Back to Journals » Infection and Drug Resistance » Volume 15
In vitro and in vivo Antimicrobial Activities of Ceftazidime/Avibactam Alone or in Combination with Aztreonam Against Carbapenem-Resistant Enterobacterales
Authors Lu G, Tang H, Xia Z, Yang W, Xu H, Liu Z, Ni S, Wang Z , Shen J
Received 5 August 2022
Accepted for publication 23 November 2022
Published 5 December 2022 Volume 2022:15 Pages 7107—7116
DOI https://doi.org/10.2147/IDR.S385240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Guoping Lu,1,2 Hao Tang,3 Zhaoxin Xia,1 Wensu Yang,1 Huaming Xu,1 Zhen Liu,1 Shenwang Ni,1 Zhaofei Wang,1 Jilu Shen1
1Department of Laboratory Medicine, The First Affiliated Hospital of Anhui Medical University; Anhui Public Health Clinical Center, Hefei, People’s Republic of China; 2Department of Laboratory Medicine, The Affiliated Fuyang Hospital of Anhui Medical University, Fuyang, People’s Republic of China; 3Department of Laboratory Medicine, The Second Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
Correspondence: Jilu Shen, The First Affiliated Hospital of Anhui Medical University; Anhui Public Health Clinical Center, Hefei, People’s Republic of China, Email [email protected]
Introduction: To examine the in vitro and in vivo antimicrobial activities of ceftazidime/avibactam (CZA) alone or in combination with aztreonam (ATM) against KPC-, NDM-, IMP-, KPC+IMP-, KPC+NDM-producing strains.
Methods: A total of 67 clinical non-repetitive carbapenem-resistant Enterobacterales (CRE) strains were selected for the microdilution broth method that was performed to analyze the minimal inhibitory concentration (MIC) and the combination antimicrobial susceptibility test using checkerboard titration method. The fractional inhibitory concentration (FIC) was calculated to determine the antimicrobial effect. The time-kill assays and the mouse infection model were used to study the bactericidal effect and therapeutic effect of CZA alone or in combination with ATM.
Results: The CZA minimal inhibitory concentration (MIC) values of CZA revealed that 29 KPC-producing strains and 1 OXA-producing strain were ≤ 4μg/mL. The CZA MIC values of 37 metal-β-lactamase (MBLs)-producing strains such as NDM-, IMP-, KPC+IMP-, KPC+NDM-producing strains were ≥ 128μg/mL, after combining with ATM, the FIC values were all below 0.51. The time-kill assays revealed that CZA at various concentrations of 2, 4 and 8 MIC showed significant bactericidal efficiency to the KPC-producing strains. For NDM-, IMP-producing strains, no colony growth was detected after 8 hours of incubation with CZA in combination with ATM. Six percent of the mice in the treatment group and 58% of the mice in the infection group died within 3 days.
Conclusion: Our in vitro results showed that CZA had a good antimicrobial effect on the KPC-producing and OXA-producing strains. CZA combined with ATM showed synergistic bacteriostatic or bactericidal activity against NDM-, IMP-, KPC+IMP-, KPC+NDM-producing strains. The combination of CZA and ATM reduced mortality and prolonged lifespan of mice infected with NDM-, IMP-, KPC+IMP-, and KPC+NDM-producing strains, which provides fundamental knowledge for improving treatment strategies and initializing clinical trials.
