide-treated group in comparison with the control animals (Figure 9). The administration of MS-NH2 and/or MS-SA had no significant effect on liver histopathological alterations or plasma biomarkers of organ injury in the current investigation (Figure 9).
A significant increase in brain and plasma and ammonia levels was evident in the TAA-induced liver failure group (Figure 10), and on the other hand, it was found that MS-NH2 and MS-SA administration (1 and 5 mg/kg, oral) significantly decreased plasma as well as brain ammonia in TAA-treated rats (Figure 10).
Significant suppression of animals’ locomotor function was evident in hyperammonemic rats as judged by decreased animal open field activity, time on the rotarod, and changes in stride patterns (Figure 11). On the other hand, it was found that MS-NH2 and MS-SA supplementation (1 and 5 mg/kg, oral) significantly decreased brain and plasma ammonia levels (Figure 10) and enhanced animals’ locomotor function (Figure 11). It is considerable to mention that the effects of MS-SA on the brain and plasma ammonia levels and locomotor activity was more significant as compared with MS-NH2 groups (Figure 11). On the other hand, the effects of MS-NH2 and MS-SA on in vivo parameters were not dose-dependent in most cases assessed in the current experimental model (Figure 11).
Discussion
Ammonium ion (NH4+) is continuously produced in the human body from amino acids and protein catabolism. The gut microbiome is also the primary source of NH4+ production. Gut-derived NH4+ has also reached the systemic circulation.
In healthy subjects, the major portion of NH4+ is metabolized in the liver tissue. On the other hand, when the liver failure occurs (eg, because of xenobiotics or severe liver disease), the NH4+ detoxification capacity of this organ is impaired. Therefore, plasma NH4+ reaches high toxic levels. The brain is the primary organ affected by hyperammonemia.
Liver failure-induced brain injury is called hepatic encephalopathy (HE) syndrome. Severe symptoms such as cognitive dysfunction, locomotor disturbances, coma, permanent brain injury, or even death could occur in HE.46 In severe cases of HE, blood or brain ammonia could reach up to 10–20 mM.20,47 On the other hand, in a clinical situation called sub-clinical HE, a constant but relatively high (100 µM to 1 mM) of NH4+ is present in the blood of patients.46,48
Both acute and sub-clinical HE could cause permanent brain damage or decrease patients’ quality of life. Based on these data, finding clinically appropriate therapeutic options could have great value. In this study, we found that oral administration of a newly synthesized succinic acid-substituted MS nanoparticle significantly scavenged ammonium ion (in vitro) and decreased plasma and brain ammonia levels in an animal model of HE (in vivo). On the other hand, the adsorption of ammonium ion to the silica antiparticles was evaluated in an in vitro condition for 3 hours (Figure 7C). It was found that the level of free ammonium ion was time-dependently decreased, and no desorption was identified during this time interval (Figure 7). Indeed, further studies with wide time intervals or administration of these particles in chronic conditions of hyperammonemia could precisely reveal the probability of ammonium desorption from MSN nanoparticles.
There are several convenient therapeutic strategies for HE. For example, non-absorbable disaccharides such as lactulose are widely administered for this complication. Several other treatments, such as antibiotics (Rifaximin and Neomycin), are also used in HE.49,50 Interestingly, it has also been found that altering gut micro-flora with prescribing probiotic agents is a safe strategy to reduce blood ammonia. Decreasing diet protein intake, zinc supplementation, and enhancing the activity of the enzymes involved in the urea cycle are other therapeutic options that are clinically used for minimizing HE adverse effects. However, all these therapeutic strategies may not be sufficient for patients with acute liver failure and severely high blood ammonia. These findings mention the importance of finding new or adjuvant therapies for hyperammonemia.
Mesoporous silica nanoparticles (MSN) are widely investigated for their use for industrial or biomedical purposes.5,51 Recently, we focused on the application of MS as antidotes in different biological systems. The effects of MS on a wide range xenobiotics, namely heavy metals, acetaminophen, phenobarbital, and iron and copper overload has been successfully tested by our research team.18,52
The current investigation was designed to synthesize a functionalized MS for ammonium ion adsorption in vitro and alleviating HE as a severe clinical complication in an animal model. We found that MS has mainly remained in the GI (Figure 8). This could significantly increase the adsorption of bacterial-derived ammonium ion in the GI. Drugs such as lactulose or non-absorbable antibiotics also affect gut bacteria and remove these microbes. However, significant adverse effects of these drugs might be associated with alteration in GI microflora. Therefore, the administration of safe agents (eg, MS particles), which only adsorb the bacterial-derived ammonia, could have a great benefit. On the other hand, using antibiotics or lactulose in very severe cases of hyperammonemia might be inevitable. In the current study, MS-SA significantly decreased serum ammonium probably through adsorption of this toxic molecule in rat gut and preventing its absorption to the systemic circulation (Figure 8). Consequently, serum and brain level of NH4+ was significantly decreased, and animal locomotor activity was significantly improved in the animal model of HE (Figures 10 and 11).
