Back to Journals » Infection and Drug Resistance » Volume 14
In vitro and in vivo Activity of Combinations of Polymyxin B with Other Antimicrobials Against Carbapenem-Resistant Acinetobacter baumannii
Authors Zhang H, Zhu Y, Yang N , Kong Q, Zheng Y, Lv N, Chen H, Yue C, Liu Y , Li J, Ye Y
Received 14 August 2021
Accepted for publication 28 October 2021
Published 5 November 2021 Volume 2021:14 Pages 4657—4666
DOI https://doi.org/10.2147/IDR.S334200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Hui Zhang, 1 Yunzhu Zhu, 1 Ning Yang, 1 Qinxiang Kong, 2 Yahong Zheng, 1 Na Lv, 1 Haoran Chen, 1 Chengcheng Yue, 1 Yanyan Liu, 1, 3, 4 Jiabin Li, 1– 4 Ying Ye 1
1Department of Infectious Disease, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 2Department of Infectious Diseases, The Chaohu Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 3Anhui Center for Surveillance of Bacterial Resistance, Hefei, Anhui, People’s Republic of China; 4Institute of Bacterial Resistance, Anhui Medical University, Hefei, Anhui, People’s Republic of China
Correspondence: Ying Ye; Jiabin Li
Department of Infectious Disease, The First Affiliated Hospital of Anhui Medical University, Jixi Road no. 218, Hefei, Anhui, People’s Republic of China
Tel +86-551-62922713
Fax +86-551-62922281
Email [email protected]; [email protected]
Purpose: To study the in vitro and in vivo antibacterial activities of polymyxin B (PB) and other five antimicrobial agents, including imipenem (IMP), meropenem (MEM), tigecycline (TGC), sulbactam (SUL), and rifampicin (RIF), alone or in combination against carbapenem-resistant Acinetobacter baumannii (CRAB).
Methods: Microbroth dilution method was used to determine the minimum inhibitory concentration (MIC) of ten strains of CRAB against six antibacterial drugs, and the checkerboard method was used to determine the fractional inhibitory concentration index (FICI). A mouse pneumonia model was established by intranasal instillation of Ab5075 to evaluate the antibacterial activity in vivo.
Results: The resistance rate of ten CRAB strains to IMP, MEM, and SUL was 100%, that to PB and TGC was 0%, and that to RIF was 20%. When PB was used in combination with the other five antibiotics in vitro, it mainly showed synergistic and additive effects on CRAB. The synergistic effect of PB and RIF was maximal, followed by MEM and IMP but was weak with SUL and TGC. In vivo, compared to the model group (untreated with antibiotics), treatment group (six antibiotics alone and PB combined with the other five antibiotics) reduced the bacterial load in the lung tissue and the serum inflammatory factors (IL-1β, IL-6, and TNF-α). The bacterial load and the inflammatory factors of the combined group decreased significantly than that of the single group (P< 0.05). The IL-6 and TNF-α values of the PB combined with the RIF group were significantly lower than the two drugs used individually.
Conclusion: The combination of PB and IMP, MEM, and RIF exerted robust in vitro synergistic effects on CRAB isolates. The combination of PB and the other five antimicrobial agents had a better effect in the mouse pneumonia model than single agent, while the combination of PB and RIF had the best effect.
