Back to Journals » Infection and Drug Resistance » Volume 12
In vitro activity and pharmacodynamic/pharmacokinetic parameters of clarithromycin and azithromycin: why they matter in the treatment of respiratory tract infections
Authors Davidson RJ
Received 11 September 2018
Accepted for publication 27 December 2018
Published 8 March 2019 Volume 2019:12 Pages 585—596
DOI https://doi.org/10.2147/IDR.S187226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eric Nulens
Ross J Davidson1–4
1Department of Pathology and Laboratory Medicine, Division of Microbiology, Queen Elizabeth II Health Sciences Center, Halifax, NS, Canada; 2Department of Medicine, 3Department of Pathology, 4Department of Microbiology & Immunology, Dalhousie University, Halifax, NS, Canada
Abstract: Clarithromycin and azithromycin are second-generation macrolides established and widely used for treating a range of upper and lower respiratory tract infections. Extensive clinical trials data indicate that these drugs are highly effective in these applications and broadly comparable in their clinical and microbiological effectiveness. However, consideration of pharmacokinetic, metabolic, and tissue-penetration data, including the significant antibacterial activity of the metabolite 14-hydroxy-clarithromycin, plus the findings of pharmacodynamic modeling, provide evidence that the long half-life and lower potency of azithromycin predispose this agent to select for resistant isolates. Comparison of the “mutant-prevention concentrations” of clarithromycin and azithromycin, and examination of large-scale epidemiological data from Canada, also support the view that these drugs differ materially in their propensity to promote resistance among bacterial strains implicated in common respiratory infections, and that clarithromycin may offer important advantages over azithromycin that should be considered when choosing a macrolide to treat these conditions.
Keywords: azithromycin, clarithromycin, 14-hydroxy-clarithromycin, mutant prevention concentration, pharmacokinetics, tissue-penetration, upper respiratory tract infection, lower respiratory tract infection
Introduction
Erythromycin, the progenitor molecule of the macrolide class, was introduced into clinical practice in the 1950s.1 Clarithromycin and azithromycin, second-generation macrolides derived from erythromycin, offer significant improvements over their parent molecule in the form of an expanded spectrum of activity and enhanced tolerability.2
Erythromycin, clarithromycin, and azithromycin exert their antibacterial effects by reversibly binding to the 50s subunit of the bacterial ribosome. This leads to an inhibition of ribonucleic acid-dependent protein synthesis by the prevention of transpeptidation and translocation reactions. The high affinity of macrolides for bacterial ribosomes, combined with the highly conserved structure of ribosomes across bacterial species, underpins the broad-spectrum activity of the macrolides.3 The macrolides are considered bacteriostatic against most susceptible organisms; however, clarithromycin and azithromycin are bactericidal against Streptococcus pyogenes, Streptococcus pneumoniae, and Haemophilus influenzae at supra-minimum inhibitory concentration (MIC) levels.2
Clinical trials data
The spectrum of activity of the macrolides identifies them as well suited for treating a range of upper and lower respiratory tract infections (LRTIs).
There is an extensive clinical trials database on macrolides involving direct comparisons between clarithromycin and azithromycin or comparisons between macrolides and other classes of antibiotics. Overall conclusions from these studies are that (a) macrolides display high rates of clinical effectiveness and (b) macrolides for the most part display clinical effectiveness against susceptible strains of infectious organisms comparable to those of other types of antibiotics. In considering these data, it must be borne in mind that individual studies were not scaled or powered to demonstrate superiority of one intervention over another. The fact that in most studies all the tested drugs were, in statistical terms, comparably effective does not mean that there may not be substantive, clinically relevant differences between agents, nor does it mean that they are in all situations interchangeable, without an impact on the clinical outcome.
Upper respiratory tract infections (URTIs)
URTIs are a high-nuisance-value condition for both patients and physicians. Patients want a quick remedy but physicians are often frustrated with regard to the choice of treatment because they lack information about the specific infectious pathogen. Many URTIs are in fact viral in origin. For those with a bacteriological etiology, however, a number of studies have clearly demonstrated the effectiveness of macrolides against the most frequently isolated bacterial causes of pharyngitis, otitis media, and sinusitis.