It is noteworthy to mention that the synthesized succinic acid-substituted MS may not only react with the ammonium ion (NH4+) but also could non-specifically interact with other groups (eg, lysine) or electrolytes. As mentioned, HE is defined as a severe elevation in plasma and brain ammonium ion levels. Hence, it is an emergency state and necessary to decrease the level of this toxic compound profoundly. The imbalance in other ions, amino acids, or electrolytes could be of the second degree of importance in HE patients. It could be compensated by appropriate medical intervention (eg, electrolyte-containing serum administration). On the other hand, the administration of MS particles did not affect the serum level of liver injury biomarkers (eg, ALT, AST, LDH, and Bilirubin) or liver histopathological alterations. These findings indicate that MS-SA does not act as a hepatoprotective agent in the current model. Indeed, MS-SA could find therapeutic value in HE, in combination with other routine hepatoprotective strategies (eg, N-acetyl cysteine supplementation).
Collectively, we found that the administration of MS particles and its functionalized derivatives could significantly lower plasma and brain ammonia levels. These effects might be mediated through adsorption of gut bacteria-derived ammonia or absorbing ammonium ion from the bloodstream. Indeed, further studies are needed to evaluate the impact of these compounds in other models of hyperammonemia and optimizing their characteristics, and finally aiming their administration in clinical settings.
Acknowledgments
This investigation was financially supported by the Vice-Chancellor of Research Affairs of Shiraz University of Medical Sciences (Grant No. 98-01-05-19528).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Bhushan B. Springer Handbook of Nanotechnology. Springer; 2017:1–19.
2. Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2019;14(1):93–126. doi:10.2217/nnm-2018-0120.
3. Farjadian F, Moghoofei M, Mirkiani S, et al. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol Adv. 2018;36(4):968–985. doi:10.1016/j.biotechadv.2018.02.016.
4. Karimi M, Ghasemi A, Mirkiani S, Basri MM, Hamblin M. Carbon Nanotubes in Drug and Gene Delivery. Morgan & Claypool Publishers; 2017.
5. Farjadian F, Roointan A, Mohammadi-Samani S, Hosseini M. Mesoporous silica nanoparticles: synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem Eng J. 2019;359:684–705. doi:10.1016/j.cej.2018.11.156.
6. Bernardos Bau A, Kourimska L. Applications of mesoporous silica materials in food: a review. Czech J Food Sci. 2013;31(2):99–107. doi:10.17221/240/2012-CJFS.
7. Ouyang G, Yang G, Sun C, Zhu W. Nanoporous structures: smaller is stronger. Small. 2008;4(9):1359–1362. doi:10.1002/smll.200800129.
8. Kresge C, Leonowicz M, Roth WJ, Vartuli J, Beck J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. nature. 1992;359(6397):710–712. doi:10.1038/359710a0.
9. Akbarian M, Tayebi L, Mohammadi-Samani S, Farjadian F. Mechanistic assessment of functionalized mesoporous silica-mediated insulin fibrillation. J Phys Chem B. 2020;124(9):1637–1652. doi:10.1021/acs.jpcb.9b10980.
10. Ghorbani M, Nowee SM. Kinetic studies of Pb and Ni adsorption onto MCM-41 amine-functionalized nano particle. Adv Environ Technol. 2015;1(2):101–104. doi:10.22104/aet.2015.269.
11. Soltani R, Dinari M, Mohammadnezhad G. Ultrasonic-assisted synthesis of novel nanocomposite of poly (vinyl alcohol) and amino-modified MCM-41: a green adsorbent for Cd (II) removal. Ultrason Sonochem. 2018;40:533–542. doi:10.1016/j.ultsonch.2017.07.045.
12. Melde BJ, Johnson BJ, Charles PT. Mesoporous silicate materials in sensing. Sensors. 2008;8(8):5202–5228. doi:10.3390/s8085202.