Keywords: Acinetobacter baumannii, carbapenem-resistant, pneumonia infection model, polymyxin B, combination treatment
Corrigendum for this paper has been published
Introduction
Acinetobacter baumannii (A. baumannii) is one of the most important pathogens in the 21st century. It is a major pathogen of nosocomial infection outbreak in the intensive care unit (ICU), with a high mortality rate. World Health Organization (WHO) classifies carbapenem-resistant Acinetobacter baumannii (CRAB) as one of the key pathogens on the global list of antibiotic-resistant bacteria to guide the research and development of new antibiotics.1 It can easily develop resistance to various antibiotics, which is related to increased risk of clinical treatment failure, prolonged hospital stay, and high healthcare costs.2 Approximately 500,000 individuals die from drug-resistant infections worldwide every year. Some studies have estimated that by 2050, antibiotic resistance will cause > 10 million deaths and that the global annual related healthcare costs would exceed 300 billion.3 Zhou et al reported an outbreak of A. baumannii resistant to imipenem (IMP) in their hospital.4 The development of multidrug and pandrug resistance of A. baumannii and the emergence of global strains is a major global concern. A. baumannii is the most famous “superbug” in China, causing ventilator-associated pneumonia (VAP), bloodstream infection, abdominal infection, central nervous system infection, urinary system infection, and skin and soft tissue infection.5,6
Currently, the treatment of CRAB is rather challenging. Previously, carbapenem antibiotics were the first choice of treatment for A. baumannii infection. Both meropenem (MEM) and IMP were approved for VAP treatment.7 However, the increased incidence of multidrug-resistant (MDR) strains has led to the use of non-traditional antibiotics.8,9 Polymyxin B (PB) and tigecycline (TGC) are the last lines of defense for CRAB treatment.10,11 The US Food and Drug Administration (FDA) has approved the use of TGC to treat complex skin infections, intra-abdominal infections, and community-acquired pneumonia caused by MDR A. baumannii (MDRAB), including CRAB.11 However, the clinical application of these drugs is limited due to the lack of large-scale clinical studies, the high cost of TGC, and the potential nephrotoxic effects of polymyxin. Sulbactam (SUL) is a serine β-lactamase inhibitor that does not inhibit any carbapenemases but shows inherent activity against A. baumannii because of its selective affinity for penicillin-binding proteins.12 Rifampicin (RIF) is a commonly used drug with antibacterial effects on both Gram-positive and Gram-negative bacteria. However, it should not be used alone because a study in a mouse model of A. baumannii-caused pneumonia showed that monotherapy leads to antibiotic resistance after 24h.13,14 Thus, adding another antibiotic to RIF is necessary to prevent the development of drug resistance.14 Therefore, some prohibited and old drugs should be re-evaluated, and novel combinations of existing drugs may be effective in the treatment of CRAB.
In 2013, Harris et al successfully established a mouse pneumonia model using a clinical strain of highly virulent A. baumannii.15 Several recent studies have evaluated the in vivo effect of combination therapy on A. baumannii - induced pneumonia using various methods.16,17 Herein, we established the pneumonia infection model in immunodeficient mice by intranasal instillation of highly virulent strain Ab5075 and studied the in vivo efficacy of PB combined with IMP, MEM, TGC, SUL, and RIF in the mouse model of A. baumannii pneumonia.
Materials and Methods
Strains
A total of nine CRAB strains and one highly virulent strain Ab5075 were isolated from clinical patients. In order to represent the strains from various clinical sources, the nine CRAB strains were derived from different body parts (including respiratory tract, blood, cerebrospinal fluid, pleural and ascites, and secretions) and various departments (including intensive care unit (ICU) and general wards). Ab5075 was a kind gift from Professor Yu Yunsong of Sir Run Shaw Hospital of Zhejiang University, Zhejiang Province, China. Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were used as control strains, stored at the Anhui Center for Surveillance of Bacterial Resistance.
Experimental Drugs
IMP, MEM, and RIF were purchased from Beijing Solarbio Technology Co., Ltd (Beijing, China). TGC and SUL were purchased from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China), and PB was procured from Sigma-Aldrich (Munich, Germany). All antibiotics except RIF were prepared in sterile water and diluted to final concentrations with cation-adjusted Mueller-Hinton broth (CaMHB). RIF was reconstituted in dimethyl sulfoxide (DMSO) and serially diluted to the desired concentration in sterile water before being diluted to the final concentration with CaMHB. The final concentration in DMSO (<1%, vol/vol) did not affect the bacterial growth.
Experimental Animals
All specific pathogen-free BALB/c female mice (weight 16–18 g, age 6–8 weeks) were bred at 20–25 °C and humidity 50±5%. All experiments involving mice were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Anhui Medical University (No. LLSC20200224).