Acute bacterial pharyngitis
Pharyngitis is a frequent reason for children aged ≤16 years to seek medical attention. Approximately 35% of these cases will be due to S. pyogenes. In adults, S. pyogenes only contributes to ∼5–10% of sore throats.4 Although a large proportion of these infections will resolve with or without treatment, antibiotic use is essential to eradicate the pathogen in order to prevent spread and re-infection and to minimize potential sequelae of the index infection.
A Cochrane analysis published in 2016 summarized studies conducted to date.5 The authors concluded that there were no clinically relevant differences in symptom resolution when comparing cephalosporins or macrolides with penicillin in the treatment of Group A Streptococcal tonsillopharyngitis. Limited evidence in adults suggests that cephalosporins may be more effective than penicillin for relapse, but the number needed to treat is high. The authors of this appraisal cautioned that the majority of trials were performed in high-income countries, where complication rates are typically low.
Most guidelines recommend penicillin V or amoxicillin as the first-line antimicrobial for patients with acute bacterial pharyngitis.6 This specific recommendation is based on the complete absence of penicillin resistance among S. pyogenes. For patients in whom penicillin therapy fails or who are allergic to penicillin, several alternatives, including macrolides, have been identified but not prioritized. There are, moreover, several articles that suggest that certain serotypes of S. pyogenes may internalize within cells, allowing them to evade beta-lactam therapy.7,8 Thus, in situations where penicillin has clearly failed, antibiotics such as macrolides that achieve both extracellular and high intracellular levels should be administered.
Acute otitis media (AOM)
AOM is a common childhood illness. Although predominantly viral in origin, bacterial infections are nevertheless common and require antibiotics for effective management. A number of trials have examined the utility of the macrolides in the treatment of AOM.
A study by Gooch et al9 randomized children (N=379) to a 10-day course of twice-daily clarithromycin, given as an oral suspension at a dosage of 7.5 mg/kg (maximum total dose 500 mg) or an oral suspension of cefaclor at a dosage of 20 mg/kg (maximum total dose 500 mg) twice daily. Among 281 evaluable patients, clinical success rates were 86% and 90%, respectively. (Success was defined as cure or cure with effusion or improvement.)
Aspin et al10 evaluated 180 pediatric patients (aged 6 months to 12 years) with AOM who were treated for 10 days with clarithromycin (15 mg/kg twice daily; N=90) in two divided doses per day (N=90), or amoxicillin/clavulanic acid (A/C) (40 mg/kg as three divided doses; N=90). Among the 172 patients (N=86 per group) eligible for outcome assessment, clinical cure/improvement was seen within 4 days of starting treatment in 93% of clarithromycin-treated patients and 95% of those treated with A/C. There was, however, a significant difference in tolerability, with gastrointestinal adverse events observed in 20% of patients in the clarithromycin group and 52% of those in the A/C arm (P<0.001). The clinical equivalence of clarithromycin and amoxicillin was reported in various early trials of pediatric AOM patients.11,12
Trials examining the efficacy of azithromycin in the treatment of AOM have produced mixed results. Aronovitz13 randomized 169 children with confirmed AOM to azithromycin oral suspension (10 mg/kg on day 1, then 5 mg/kg on days 2–5), or an A/C suspension dosed at 40 mg/kg/day in three divided doses for 10 days. Analysis at day 11 demonstrated statistically comparable rates of cure or improvement: azithromycin, 87.8%; A/C, 100.0%. By contrast, Dagan et al14 reported some indication of superior effect from A/C. They randomized 238 pediatric patients with AOM to receive A/C (45/6.4 mg/kg/day in two divided doses for 10 days) or azithromycin (10 mg/kg on day 1, then 5 mg/kg daily for the next 4 days). A/C was significantly more likely than azithromycin to eradicate all bacterial pathogens from the middle ear fluid (83% vs 49%; P=0.001) and showed a similar superiority for the eradication of H. influenzae (87% vs 39%; P=0.0001). A/C was also more likely than azithromycin to eradicate S. pneumoniae, although not significantly so (P=0.095). Signs and symptoms (assessed on days 12–14) were more likely to have resolved completely or improved in culture-positive patients who received A/C (86% vs 70%; P=0.023); a similar difference in effect was apparent in patients with H. influenzae infections (91% vs 65%; P=0.010).