Keywords: combination therapy, FIC, ceftazidime/avibactam, carbapenem-resistant Enterobacterales
With the clinical applications of antibiotics, glucocorticoids, antitumor drugs, and trauma surgery, the prevalence of carbapenem-resistant Enterobacterales (CRE) strains has been reported globally.1 Except for tigecycline, polymyxin, and ceftazidime/avibactam (CZA), other antibiotics showed very limited antimicrobial efficacy against CRE strains. Predominant spreading of carbapenemase-encoding plasmids, accompanied by the emergence of phenotypically diverse derivatives, may contribute to the widespread of CRE.2 The high morbidity and mortality rates of CRE infections pose serious clinical challenges due to limited treatment options. In addition, the emergence and continued spread of multidrug-resistant bacteria has become a major global public health threat. Another solution is to recombine failed antibiotics,3 which has been proven not only cost-effective but also clinically effective.4 In this paper, the microdilution broth method was performed to analyze the minimal inhibitory concentration (MIC), the combination antimicrobial susceptibility test using checkerboard titration method. The time-kill assays and drug treatment strain infection animal test method were used to evaluate the in vitro bactericidal effect and in vivo therapeutic effect of single drug or combination drugs, which provided a reliable basis for clinical drug use.
Materials and Methods
Bacterial Strains
Sixty-seven CRE strains were isolated from clinical samples, among which there were 39 strains of Klebsiella pneumoniae, 11 strains of Escherichia coli, 13 strains of Enterobacter cloacae, 2 strains of Citrobacter freundii, 1 strain of Klebsiella oxytoca, and 1 strain of Serratia marcescens. By bacterial enzyme type classification, there were 29 KPC-producing strains, 1 OXA-producing strain, 32 NDM-producing strains, 1 IMP-producing strain, 3 KPC+NDM-producing strains and 1 KPC+IMP-producing strains. The clinical samples were not specifically isolated for this research and they were part of the routine hospital laboratory procedure. This research will not affect the health and privacy of the patient. All strains were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). The control strains used in this study were Escherichia coli ATCC25922, ATCC BAA-1705, ATCC2146, and ATCC2524.
Reagent Consumables
Cation-adjusted Mueller–Hinton broth was purchased from British OXOID Company for antibiotic susceptibility test. Antibacterial paper was also purchased from British OXOID company. Ceftazidime was purchased from Beijing Solarbio Science & Technology Co., Ltd. Avibactam and aztreonam (ATM) were purchased from Shanghai yuanye Bio-Technology Co., Ltd. The consumables corresponding to PCR amplification test were from Shanghai Shenggong Biological Co., Ltd. Carbapenemase detection kit (NG-Test® CARBA 5) was purchased from Changsha Zhongsheng Zhongjie Biotechnology Co., Ltd.
Detection of Carbapenemase
All strains were analyzed by carbapenemase phenotypic screening test (modified carbapenem inactivation methods, mCIM and EDTA-modified carbapenem inactivation methods, eCIM) according to CLSI. Then the rapid detection method of carbapenemase (colloidal gold immunochromatography) was used to detect the enzyme type, and finally the gene type of carbapenemases was analyzed by PCR amplification and DNA sequencing. Metal-β-lactamase positive: ≥5-mm increase in zone diameter for eCIM vs zone diameter for mCIM when mCIM test is positive. Metal-β-lactamase negative: ≤4-mm increase in zone diameter for eCIM vs zone diameter for mCIM when mCIM test is positive. The rapid detection method of carbapenemase (colloidal gold immunochromatography) has been preliminarily used in clinical practice. Carbapenemase detection kit (NG-Test® CARBA 5) can be used to detect five enzyme types: KPC, OXA, VIM, IMP and NDM. Its methodological principle is colloidal gold immunochromatography. Testing steps: The sample and extraction buffer were thoroughly mixed in an Eppendorf tube and left at room temperature for 10 minutes, followed by loading onto the detection strip. After incubating at room temperature for 15 minutes, the result were recorded. A red line in the C-line region of the detection strip and one or more red lines in the detection regions of KPC, OXA, VIM, IMP and NDM were interpreted as positive results, and the specimen contained one or more carbapenemases.
Checkerboard Titration
The MICs of CZA and ATM in 67 CRE strains were determined by microbroth dilution method, for which the quality control strain of drug susceptibility test was Escherichia coli ATCC 25922. The tests and results were interpreted in accordance with the norms and breakpoints recommended by the Clinical & Laboratory Standards Institute (CLSI) guideline (2020 M100s-30th).