Antibiotic Susceptibility Tests
According to the microbroth dilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) in 2019,18 the minimum inhibitory concentration (MIC) of ten strains of A. baumannii was determined against six antibacterial drugs. According to the multiple dilution method, a series of antimicrobial solutions with decreasing concentration gradient was obtained in each row of the 96-well plate. A volume of 100ul/well bacterial suspension with 5×105 colony-forming unit (CFU)/mL prepared in CaMHB was inoculated in 96-well plates. Also, positive control (only bacterial liquid) and negative control (only CaMHB) were added. After the sample was added, the plate was incubated at 37 °C for 16–20 h. Due to the lack of a standard for the MIC breakpoint of TGC against Acinetobacter on CLSI, we referred to the FDA standard for TGC against E. coli.19
Checkerboard Assays
A microdilution method associated with checkerboard was used to detect the in vitro antibacterial activity of PB combined with the other five antibacterial drugs. The final concentration range of each antimicrobial drug varied based on the MIC of each strain. The concentration of the final bacterial suspension was adjusted to 5×105 CFU/mL in a 100 uL final volume. The assay was performed in triplicate for each isolate. The fractional inhibitory concentrations index (FICI) was used to evaluate the synergistic effect of two antibacterial drugs. FICI=MICA drug combination/MICA drug single use+MICB drug combination/MICB drug single use. FICI≤0.5 is defined as synergy; 0.5<FICI≤1 is defined as an addition; 1<FICI≤4 is defined as irrelevant; FICI>4 is defined as antagonism.20
Mouse Model of CRAB Pneumonia
Because A. baumannii is a conditional pathogen, most of the strains have weak virulence. Therefore, we selected the highly virulent strain Ab 5075 to establish a stable pneumonia model through intranasal infection of mice. After 1 week of adaptation, mice with transient neutropenia were induced by intraperitoneal injection of cyclophosphamide (300 mg/kg body weight) in a volume of 0.2 mL 4 days before infection with A. baumannii. The intraperitoneal injection of chloral hydrate was administered in a volume of 0.08–0.1 mL at a concentration of 100 mg/mL. After deep anesthesia, the mice were inoculated intranasally with 5×107 CFU/mL Ab5075 bacterial suspension of 1.2 mL/kg.21 After standing vertically for 4 min, the mice were maintained at a 30° recumbent position until they regained consciousness.
Study Groups and Treatment Protocol
The mice were divided into three groups. The control group was not inoculated with bacteria, the model group was inoculated with bacteria and not treated, and the treatment group was treated after the inoculation of bacteria. The treatment group was divided into 11 subgroups, with 6 mice in each subgroup: PB, IMP, MEM, TGC, SUL, RIF, PB + IMP, PB + MEM, PB + TGC, PB + SUL group, PB + RIF. Mice were administered 4 h after the infection, and the treatment groups were administered intraperitoneal injections of 0.2 mL of the antibacterial drugs. The specific dose and frequency of each drug were as follows: IMP, 40 mg/kg, Q8h; MEM, 20 mg/kg, Q8h; TGC, 5 mg/kg, Q12h; SUL, 40 mg/kg, Q8h; RIF, 10 mg/kg, QD; PB, 1.5 m/kg, Q8h. These doses were selected according to previous pharmacokinetic and pharmacodynamic data from experimental models.22,23 Groups A and group B were injected 0.2 mL of sterile normal saline subcutaneously at the same time.
Observation Indicators
The body weight changes and clinical scores of mice were recorded. The clinical scores of each mouse were 0 (normal, active, healthy), 1.0 (slightly ill and slightly wrinkled fur), 2.0 (sick, wrinkled fur, slow movement, hunchback), 3.0 (very uncomfortable, wrinkled fur, very slow movement, hunchback, and close eyes), 4.0 (dying), and 5.0 (death).24
Pathological examination of the lung revealed that the mice in the model group were autopsied at 4, 24 and 48 h after inoculation, and the mice in the treatment group were autopsied after 48 h of treatment. The whole left lung of the mouse was excised aseptically, and the pathological diagnosis was pneumonia. After the sterile lung specimens were fixed with 10% formaldehyde for 24 h, the left lung tissue was dehydrated, transparent, soaked in wax, and embedded using conventional methods. Then, 5-mm-thick sections were stained with hematoxylin-eosin and observed under a light microscope.
Lung histopathology under a light microscope: the general observation of lung tissue (color, mass, volume, bleeding point, exudate), infiltration of lymphocytes and inflammatory cells under and around bronchial mucosa, and exfoliation and proliferation of small airway epithelium.