The Canadian anti-infective guidelines for community-acquired infections15 recommend amoxicillin as first-line therapy for both children and adults, a position seconded by Forgie et al16 and shared by US guidelines.17 Clarithromycin is listed as a second-line agent for those who fail or for penicillin-allergic patients. UK guidelines released for consultation in 2017 conform to broadly the same pattern but consider clarithromycin the drug of choice for patients with an allergy to penicillin.18
Acute bacterial sinusitis
Acute bacterial sinusitis can be a very difficult diagnosis to make, with physicians having to rely on their clinical acumen and indicators such as the duration and severity of disease to help guide their management of the condition. Acute sinusitis is primarily viral, with a small percentage of affected patients progressing to bacterial disease.
An early study by Dubois et al19 evaluated clarithromycin (500 mg twice daily) and A/C (500 mg three times daily) in a single-blind, randomized study of 497 outpatients with acute maxillary sinusitis. S. pneumoniae was isolated from 22% of patients, Staphylococcus aureus from 16%, H. influenzae from 10%, and Moraxella catarrhalis from 7%. Clinical success (cure or improvement) was recorded for 97% of clarithromycin recipients (128/132) vs 93% of A/C recipients (119/128), with corresponding bacteriologic cure rates of 87% and 90%.
Adelglass et al20 evaluated 236 adult patients with acute sinusitis randomized in a double-blind study to 500 mg oral levofloxacin once daily (N=119) or 500 mg oral clarithromycin twice daily (N=117) for 10–14 days. Clinical response rates (cured plus improved) at days 2–5 for clinically evaluable patients were 96.0% for levofloxacin (N=98) and 93.5% for clarithromycin (N=93).
Riffer et al21 compared clarithromycin extended-release (ER) with A/C in a multicenter study involving 437 patients aged ≥12 years diagnosed with acute bacterial sinusitis. Clinical cure rates (96% for each treatment group), radiological success rates, and pathogen eradication rates were identical in the two arms. However, clarithromycin ER (1,000 mg once daily) was associated with symptomatic improvement or relief as early as day 2 after initiation of treatment. Clarithromycin ER was also associated with a significantly higher resolution rate for sinus pressure (P=0.027) and improvement/resolution of nasal congestion (P=0.035). There was also a statistically significantly higher resolution/improvement rate of purulent nasal discharge with clarithromycin ER at the test-of-cure visit (P=0.01); the resolution/cure rate was similar, and high, in both groups (>94%).
Casiano22 enrolled 78 patients in a multicenter, blinded study comparing a single daily dose of azithromycin for 5 days (500 mg on day 1, 250 mg/day thereafter) with amoxicillin (500 mg three times daily) for 10 days in the treatment of acute bacterial maxillary sinusitis and reported almost identical rates of clinical cure from the 38 evaluable patients (azithromycin 73.9%; amoxicillin 73.3%).
A meta-review published in 2012 examined the efficacy and safety of clarithromycin in pediatric patients with URTIs.23 The authors evaluated 24 studies and concluded on the basis of what they described as “high quality evidence” that clarithromycin was therapeutically equivalent to other antibiotics studied with respect to clinical cure (RR: 1.02; 95% CI: 0.98–1.06; P=not significant [NS]), clinical success (RR: 1.01; 95% CI: 0.99–1.03; P=NS), and relapse risk (RR: 1.34; 95% CI: 0.81–2.21; P=NS). They also concluded that clarithromycin was superior to other antibiotics in relation to bacterial eradication and that it exhibited a low risk for adverse events (RR: 0.77; 95% CI: 0.65–0.90; P=0.001).