The concentration range of CZA was 0.25/4-128/4 µg/mL (the concentration of avibactam was fixed at 4 µg/mL), and the concentration of ATM was 0.06–128 µg/mL. The checkerboard titration method was the same described previously.5
The fractional inhibitory concentration (FIC) was used for judging the results of the combined drug susceptibility test. FIC = MIC of drug A in combination/MIC of drug A alone + MIC of drug B in combination/MIC drug B alone. FIC ≤0.5 was considered as synergistic effect, 0.5≤1 was considered as additive effect, 1<FIC≤2 was considered as irrelevant effect, FIC >2 was considered as antagonistic effect.6
Time-Kill Assay
According to the MICs of CZA against KPC-producing bacteria, the bactericidal effect of CZA at 0.5, 1, 2, 4 and 8 MIC was studied by time-kill assay. According to the drug susceptibility test results of CZA combined with ATM and the method recommended in the literature,6 the following stains were randomly selected for the synergistic bactericidal effect: 1NDM-producing strain of Escherichia coli, 1 IMP-producing strain of Enterobacter cloacae. Briefly, 2–3 colonies were added to 2 mL of Mueller–Hinton broth and cultured overnight at 35°C for 18–20 h. After adjusted the bacteria solution to a McFarland’s turbidity of 0.5, the bacteria solution was diluted for 10,000 times by Mueller–Hinton broth (T-2 count was about 104 CFU/mL) and cultured in a shaker for 2 h (T+0, about 105 CFU/mL). Mueller–Hinton broth containing 1×105 CFU/mL of bacteria was mixed with single or combined antimicrobials and incubated overnight at 35°C with continuous shaking. The same broth without antibiotics was used as a growth control. Broth samples were serially 10-fold diluted at 0, 2, 4, 6, 8, 10, and 24 hours and plated on Mueller–Hinton plates with 100µL of each diluted sample in triplicate, respectively. After overnight incubation at 35°C, colonies were counted and averaged. The experiment was repeated three times, the count of bacterial solution per mL was expressed as Nt, and the data were converted to Log10Nt. GraphPad Prism 5 software was used to analyze the data and plot.
The Effect of CZA Combined with ATM Against Metalloenzyme-Producing CRE Strains in vivo
Six-week-old male C57 mice were purchased from Henan Skbex Biotechnology Co., Ltd. Animal experiments were performed following the Animal Ethics Committee of Anhui Medical University and national guidelines and regulations. The institution granting the approval is Anhui Medical University. Mice were intraperitoneally infected with 5×107 CFU of the bacteria. Ten metal-β-lactamases (MBLs)-producing strains of NDM-, IMP-, KPC+IMP-, KPC+NDM-producing strains were selected. Ten mice injected with each strain of MBLs-producing strain were used as infection group and treatment group, 5 mice in each group. Four hours after infection, PBS (infection group) or CZA combined with ATM (treatment group, ceftazidime/avibactam dosage was 0.375 mg/g body weight in 0.1 mL PBS, ATM dosage was 0.1875 mg/g body weight in 0.1 mL PBS) were subcutaneously injected every 8 h for 10 days. Mice survival was measured at designed time points to assess the efficacy of CZA combined with ATM.
Results
Susceptibility Tests for CRE Strains
The CZA minimal inhibitory concentration (MIC) values of CZA revealed that 29 KPC-producing strains and 1 OXA-producing strain were ≤4µg/mL. However, the CZA MIC values of 37 metal-β-lactamases (MBLs)-producing strains of NDM-, IMP-, KPC+IMP-, KPC+NDM-producing strains were ≥128µg/mL, indicating these strains were resistant to CZA treatment (Table 1).
 |
Table 1 Modal MICs of CZA and ATM |
Combined Drug Susceptibility
Thirty-one CZA and ATM-resistant strains were selected for combined drug susceptibility test. Our results showed that the FIC values of CZA combined with ATM were all below 0.51. After the combination treatment, the MIC values were reduced to their respective sensitive ranges, which showed a good synergistic effect (Table 2).