13. Farjadian F, Hosseini M, Ghasemi S, Tamami B. Phosphinite-functionalized silica and hexagonal mesoporous silica containing palladium nanoparticles in Heck coupling reaction: synthesis, characterization, and catalytic activity. RSC Adv. 2015;5(97):79976–79987. doi:10.1039/C5RA16131B.
14. Popova M, Szegedi A, Yoncheva K, et al. New method for preparation of delivery systems of poorly soluble drugs on the basis of functionalized mesoporous MCM-41 nanoparticles. Micropor Mesopor Mater. 2014;198:247–255. doi:10.1016/j.micromeso.2014.07.044.
15. Nhavene EPF, Andrade GF, Faria JAQA, Gomes DA, Sousa E. Biodegradable polymers grafted onto multifunctional mesoporous silica nanoparticles for gene delivery. ChemEngineering. 2018;2(2):24. doi:10.3390/chemengineering2020024.
16. Dimos K, Stathi P, Karakassides MA, Deligiannakis Y. Synthesis and characterization of hybrid MCM-41 materials for heavy metal adsorption. Micropor Mesopor Mater. 2009;126(1–2):65–71. doi:10.1016/j.micromeso.2009.05.021.
17. Dinh Du D, Hieu NT, To TC, et al. Aminopropyl functionalised MCM-41: synthesis and application for adsorption of Pb (II) and Cd (II). Adv Mater Sci Eng. 2019:2019. doi:10.1155/2019/8573451.
18. Farjadian F, Ghasemi S, Heidari R, Mohammadi-Samani S. In vitro and in vivo assessment of EDTA-modified silica nano-spheres with supreme capacity of iron capture as a novel antidote agent. Nanomed Nanotechnol Biol Med. 2017;13(2):745–753. doi:10.1016/j.nano.2016.10.012.
19. Taqanaki ER, Heidari R, Monfared M, Tayebi L, Azadi A, Farjadian F. EDTA-modified mesoporous silica as supra adsorbent of copper ions with novel approach as an antidote agent in copper toxicity. Int J Nanomedicine. 2019;14:7781–7792. doi:10.2147/IJN.S218760.
20. Bhatia V, Singh R, Acharya SK. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut. 2006;55(1):98–104. doi:10.1136/gut.2004.061754.
21. Romero-Gómez M. Pharmacotherapy of hepatic encephalopathy in cirrhosis. Expert Opin Pharmacother. 2010;11(8):1317–1327. doi:10.1517/14656561003724721.
22. Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7(9):515. doi:10.1038/nrgastro.2010.116.
23. Shawcross D, Wright G, Damink SO, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22(1):125–138. doi:10.1007/s11011-006-9042-1.
24. Rodrigo R, Cauli O, Gomez–Pinedo U, et al. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139(2):675–684. doi:10.1053/j.gastro.2010.03.040.
25. Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52(3):737–741. doi:10.1007/s10620-006-9442-4.
26. Greenberger NJ, Carley J, Schenker S, Bettinger I, Stamnes C, Beyer P. Effect of vegetable and animal protein diets in chronic hepatic encephalopathy. Am J Dig Dis. 1977;22(10):845–855. doi:10.1007/BF01076158.
27. Riggio O, Ariosto F, Merli M, et al. Short-term oral zinc supplementation does not improve chronic hepatic encephalopathy. Dig Dis Sci. 1991;36(9):1204–1208. doi:10.1007/BF01307509.
28. Stauch S, Kircheis G, Adler G, et al. Oral L-ornithine-L-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled double-blind study. J Hepatol. 1998;28(5):856–864. doi:10.1016/s0168-8278(98)80237-7.
29. Naylor CD, O’Rourke K, Detsky AS, Baker JP. Parenteral nutrition with branched-chain amino acids in hepatic encephalopathy: a meta-analysis. Gastroenterology. 1989;97(4):1033–1042. doi:10.1016/0016-5085(89)91517-5.
30. Bosoi CR, Parent‐Robitaille C, Anderson K, Tremblay M, Rose CF. AST‐120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct–ligated rats. Hepatology. 2011;53(6):1995–2002. doi:10.1002/hep.24273.
31. Solorzano L. Determination of ammonia in natural waters by the phenolhypochlorite method Limnology and oceanography. Limnol Oceanogr. 1969;14(5):799–801. doi:10.4319/lo.1969.14.5.0799.
32. Bolleter W, Bushman C, Tidwell PW. Spectrophotometric determination of ammonia as indophenol. Anal Chem. 1961;33(4):592–594. doi:10.1021/ac60172a034.