The Canadian anti-infective guidelines for community acquired infections15 recommend amoxicillin for sinusitis patients whose condition has not resolved within 5–7 days with ancillary treatment. Clarithromycin is listed as a second-line agent for those patients who are penicillin-allergic or for whom first-line measures fail.24
UK guidelines state that short-term antibiotics (2 weeks) can be used for acute exacerbations of rhinosinusitis (grade of recommendation D).25 The guidelines make no specific antimicrobial choices at this point but go on to state that trials of long-term oral antibiotics (12 weeks), especially macrolides, have demonstrated symptomatic and objective improvements similar to those achieved with endoscopic sinus surgery (grade of recommendation A). The improvement shown increases with time and may relate to anti-inflammatory or immunological properties of macrolides.26–30
Lower respiratory tract infections
A number of trials have examined the efficacy of clarithromycin and azithromycin for LRTIs, specifically community-acquired pneumonia (CAP) and acute exacerbation of chronic bronchitis. Most of the studies involved patients who were not hospitalized.
Community-acquired pneumonia
Clarithromycin and erythromycin have been evaluated for their efficacy and safety in CAP in a series of clinical trials.31–39 The central conclusion of those studies is that the two macrolides are highly effective and closely comparable in this situation, with rates of clinical cure/improvement consistently exceeding 90%. This verdict was substantiated in a Cochrane review by Pakhale et al40 in 2014 which examined 11 randomized controlled trials that included 3,352 patients aged >12 years with a diagnosis of CAP and found no overall significant difference in the efficacy of the various antibiotics examined.
Also in 2014, Sligl et al41 reported that, in observational data collected from 28 studies in almost 10,000 critically ill patients with CAP, macrolide therapy (vs non-macrolides) was associated with at least a 3% absolute lower risk of death (18% RR reduction), a benefit which the authors considered made a strong case for macrolides as first-line therapy for CAP.
Macrolides feature prominently in the latest Canadian/US and UK guidelines for management of adult outpatients with CAP:42,43 they are recommended for all patients with mild-to-moderate disease with no comorbidities and recommended (in combination with a beta-lactam) when comorbidities are present. The physician’s choice should always be guided by the patient’s antibiotic history.
Pediatric guidelines rely heavily on age as indicators of etiology and recommended management.44,45 Antimicrobial therapy is typically not routinely required for preschool-aged children with CAP, because viral pathogens are responsible for the great majority of clinical diseases in that age group. Amoxicillin is typically used as the first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild-to-moderate CAP suspected to be of bacterial origin. Macrolide antibiotics should be prescribed for the treatment of children (primarily school-aged) and adolescents evaluated in an outpatient setting with findings compatible with CAP caused by atypical pathogens.
Acute exacerbations of COPD
Bradbury46 reported that the clinical responses to azithromycin and clarithromycin were very similar in 510 patients with a diagnosis of acute bronchitis, acute exacerbations of chronic bronchitis (AECB), or pneumonia. The two macrolides also showed high and comparable levels of clinical and bacteriological success in a very large eight-country study in 322 adult outpatients with AECB.47
A meta-analysis of 19 trials (N=7,405) compared the short- and long-term efficacy of the macrolides to the quinolones and A/C in acute exacerbations of COPD in adults.48 No statistically robust differences were identified regarding treatment success in intention-to-treat and clinically evaluable patients between (a) macrolides and quinolones, (b) A/C and quinolones, or (c) A/C and macrolides. There was also no difference in hospitalization rates between patients treated with macrolides and those treated with quinolones (N=2581; OR: 1.37; 95% CI: 0.75–2.50) or in mortality rates (N=2,627; OR: 1.96; 95% CI: 0.45–8.51). Fewer quinolone-treated patients experienced a repeat episode of acute bacterial exacerbation of COPD after resolution of their initial episode compared with macrolide recipients during the 26-week period following therapy. Adverse events were similar between macrolides and quinolones (N=4,081; OR: 1.11; 95% CI: 0.94–1.32) and were higher with A/C than with either of those classes of antibiotics.