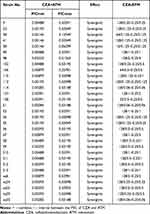 |
Table 2 The FIC Value and Ratio Range of CZA Combined with ATM |
Time-Kill Test
Our time-kill test showed that the KPC-producing strains rebounded at 10 hours after 0.5 and 1MIC of CZA treatment. When treated with 2, 4 and 8 MIC CZA, the number of bacteria declined steadily within 2 hours, and no colonies were detected after 10 hours, showing significant bactericidal efficacy against KPC-producing strains. Besides, with 2, 4 and 8 MIC CZA treatment, no colony growth was detected for most strains after 24 hours of culture (Figure 1). The combination of CZA and ATM showed significant bactericidal efficacy against NDM-, IMP-producing strains. No colony growth was detected after 8 hours of incubation (Figures 2 and 3).
 |
Figure 1 Bactericidal curve plots of ceftazidime/avibactam at various concentrations against KPC-producing Klebsiella pneumoniae No. 18. |
 |
Figure 2 Bactericidal curve plots of ceftazidime/avibactam combined with aztreonam against NDM-producing Escherichia coil No. 90. |
 |
Figure 3 Bactericidal curve plots of ceftazidime/avibactam combined with aztreonam against IMP-producing Enterobacter cloacae No. 58. |
In vivo Therapeutic Efficacy of Ceftazidime–Avibactam Combined with Aztreonam Against CRE Strain Infection
Mice were infected with 5×107 CFU of bacteria and treated with PBS or CZA combined with ATM for 10 days. Six percent (3/50) of the mice in the treatment group and 58% (29/50) of the mice in the infection group died within 3 days. And 26% (13/50) of the mice in the treatment group and 74% (37/50) of the mice in the infection group died within 10 days. After 15 days, 26% (13/50) of the mice in the infected group and 60% (30/50) of the mice in the treated group were still alive and euthanized (Figure 4).
Discussion
At present, the infections caused by carbapenemase-producing Enterobacterales are very severe and have a high mortality rate, making CRE a worldwide public health threat. Since the first case of KPC-positive Klebsiella pneumoniae was discovered in 1996, outbreaks or epidemics of CRE strains with different resistant enzyme types have been reported around the world. CRE is not only spread among the same strains but also among different strains. The first case of Klebsiella pneumoniae carbapenemase KPC-2 transfer from Klebsiella pneumoniae to Escherichia coli was reported in Europe in 2011. The KPC-positive plasmids were identical in both species, indicating horizontal plasmid transfer.7 The environmental contaminating CRE strains has also brought serious challenges through environmental transmission. The environmental spread of carbapenemase-producing Enterobacterales in Swiss rivers has been reported.8
There are three main mechanisms of CRE resistance to carbapenems: carbapenemase production, overexpression of the efflux pump, and membrane porin mutation. Among them, the production of carbapenemase is the major resistance mechanism. Three groups of carbapenemases are responsible for carbapenem resistance: KPC (Ambler class A), MBLs (metal-β-lactamases, Ambler class B) including NDM, VIM, IMP etc, and OXA (Ambler class D) such as OXA-48. All of these enzymes are plasmid-mediated, which facilitate the horizontal transfer and global spread of the strains.9