Lung colony count: The lung tissues of the model group were excised 48 h after inoculation, and those of the treatment group were taken out after 48 h of treatment. The lungs were excised, weighed, and homogenized in 1 mL saline. The original solution was diluted continuously five times with normal saline, and then with different concentrations of diluent (10 mL×3) were dispensed on the agar plate and placed at 37 °C for 12 h. The data are expressed as means±standard deviation (SD) log10 CFU/g lung.
Mouse serum inflammation index measurement: Whole blood was obtained by mouse eyeball enucleation method, and serum was collected after centrifugation. The mice in the model group obtained whole blood by removing the eyeballs 48 h after infection, and the mice in the treatment group obtained whole blood by removing the eyeballs 48 h after the treatment. Enzyme-linked immunosorbent assay (ELLSA) measured the values of serum inflammatory indexes: IL-6, IL-1β, and TNF-α.
Statistical Analysis
The statistical results were analyzed using SPSS 20.0 software. Continuous variables are expressed as 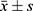 and grouping variables are expressed as percentages. Compared to the mean value of continuous variables of normal distribution, t-test was used in the two groups, and a bilateral test was used in all tests. P<0.05 indicated statistical significance.
and grouping variables are expressed as percentages. Compared to the mean value of continuous variables of normal distribution, t-test was used in the two groups, and a bilateral test was used in all tests. P<0.05 indicated statistical significance.
Results
Antibiotic Susceptibility Tests
The results showed that ten CRAB strains tested in this study were resistant to IMP, MEM, and SUL (resistance rate: 100%) and sensitive to TGC and PB (sensitivity rate: 100%). CRAB is 80% sensitive to RIF. The MICs of six single drugs against ten strains are shown in Table 1.
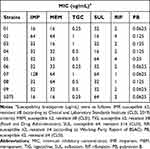 |
Table 1 MIC of Ten Carbapenem-Resistant Acinetobacter baumannii Against Six Antibacterial Drugs |
In vitro Antibacterial Activity of Antimicrobial Agents in Combination
The results showed that PB combined with the other five antibiotics showed synergistic and additive effects on CRAB with little irrelevant effect and no antagonistic effect on CRAB. The synergistic effect of PB and RIF was maximal (8/10), followed by MEM and IMP (6/10 and 5/10, respectively), and a weak synergistic effect of SUL and TGC (3/10 and 1/10, respectively) (Table 2).
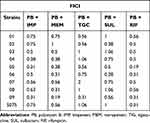 |
Table 2 FICI of PB Combined with IMP, MEM, TGC, SUL, and RIF |
In vivo Antibacterial Activity of Antimicrobial Agents
General Situation of Modeling Mice
After intranasal infusion of A. baumannii for 4 h, no significant change was detected in the state of mice. However, after 24 h, the model group showed pathological changes such as decreased body temperature, decreased appetite, slow movement, and hair wrinkles. Compared to the control group, significant differences were observed in the body weight, and clinical score detected at 24 and 48 h between groups A and B (P<0.05; Table 3).
Pathological Changes in the Pneumonia Model Before and After Drug Treatment
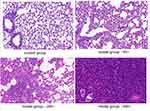
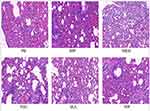
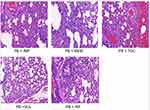
The microscopic structure of the lung tissue section of the mouse is shown in Figures 1–3. In the model group, the alveolar structure was disordered, the alveolar wall was broken, the alveolar cavity fusion was enlarged, a large number of macrophages and neutrophils infiltrated the alveoli, and there were secretions in some alveolar cavities after nasal inoculation. The degree of inflammation in the treatment group was significantly less than that in the model group. Compared to the corresponding monotherapy group, the combination group had fewer inflammatory cells and less exudate.
Lung Colony Count
Table 4 shows the bacterial load in the lungs of each group. The pulmonary bacterial load in the treatment group was significantly lower than that in the model groups except for PB, TGC, and PB+TGC (P<0.05).
 |
Table 4 Comparison of Bacterial Load in the Lungs of Mice and Serum Inflammatory Index of Mice Between Groups |
Compared to antibiotics alone, the bacterial load decreased in the combination group. Interestingly, significant differences were detected in the lung colony count between PB+MEM and PB+RIF groups compared to the corresponding single drug group (P<0.05). Also, a significant difference was noted in the pulmonary bacterial load between the PB+IMP and PB groups (P< 0.05).