33. Farjadian F, Rezaeifard S, Naeimi M, et al. Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly (vinylcaprolactam). Int J Nanomedicine. 2019;14:6901–6915. doi:10.2147/IJN.S214467.
34. Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15(25):3086. doi:10.3748/wjg.15.3086.
35. Jamshidzadeh A, Abdoli N, Niknahad H, et al. Taurine alleviates brain tissue markers of oxidative stress in a rat model of hepatic encephalopathy. Trends Pharma Sci. 2017;3(3):181–192.
36. Ali S, Ansari KA, Jafry M, Kabeer H, Diwakar G. Nardostachys jatamansi protects against liver damage induced by thioacetamide in rats. J Ethnopharmacol. 2000;71(3):359–363. doi:10.1016/S0378-8741(99)00153-1.
37. Ommati MM, Farshad O, Mousavi K, Khalili M, Jamshidzadeh A, Heidari R. Chlorogenic acid supplementation improves skeletal muscle mitochondrial function in a rat model of resistance training. Biologia. 2020;75(8):1221–1230. doi:10.2478/s11756-020-00429-7.
38. Carter RJ, Morton J, Dunnett SB. Motor coordination and balance in rodents. Curr Protocols Neurosci. 2001;15(1):
39. Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883(2):165–177. doi:10.1016/s0006-8993(00)02778-5.
40. Ommati MM, Heidari R, Ghanbarinejad V, Abdoli N, Niknahad H. Taurine treatment provides neuroprotection in a mouse model of manganism. Biol Trace Elem Res. 2019;190(2):384–395. doi:10.1007/s12011-018-1552-2.
41. Heidari R, Jafari F, Khodaei F, Shirazi Yeganeh B, Niknahad H. Mechanism of valproic acid‐induced Fanconi syndrome involves mitochondrial dysfunction and oxidative stress in rat kidney. Nephrology. 2018;23(4):351–361. doi:10.1111/nep.13012.
42. Ommati MM, Heidari R, Ghanbarinejad V, Aminian A, Abdoli N, Niknahad H. The neuroprotective properties of carnosine in a mouse model of manganism is mediated via mitochondria regulating and antioxidative mechanisms. Nutr Neurosci. 2020;23(9):731–743. doi:10.1080/1028415X.2018.1552399.
43. Heidari R, Jamshidzadeh A, Niknahad H, et al. Effect of taurine on chronic and acute liver injury: focus on blood and brain ammonia. Toxicol Rep. 2016;3:870–879. doi:10.1016/j.toxrep.2016.04.002.
44. Ommati MM, Jamshidzadeh A, Niknahad H, et al. N-acetylcysteine treatment blunts liver failure-associated impairment of locomotor activity. Pharm Nutr. 2017;5(4):141–147. doi:10.1016/j.phanu.2017.10.003.
45. Heidari R, Behnamrad S, Khodami Z, Ommati MM, Azarpira N, Vazin A. The nephroprotective properties of taurine in colistin-treated mice is mediated through the regulation of mitochondrial function and mitigation of oxidative stress. Biomed Pharmacother. 2019;109:103–111. doi:10.1016/j.biopha.2018.10.093.
46. Heidari R. Brain mitochondria as potential therapeutic targets for managing hepatic encephalopathy. Life Sci. 2019;218:65–80. doi:10.1016/j.lfs.2018.12.030.
47. Butterworth RF. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab Brain Dis. 2002;17(4):221–227. doi:10.1023/a:1021989230535.
48. Heidari R, Niknahad H. The role and study of mitochondrial impairment and oxidative stress in cholestasis. In: Vinken M, editor. Experimental Cholestasis Research. New York: Springer New York; 2019:117–132.
49. Hadjihambi A, Jalan R. Hepatic encephalopathy: new treatments. Clin Liver Dis. 2015;5(5):109. doi:10.1002/cld.468.
50. Toris GT, Bikis CN, Tsourouflis GS, Theocharis SE. Hepatic encephalopathy: an updated approach from pathogenesis to treatment. Med Sci Monit. 2011;17(2):RA53. doi:10.12659/msm.881387.
51. Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24(12):1504–1534. doi:10.1002/adma.201104763.
52. Farjadian F, Azadi S, Mohammadi-Samani S, Ashrafi H, Azadi A. A novel approach to the application of hexagonal mesoporous silica in solid-phase extraction of drugs. Heliyon. 2018;4(11):e00930. doi:10.1016/j.heliyon.2018.e00930.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.