KPC-2, NDM, and OXA-48-like carbaenase strains were the predominant CRE clinical isolates in China. In adult and pediatric isolated Enterobacterales, the most prevalent carbapenemase genes are blaKPC-2 and blaNDM.10 KPC-producing Enterobacterales are mainly endemic in the United States, Colombia, Argentina, Greece, and Italy. While NDM-producing Enterobacterales are mainly common in India, and OXA-48- producing Enterobacterales are prevalent in Turkey, Malta, the Middle East, and North Africa. Additionally, blaNDM and blaOXA-48-like co-expressed isolates were found in 28% of the CRKP isolates in Italy.11 In Greece, the endemic strain is CRKP, and NDM-positive strains ranked secondly and continued to rise.12
Most CRE strains are resistant to cephalosporins, carbapenems, aminoglycosides, and fluoroquinolones, but are sensitive to colistin and tigecycline, which are important last-line antibiotics for the treatment of MDR CRKP infections. However, the application of these antibiotics is associated with the emergence of drug resistance during treatment, limiting treatment options against MDR CRKP infections. Tigecycline is also considered the last resort for the treatment of CRKP infections, but increasing resistance has been reported with the increase clinical application of tigecycline.13 Tigecycline can be used for intraperitoneal and soft tissue infections, but it has nephrotoxicity and lacks in vitro synergy.14 Previous study reported that CZA had a higher success rate and lower mortality than colistin.15
Launched in China in September 2019, CZA is a novel anti-serine β-lactamase (SBLs) drug with good activity against Gram-negative bacteria, especially Enterobacteriaceae. The high resistance rate of CZA is associated with carbapenem resistance. β-Lactamase-associated mutation is the major mechanism of CZA resistance.16,17 For the treatment of CZA-resistant strains, other effective antimicrobial agents or the combination of CZA with other antimicrobial agents should be considered.18 NDM-producing Enterobacteriaceae also contains serine β-lactamases, and one potential treatment option is to combine ATM with CZA. This combination introduces a β-lactamase inhibitor (AVI) that “protects” ATM from the hydrolysis of broad-spectrum β-lactamases and AmpCs that frequently accompanied by NDM-producing strains. The bactericidal effect is likely achieved through the action of ATM, a monomeric substance that is not hydrolyzed by NDM.
In this study, CZA showed good in vitro bactericidal efficacy on KPC-producing strains and OXA-producing strain, which had MIC values less than 4 µg/mL, indicating these strains were sensitive. However, the CZA MIC values of 37 metal-β-lactamases (MBLs)-producing strains of NDM-, IMP-, KPC+IMP-, KPC+NDM-producing strains were ≥128µg/mL, indicating drug resistance. After the combination of the two drugs, the MIC values could decrease to within their respective sensitive ranges, the FIC values were all below 0.51, suggesting a good synergistic effect.19–22
Our time-kill assays showed rebound growth of KPC-producing strains at 10 hours after 0.5 and 1MIC of CZA treatment. At 2 MIC of CZA, the strains steadily decreased in the first 2 hours of treatment. No colonies were detected after 10 hours of treatment. For 4 and 8 MIC, no colonies were detected within 10 hours, indicating good bactericidal efficacy of CZA with proper dosages against KPC-producing strains. For NDM-, IMP-producing bacteria, no colony growth was detected after 8 hours incubation with the combination of CZA and ATM, showing good bactericidal efficacy of the combination. Therefore, CZA combined with ATM regimen can be used for CRE strains of NDM-, IMP-producing in clinical practice.