Data from a separate meta-analysis suggest that macrolide-based prophylaxis may be effective for reducing incident COPD exacerbations in severe disease.49 (See also references.50–52)
Clarithromycin and azithromycin: How do they differ?
Direct comparisons between clarithromycin and azithromycin or between them and other comparator agents in clinical trials generally show no robust difference in efficacy. As noted previously, this is not wholly surprising as clinical trials are typically not designed to deliver the statistical power required to demonstrate superiority. This does not mean, however, that there are not clinically relevant properties or criteria that differentiate these molecules.
In vitro activity
Differentiation between clarithromycin and azithromycin must begin with appraisal of their in vitro activity against common respiratory tract pathogens. At the very least, these should include S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and S. pyogenes, as these pathogens account for the majority of bacterial URTIs and LRTIs.53–58 Against susceptible S. pneumoniae, clarithromycin shows the greatest potency, while erythromycin and azithromycin are approximately equivalent in activity (Table 1).
Ednie et al59 demonstrated that, among 120 clinical isolates of S. pneumoniae, the MIC90 values for clarithromycin and erythromycin against penicillin-susceptible strains were 0.06 µg/mL and reported that clarithromycin and azithromycin displayed bactericidal activity at twice their MIC90, whereas erythromycin was only bactericidal at eight times its MIC90 of 0.125 µg/mL. All these drugs displayed reduced activity against penicillin-intermediate and -resistant isolates but the differential in favor of clarithromycin seen in penicillin-susceptible strains was preserved (Table 1).
All three agents have excellent activity against the atypical pathogens M. pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophilia (Table 1).59–65
Contribution of 14-hydroxy-clarithromycin to antimicrobial effect
When the in vitro activities of the macrolides are examined against H. influenzae, azithromycin appears to be the most active, exhibiting a range of MICs between 0.5 and 2.0 µg/mL, whereas the MIC range of clarithromycin is 4–16 µg/mL (Table 1). Depending on the study, the MIC90 for azithromycin is typically 1 or 2 µg/mL, compared with 8–16 µg/mL for clarithromycin.
However, researchers have suggested that the active metabolite of clarithromycin, 14-hydroxy-clarithromycin (14-HC), can act synergistically both in vitro and in vivo with its parent molecule, enhancing the anti-Haemophilus activity of the macrolide.39,66–71 Using in vitro checkerboard and kill-curve bactericidal assays, Bergeron et al69 and Hoover et al70 were able to demonstrate synergy between clarithromycin and 14-HC against some strains of Haemophilus, as well as Enterococcus and Staphylococci.
Bergeron et al69 reported that the bactericidal effect of clarithromycin and 14-HC combinations was additive in 92% of all strains of H. influenzae and synergistic in the other 8%, with no evidence of influence from the presence/absence of a beta-lactamase. Hardy et al39 demonstrated, in a number of time-kill experiments, that the combination of parent compound and metabolite at even a quarter and a half of their individual MICs reduced bacterial counts by >5 log colony-forming units (CFUs).
Thus it appears that, in vivo, clarithromycin and its 14-hydroxy metabolite are more active against H. influenzae, and perhaps other pathogens, than traditional in vitro MIC testing would suggest.
Pharmacokinetics
Clarithromycin and azithromycin are also differentiated by their pharmacokinetic profiles (Table 2).72–80 Clarithromycin is well absorbed from the gastrointestinal tract, although it undergoes substantial first-pass metabolism that reduces systemic bioavailability to 55% after a 250-mg dose in healthy volunteers. The maximum clarithromycin plasma concentration in healthy volunteers is ∼0.62–0.84 mg/L following single-dose administration of 250 mg and 1.77–1.89 mg/L after administration of 500 mg. The time to reach maximum clarithromycin plasma concentration is ∼3 hours. The areas under the plasma concentration–time curves (AUCs) are ∼4 and 11 mg/L×hour after doses of 250 and 500 mg, respectively in Western volunteers. Clarithromycin also undergoes rapid biotransformation to produce the microbiologically active 14-HC metabolite referenced above, which achieves peak plasma concentrations of 0.4 and 0.8 mg/L within 3 hours of administering a 250- or 500-mg dose, respectively.