Serum Inflammatory Index
The index value of the serum inflammatory factors (IL-1 β, IL-6, and TNF-α) in the model group was significantly higher than that in the control group (P<0.05). Compared to the model group, the values of serum inflammatory factors were significantly decreased in the treatment group (P<0.05). The combination group compared to the single-drug group in the values of IL-1β did not show a significant difference except between the PB+TGC and TGC groups (P< 0.05) and between PB+RIF and RIF groups (P<0.05). The values of IL-6 and TNF-α were significantly lower in PB + RIF group than those in PB and RIF groups (P< 0.05). The inflammatory indexes of the other combination groups were decreased, but no significant difference was detected between the combined group and the corresponding single drug group (P>0.05) (Table 4).
Discussion
In recent decades, the number of infections caused by antibiotic-resistant bacteria has increased rapidly, and hence, there is an urgent need for new therapeutic targets and antimicrobials. The decrease in sensitivity to these drugs might be related to the widespread clinical use of antibiotics. A. baumannii is becoming a major pathogen with the ability to cause nosocomial infection.25 Previously, carbapenem was the most commonly used antibiotic in the treatment of A. baumannii infection. However, carbapenem-resistant strains are increasing worldwide. Therefore, carbapenem monotherapy is not a treatment option for serious infections caused by drug-resistant A. baumannii. Therefore, several national and international programs have been implemented, including the EU innovative Medicine Initiative (IMI), the Joint Antimicrobial Resistance Planning Initiative (JPIAMR), and Spain’s National Drug Resistance Plan (PRAN).26 The proposed strategy was to combine two or more known antimicrobial agents to increase their antibacterial activity compared to their single antimicrobial agents. Another advantage of this method is to avoid or minimize the occurrence of drug-resistant mutants. The combination therapy of polymyxin and other antibiotics has a synergistic effect on the resistant strains of A. baumannii by increasing the killing rate of bacteria and reducing drug resistance. Some studies also investigated the effects of previously used antibiotics and chemically modified antibiotic conjugates, such as cephalosporin, a β-lactam antibiotic, and found synergistic antibacterial activity.27,28
A review highlighted the potential clinical utility of combining non-antibiotic FDA-approved drugs with antibiotics such as polymyxin B for the treatment of XDR bacterial infections.29 Hirsch et al observed a lower clinical success rate when the polymyxins were used as monotherapy but a higher rate when used in combination.30 A randomized controlled trial studied the use of colistin alone vs colistin plus meropenem for the treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria. No significant difference was observed between colistin monotherapy and combination therapy with respect to clinical failure at 14 days after randomization. Nonetheless, combination therapy decreased the incidence of mild renal failure.31 Our study showed that A. baumannii is sensitive to PB (MIC90: 0.125 ug/mL), TGC (MIC90: 1 ug/mL), and RIF (MIC90: ug/mL). The combination experiment showed that the synergistic effect of PB and RIF was the strongest, followed by MEM and IMP and weak with SUL and TGC. Intriguingly, the combination based on PB did not exert any antagonistic effect.
Some antimicrobials could not inhibit or kill bacteria but may enhance the combined antibacterial activity of other antimicrobials. On the other hand, some antimicrobial agents that can inhibit or kill bacteria may quickly develop drug resistance, require high doses, and be toxic. PB and TGC have good antibacterial activity against A. baumannii. PB inhibits bacterial growth by increasing membrane permeability.32 However, because of nephrotoxicity and neurotoxicity, PB is best used in clinical practice to reduce the dose to decrease the toxicity. TGC is a novel glycyl prostacyclin antimicrobial agent, which acts on 30S ribosomal subunit to inhibit bacterial protein translation and prevent amino acids from merging and prolonging peptide chains. These antibacterial effects regulated by varied factors include drug-resistant nodule cell division (RND)-type transporters and other efflux pumps, with a wide range of activities against Gram-positive and Gram-negative bacteria.33 Randomized trials have shown that TGC alone may increase the risk of death, further suggesting that TGC is best used in combination therapy.34 RIF is a safe, effective, and economical drug widely used in the clinic. It reduces the cost of treatment and improves the inhibitory activity against MDRAB.