Six percent (3/50) of the mice in the treatment group and 58% (29/50) of the mice in the infection group died within 3 days. And, 26% (13/50) of the mice in the treatment group and 74% (37/50) of the mice in the infection group died within 10 days. After 15 days, 26% (13/50) of the mice in the infected group and 60% (30/50) of the mice in the treated group were still alive. These results showed that CZA combined with ATM could prolong the life span and reduce the mortality of mice with NDM-, IMP-, KPC+IMP-, or KPC+NDM-producing strains infection.21
In summary, CZA has a good bactericidal effect on KPC and OXA-producing strains, and has a good synergistic bactericidal effect on NDM-, IMP-, KPC+IMP-, and KPC+NDM-producing bacteria when combined with ATM. No adverse events related to CZA were observed in the study population.23–25 However, CZA is not effective as a rescue treatment for MDR-GNB infection.26 Therefore in clinical practice, CZA or CZA combined with ATM should be used as an early treatment for CRE infection.27
Funding
This work was funded through a grant from Anhui Provincial Department of Education for University cooperative research and public health collaborative innovation project in Anhui Province in 2020 (Grant No. GXXT-2020-016) and a grant from Anhui Provincial Health Commission for key scientific research projects in 2021 (Grant No.AHWJ2021a011) and major natural science research projects of colleges and universities in Anhui Province in 2021 (Grant No. KJ2021ZD0032).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Di. 2019;38(12):2275–2281. doi:10.1007/s10096-019-03673-1
2. Seo H, Lee SC, Chung H, et al. Clinical and microbiological analysis of risk factors for mortality in patients with carbapenem-resistant Enterobacteriaceae bacteremia. Int J Antimicrob Agents. 2020;56(4):106126. doi:10.1016/j.ijantimicag.2020.106126
3. Sun J, Wang B, Warden AR, et al. Overcoming multidrug-resistance in bacteria with a two-step process to repurpose and recombine established drugs. Anal Chem. 2019;91(21):13562–13569. doi:10.1021/acs.analchem.9b02690
4. Seok H, Choi JY, Wi YM, et al. Fosfomycin resistance in Escherichia coli isolates from South Korea and in vitro activity of fosfomycin alone and in combination with other antibiotics. Antibiotics. 2020;9(3):112. doi:10.3390/antibiotics9030112
5. Isber C, Stockman DL, Daoud Z. Quadruple-checkerboard: a modification of the three-dimensional checkerboard for studying drug combinations. J Vis Exp. 2021;173:e62311.
6. Zhang W, Guo Y, Li J, et al. In vitro and in vivo bactericidal activity of ceftazidime-avibactam against Carbapenemase–producing Klebsiella pneumoniae. Antimicrob Resist Infect Control. 2018;7(1):142. doi:10.1186/s13756-018-0435-9
7. Richter SN, Frasson I, Bergo C, et al. Transfer of KPC-2 Carbapenemase from Klebsiella pneumoniae to Escherichia coli in a patient: first case in Europe. J Clin Microbiol. 2011;49(5):2040–2042. doi:10.1128/JCM.00133-11
8. Bleichenbacher S, Stevens M, Zurfluh K, et al. Environmental dissemination of carbapenemase-producing Enterobacteriaceae in rivers in Switzerland. Environ Pollut. 2020;265(Pt B):115081. doi:10.1016/j.envpol.2020.115081
9. Suay-Garcia B, Perez-Gracia MT. Present and future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics. 2019;8(3):122. doi:10.3390/antibiotics8030122
10. Han R, Shi Q, Wu S, et al. Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. doi:10.3389/fcimb.2020.00314
11. Veeraraghavan B, Shankar C, Karunasree S, et al. Carbapenem resistant Klebsiella pneumoniae isolated from bloodstream infection: Indian experience. Pathog Glob Health. 2017;111(5):240–246. doi:10.1080/20477724.2017.1340128
12. Galani I, Karaiskos I, Karantani I, et al. Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014 to 2016. Euro Surveill. 2018;23(31). doi:10.2807/1560-7917.ES.2018.23.30.1700775