  | Table 2 Pharmacokinetics of clarithromycin and azithromycin in plasma. (Derived from data published in references.75–78,80) Abbreviations: AUC, area under the plasma concentration–time curve; Cmax, maximum plasma concentration; ND, not determined; tmax, time to reach maximum plasma concentration. |
Approximately 37% of a single oral dose of 500 mg azithromycin is bioavailable and produces a peak serum concentration of 0.4 mg/L. Multiple-dose regimens (two 500-mg doses separated by 12 hours and followed a 500-mg dose once daily for 5 days, or two 250-mg doses separated by 12 hours and followed by a 250-mg dose once daily for 9 days) produce only slight increases in peak serum concentrations.73,80,81
Half-life
An early indication that pharmacokinetic differences between these molecules were clinically relevant came from observations by Kastner and Guggenbichler82 on patterns of oropharyngeal flora in 156 children taking open-label macrolides. These children were randomly assigned to receive azithromycin, clarithromycin, erythromycin, roxithromycin, or josamycin. Throat swabs were obtained prior to treatment and repeated weekly for 6 weeks. As many as 90% of children harbored macrolide-resistant strains in their oral flora 1 week posttreatment. By 6 weeks after treatment, the percentage of patients colonized by resistant organisms had decreased very substantially for clarithromycin and most other macrolides. For azithromycin, however, the prevalence of resistant organisms remained close to the week 1 rate (Figure 1) and re-infection was documented in 7/60 children (11.6%). The authors attributed the significant difference in colonization between azithromycin and the other macrolides to the prolonged elimination half-life of azithromycin (≈68 hours) (Table 2). They conjectured that property of the drug contributed to the emergence of macrolide resistance by promoting the persistence of “sub-inhibitory serum and tissue concentrations over a period of several weeks posttreatment”.
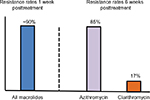  | Figure 1 Differential effects of randomized, open-label macrolide assignment on the prevalence of macrolide-resistant strains in the oral flora of children. Notes: One week after treatment, ~90% of patients treated with azithromycin, clarithromycin, erythromycin, roxithromycin, or josamycin harbored resistant strains. By 6 weeks posttreatment, the percentage of resistant organisms had decreased to 17% for clarithromycin but for azithromycin the prevalence of resistant organisms remained very high (85%) and was associated with a substantial re-infection rate (11.6%). (Derived from data published in reference.82) |
Macrolide penetration to target tissues
In relation to these data, it should be noted that the macrolides as a group are extensively distributed in body tissues, with tissue concentrations typically substantially exceeding serum concentrations. These tissue concentrations are pertinent to antibacterial effect in a range of infectious conditions and differences are discernible here also between azithromycin and clarithromycin.
Macrolide concentrations in epithelial lining fluid (ELF)
Rodvold et al77 reported that clarithromycin (500 mg twice daily for 9 days) and its active metabolite 14-HC were concentrated in ELF and alveolar macrophages of healthy adult volunteers to much higher levels than azithromycin (500 mg on day 1 then 250 mg/day for 4 days) (Figure 2). Patel et al78 reported similar findings with the same dosing schedules, with concentrations of clarithromycin in ELF of 34.02±5.16 mg/mL at 4 hours, 20.63±4.49 mg/mL at 8 hours, 23.01±11.9 mg/mL at 12 hours, and 4.17±0.29 mg/mL at 24 hours. By contrast, azithromycin concentrations were detectable in only 2 of 41 healthy volunteers. Administration of a single 200 mg dose of clarithromycin was documented by Kikuchi et al83 as producing an area under the concentration-time curve from 0 to 10 (AUC0–10) of 7.37±2.07 mg/h/L for bronchial ELF, compared with a serum AUC0–10 of 2.10±0.49 mg/h/L (P<0.01).