BALB/c strain (inbred strain) mice are susceptible to pneumonia.35 In this study, we used BALB/c mice to establish a model of A. baumannii pneumonia using intranasal instillation of the highly virulent type Ab5075. Before the establishment of the model, cyclophosphamide was used to reduce the neutrophils of mice, resulting in an immune deficiency in mice. After nasal drip of bacterial solution and prolonged infection time, the bodyweight of mice decreased gradually, and the clinical score gradually increased. At 24 h and 48 h after infection, some inflammatory cells (mainly neutrophils and macrophages) were observed in the lung. This pneumonia model could help us study the treatment of CRAB lung infection.
After the successful establishment of the mouse pneumonia model, the effects of drug treatment and pathological changes of lung tissue were observed. The drug sensitivity test in vitro showed that PB, TGC, and RIF had inhibitory effects on A. baumannii, PB and RIF, MEM, and IMP exerted a synergistic antibacterial effect. The in vivo experiment showed that the degree of pulmonary inflammation in the treatment group was less than that in the model group, and the inflammatory cells and exudate decreased significantly in the combination group compared to the corresponding single drug group.
The pulmonary bacterial load in the treatment group was lower than that in the model group. We found that the PB and TGC groups and the combined use of the two drugs could reduce the pulmonary bacterial load, albeit not significantly. The pulmonary bacterial load in the other treatment groups was significantly lower than that in the model group (P<0.05). We also found that the pulmonary bacterial load of PB combined with MEM and RIF was significantly lower than that of any of the two drugs independently (P<0.05). Also, a significant difference was detected in the pulmonary bacterial load between PB combined with IMP and PB alone (P<0.05). Combined with our combined drug sensitivity test in vitro, PB combined with IMP, MEM, and RIF exerted a satisfactory synergistic effect.
The levels of serum inflammatory factors (IL-1 β, IL-6, and TNF-α) in the treatment group were significantly lower than those in the model group (P<0.05). Compared to the corresponding single drug group, the index of serum inflammatory factor decreased in the combination group, but not significantly (P>0.05). Interestingly, the values of IL-6 and TNF-α in the PB combined with RIF group were significantly higher than those in the PB and RIF groups. In addition, a good synergistic effect was observed between the in vitro combined with drug sensitivity test and lung colony count test. Some studies demonstrated the in vitro and in vivo bactericidal activity of RIF against MDRAB in experimental pneumonia models. In addition, RIF combined with IMP or SUL is effective in the treatment of experimental pneumonia and meningitis caused by IMP-resistant A. baumannii. We also found a significant difference in IL-1 β between PB combined with TGC group and TGC group. TGC is a new antimicrobial agent with broad-spectrum antibacterial activity against various organisms that could penetrate into the lung tissue.36 However, in the combined drug sensitivity test in vitro, we found that the synergistic effect of PB and TGC was not distinct, and no statistically significant difference was observed between the two drugs in reducing the lung colony count. Therefore, the conclusion of drug sensitivity tests in vitro was not consistent with that of animal experiments in vivo.
Nevertheless, the present study has some limitations. First, the number of strains tested in vitro is small, which dose not represent the resistance of all CRAB strains to the six antibiotics, and there were some limitations in evaluating the synergistic effect of antimicrobials. Second, all strains were from the same medical institution and could not represent the drug resistance of strains from other medical institutions. In the future, we should expand the number of strains and include the maximum number of medical institutions so that our results can be applied to other medical institutions. Third, the pharmacokinetics of mice and humans are different, thereby necessitating the investigation of the corresponding human doses and adverse reactions related to the local administration in clinical trials. Finally, our pneumonia infection model could not represent the other types of models, and the synergistic effects of other animal infection models (including meningitis and sepsis models) should be explored further. This would expand the scope of application of the combination and lay a foundation for its clinical application.