13. Maraki S, Mavromanolaki VE, Moraitis P, et al. Ceftazidime-avibactam, meropenen-vaborbactam, and imipenem-relebactam in combination with aztreonam against multidrug-resistant, metallo-beta-lactamase-producing Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 2021;40(8):1755–1759. doi:10.1007/s10096-021-04197-3
14. Meini S, Viaggi B, Tascini C, Mono V. combo regimens with novel beta-lactam/beta-lactamase inhibitor combinations for the treatment of infections due to carbapenemase-producing Enterobacterales: insights from the literature. Infection. 2021;49(3):411–421. doi:10.1007/s15010-021-01577-x
15. Hakeam HA, Alsahli H, Albabtain L, et al. Effectiveness of ceftazidime-avibactam versus colistin in treating carbapenem-resistant Enterobacteriaceae bacteremia. Int J Infect Dis. 2021;109:1–7. doi:10.1016/j.ijid.2021.05.079
16. Di Pilato V, Aiezza N, Viaggi V, et al. KPC-53, a KPC-3 variant of clinical origin associated with reduced susceptibility to ceftazidime-avibactam. Antimicrob Agents Chemother. 2020;65(1):e01429. doi:10.1128/AAC.01429-20
17. Li D, Liao W, Huang H, et al. Emergence of hypervirulent ceftazidime/avibactam-resistant Klebsiella pneumoniae isolates in a Chinese tertiary hospital. Infect Drug Resist. 2020;13(2673–2680). doi:10.2147/IDR.S257477
18. Wang Y, Wang J, Wang R, et al. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist. 2020;22:18–27. doi:10.1016/j.jgar.2019.12.009
19. Wenzler E, Deraedt MF, Harrington AT, et al. Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-β-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis. 2017;88(4):352–354. doi:10.1016/j.diagmicrobio.2017.05.009
20. Yao H, Liu J, Jiang X, et al. Analysis of the clinical effect of combined drug susceptibility to guide medication for carbapenem-resistant Klebsiella pneumoniae patients based on the Kirby-Bauer disk diffusion method. Infect Drug Resist. 2021;14:79–87. doi:10.2147/IDR.S282386
21. Falcone M, Menichetti F, Cattaneo D, et al. Pragmatic options for dose optimization of ceftazidime/avibactam with aztreonam in complex patients. J Antimicrob Chemother. 2021;76(4):1025–1031. doi:10.1093/jac/dkaa549
22. Yasmin M, Fouts DE, Jacobs MR, et al. Monitoring ceftazidime-avibactam and aztreonam concentrations in the treatment of a bloodstream infection caused by a multidrug-resistant Enterobacter sp. carrying both Klebsiella pneumoniae Carbapenemase–4 and New Delhi Metallo-β-Lactamase–1. Clin Infect Dis. 2020;71(4):1095–1098. doi:10.1093/cid/ciz1155
23. Vena A, Giacobbe DR, Castaldo N, et al. Clinical experience with ceftazidime-avibactam for the treatment of infections due to multidrug-resistant gram-negative bacteria other than carbapenem-resistant enterobacterales. Antibiotics. 2020;9(2):71. doi:10.3390/antibiotics9020071
24. Cultrera R, Libanore M, Barozzi A, et al. Ceftolozane/tazobactam and ceftazidime/avibactam for multidrug-resistant gram-negative infections in immunocompetent patients: a single-center retrospective study. Antibiotics. 2020;9(10):640. doi:10.3390/antibiotics9100640
25. Nwankwo L, Butt Z, Schelenz S. Experience of Ceftazidime/avibactam in a UK tertiary cardiopulmonary specialist center. Expert Rev Anti Infect Ther. 2021;19(1):101–108. doi:10.1080/14787210.2020.1810568
26. Kuang H, Zhong C, Wang Y, et al. Clinical characteristics and outcomes of patients with multidrug-resistant Gram-negative bacterial infections treated with ceftazidime/avibactam. J Glob Antimicrob Resist. 2020;23:404–407. doi:10.1016/j.jgar.2020.10.023
27. Castanheira M, Mills JC, Costello SE, et al. Ceftazidime-avibactam activity tested against Enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of beta-lactamase-producing strains. Antimicrob Agents Chemother. 2015;59(6):3509–3517. doi:10.1128/AAC.00163-15
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