Conclusions
This study provided effective in vitro and in vivo approaches for the emergency issues, such as pulmonary infection caused by CRAB. The combination of PB and IMP, MEM, and RIF exerted a robust in vitro synergistic effect on CRAB isolates. The combination of PB had a better effect in the mouse pneumonia model than a single agent, while the combination of PB and RIF had the best effect. Our in vitro and in vivo models provided replicable and comprehensive data for informed optimal treatment management in patients resistant to carbapenem. Although additional clinical studies are needed, single treatment for these infections is not recommended.
Funding
This study was supported by the Scientific Research Project of Anhui Provincial Health Committee (No. AHWJ 2021b096), the National Natural Science Foundation of China (No. 81973983), the National Science and Technology Major Project (No. 2017ZX10204401), the Borrowing and Transferring Subsidy Project in 2019, Hefei (No. J2019Y04), Collaborative Tackling and Public Health Collaborative Innovation Project in Anhui Province (No. GXXT-2020-018), and Natural Science Research Project of Universities in Anhui Province (No. KJ2020A0176).
Disclosure
The authors declare that this study was conducted in the absence of any commercial or financial correlations that could be construed as a potential conflict of interest.
References
1. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/S1473-3099(17)30753-3
2. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582.
3. Neill JO. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist. 2014;4:1–16.
4. Zhou H, Yang Q, Yu Y, Wei Z, Li L. Clonal spread of imipenem- resistant Acinetobacter baumannii among different cities of China. J Clin Microbiol. 2007;45(12):4054–4057. doi:10.1128/JCM.00343-07
5. Fournier PE, Richet H, Weinstein RA. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42(5):692–699. doi:10.1086/500202
6. Antunes LCS, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71(3):292–301. doi:10.1111/2049-632X.12125
7. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator - associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi:10.1164/rccm.200405-644ST
8. Mangoni ED, Signoriello G, Andini R, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57(3):349–358. doi:10.1093/cid/cit253
9. Chuang Y, Cheng C, Sheng W, et al. Effectiveness of tigecycline-based versus colistin- based therapy for treatment of pneumonia caused by multidrug- resistant Acinetobacter baumannii in a critical setting: a matched cohort analysis. BMC Infect Dis. 2014;14:102. doi:10.1186/1471-2334-14-102
10. Galani I, Kontopidou F, Souli M, et al. Colistin susceptibility testing by Etest and disk diffusion methods. Int J Antimicrob Ag. 2008;31(5):434–439. doi:10.1016/j.ijantimicag.2008.01.011
11. MacGowan AP. Tigecycline pharmacokinetic/pharmacodynamic update. J Antimicrob Chemoth. 2008;62(Supplement 1):i11–6. doi:10.1093/jac/dkn242
12. Urban C, Go E, Mariano N, et al. Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J Infect Dis. 1993;167(2):448–451. doi:10.1093/infdis/167.2.448
13. Montero A, Ariza J, Corbella X, et al. Efficacy of colistin versus beta-lactams, aminoglycosides, and rifampin as monotherapy in a mouse model of pneumonia caused by multiresistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2002;46(6):1946–1952. doi:10.1128/AAC.46.6.1946-1952.2002
14. Pachón-Ibáñez ME, Fernández-Cuenca F, Docobo-Pérez F, Pachón J, Pascual A. Prevention of rifampicin resistance in Acinetobacter baumannii in an experimental pneumonia murine model, using rifampicin associated with imipenem or sulbactam. J Antimicrob Chemother. 2006;58(3):689–692. doi:10.1093/jac/dkl303
15. Harris G, Kuo Lee R, Lam CK, et al. A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob Agents Chemother. 2013;57(8):3601–3613. doi:10.1128/AAC.00944-13
16. Ma X, Guo Y, Wu Y, Gong W, Sun J, Huang Z. In vivo bactericidal effect of colistin–linezolid combination in a murine model of MDR and XDR Acinetobacter baumannii pneumonia. Sci Rep-Uk. 2020;10(1):1–8.
17. Ku NS, Lee S, Lim YS, et al. In vivo efficacy of combination of colistin with fosfomycin or minocycline in a mouse model of multidrug-resistant Acinetobacter baumannii pneumonia. Sci Rep-Uk. 2019;9(1):1–7.
18. Institute CALS. Performance standards for antimicrobial susceptibility testing. Clin Microbiol Newsletter. 2019;23(6):M100–M111.
19. Jones RN, Ferraro MJ, Reller LB, Schreckenberger PC, Swenson JM, Sader HS. Multicenter studies of tigecycline disk diffusion susceptibility results for Acinetobacter spp. J Clin Microbiol. 2007;45(1):227–230. doi:10.1128/JCM.01588-06
20. Phee L, Hornsey M, Wareham DW. In vitro activity of daptomycin in combination with low-dose colistin against a diverse collection of Gram-negative bacterial pathogens. Eur J Clin Microbiol. 2013;32(10):1291–1294. doi:10.1007/s10096-013-1875-z
21. Manepalli S, Gandhi JA, Ekhar VV, Asplund MB, Coelho C, Martinez LR. Characterization of a cyclophosphamide-induced murine model of immunosuppression to study Acinetobacter baumannii pathogenesis. J Med Microbiol. 2013;62(11):1747–1754. doi:10.1099/jmm.0.060004-0
22. Miyazaki S, Fujikawa T, Kanazawa K, Yamaguchi K. In vitro and in vivo activities of meropenem and comparable antimicrobial agents against Haemophilus influenzae, including -lactamase-negative ampicillin-resistant strains. J Antimicrob Chemother. 2001;48(5):723–726. doi:10.1093/jac/48.5.723
23. Song JY, Cheong HJ, Lee J, Sung AK, Kim WJ. Efficacy of monotherapy and combined antibiotic therapy for carbapenem-resistant Acinetobacter baumannii pneumonia in an immunosuppressed mouse model. Int J Antimicrob Ag. 2009;33(1):33–39. doi:10.1016/j.ijantimicag.2008.07.008
24. van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun. 2007;75(12):5597–5608. doi:10.1128/IAI.00762-07
25. Ozturk S, Ustun C, Pehlivan S, Ucak H. Acute generalized exanthematous pustulosis associated with tigecycline. Ann Dermatol. 2014;26(2):246–249. doi:10.5021/ad.2014.26.2.246
26. Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis. 2016;3(1):15–21.
27. Li WY, Brien-Simpson NMO, Holden JA, et al. Holden Covalent conjugation of cationic antimicrobial peptides with a β‐lactam antibiotic core[J]. Pept Sci. 2018;110(3). doi:10.1002/pep2.24059
28. Desgranges S, Ruddle CC, Burke LP, et al. β-Lactam-host defence peptide conjugates as antibiotic prodrug candidates targeting resistant bacteria. RSC Adv. 2012;2(6):2480–2492. doi:10.1039/c2ra01351g
29. Schneider EK, Reyes-Ortega F, Velkov T, Li J. Antibiotic-non-antibiotic combinations for combating extremely drug-resistant Gram-negative “superbugs”. Essays Biochem. 2017;61(1):115–125. doi:10.1042/EBC20160058
30. Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010;65(6):1119–1125. doi:10.1093/jac/dkq108
31. Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi:10.1016/S1473-3099(18)30099-9
32. Liu Q, Li W, Feng Y, Tao C. Efficacy and safety of polymyxins for the treatment of Acinectobacter baumannii infection: a systematic review and meta-analysis. PLoS One. 2014;9(6):e98091. doi:10.1371/journal.pone.0098091
33. Sun Y, Cai Y, Liu X, Bai N, Liang B, Wang R. The emergence of clinical resistance to tigecycline. Int J Antimicrob Agents. 2013;41(2):110–116. doi:10.1016/j.ijantimicag.2012.09.005
34. Shen F, Han Q, Xie D, Fang M, Zeng H, Deng Y. Efficacy and safety of tigecycline for the treatment of severe infectious diseases: an updated meta-analysis of RCTs. Int J Infect Dis. 2015;39:25–33. doi:10.1016/j.ijid.2015.08.009
35. Chiang S, Tang H, Chen C, et al. Acid aspiration provokes the pneumonia caused by multidrug-resistant Acinetobacter baumannii in BALB/c mice. Int J Infect Dis. 2013;17(6):e454–60. doi:10.1016/j.ijid.2013.01.022
36. Conte JE, Golden JA, Kelly MG, Zurlinden E. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int J Antimicrob Agents. 2005;25(6):523–529. doi:10.1016/j.ijantimicag.2005.02.013
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.