  | Figure 2 Comparison of achieved concentrations of azithromycin (500 mg on day 1 then 250 mg/day for 4 days) and clarithromycin (500 mg twice daily for 9 days) in (A) plasma, (B) epithelial lining fluid (ELF), and (C) alveolar macrophages of healthy adult volunteers at 4 and 24 hours after last drug administration. Notes: All comparisons P<0.05 vs azithromycin except ELF at 24 hours. The mean ratio of clarithromycin to 14-HC in plasma was 4.7:1 at 4 hours and decreased to 1.2:1 at 24 hours (data not shown). (Derived from data published in reference.77) |
Macrolide concentrations in middle ear fluid
Gan et al84 reported that mean middle ear fluid concentrations of clarithromycin (7.5 mg/kg/12 hours for six doses) in 32 children varied from 3.0 to 8.3 µg/g during the dosing interval; the range for 14-HC was 1.5–3.8 µg/g. For both parent drug and metabolite, these ranges reliably exceeded the mean plasma concentrations. At 12 hours after dosing, the ratios of middle ear fluid concentration to plasma concentration were 8.8 for clarithromycin and 3.8 for 14-HC.
Penetration of clarithromycin into the middle ear fluid was further examined in children with AOM treated with a dose of 7.5 mg/kg every 12 hours for 7 days. A mean clarithromycin concentration of 8.3 µg/g was detected in effusion samples obtained 4 hours after the sixth dose. The serum drug concentration at that time was 3.4 µg/mL. Similarly, the mean middle ear concentration of the 14-hydroxy metabolite of clarithromycin was 2.9 µg/g, compared with a serum concentration of 1.8 µg/mL. At 12 hours after the sixth dose, the mean concentrations in middle ear fluid were 7.4 µg/g for clarithromycin and 3.8 µg/g for 14-HC.85
In 16 evaluable patients with AOM with effusion, azithromycin (10 mg/kg as a single dose 12, 24, or 48 hours before the insertion of tympanostomy tubes, then once daily for 5 days: 10 mg/kg on day 1, then 5 mg/kg/day) penetrated middle ear exudates, with group mean concentrations of ~8.61 and 9.40 µg/mL at 24 and 48 hours after administration. Plasma concentrations during the same time period ranged from 0.013 to 0.034 µg/mL.86
Sinus penetration of macrolides
Margaritis et al87 examined the penetration of clarithromycin and azithromycin in 36 adult outpatients with acute bacterial rhino-sinusitis. They collected serial sinus fluid aspirates and serum samples at 2, 4, 6, 8, and 12 hours or 2, 6, 12, and 24 hours after administration of three doses of oral clarithromycin (500 mg twice daily) or two doses of oral azithromycin (500 mg once daily). The mean clarithromycin sinus fluid concentration significantly exceeded the corresponding azithromycin concentration (2.47 vs 0.65 mg/L; P<0.05), as did clarithromycin levels at 2, 6, and 12 hours after dosing.
Pharmacodynamic modeling
After the report of Kastner and Guggenbichler,82 outlined above, several other research groups accumulated data suggesting that the long half-life and lower potency of azithromycin (based on MIC to tissue concentration ratios) might predispose this agent to select for resistant isolates. Zhanel et al88 published two studies that examined the ability of clarithromycin and azithromycin to eradicate both susceptible and resistant isolates of S. pneumoniae at therapeutic drug concentrations.88,89
Noreddin et al89 suggested that achievement of high clarithromycin concentrations in ELF relative to serum drug concentration would result in inhibition of both susceptible and low-level macrolide-resistant (mefA) S. pneumoniae isolates (MIC 1–8 µg/mL). They demonstrated that simulation of clinically achievable total and free-drug concentrations of clarithromycin in ELF (based on a 500-mg twice-daily oral regimen) completely eradicated macrolide-susceptible and mefA strains of S. pneumoniae, with clarithromycin MICs ranging from 1 to 8 µg/mL and 1 to 4 µg/mL, respectively. This was relevant because the majority of macrolide-resistant (mefA) S. pneumoniae isolates in North America have MICs ranging between 1 and 8 µg/mL. This study provided some explanation for the infrequency of clinical failures with macrolides in the treatment of respiratory infections, despite reports of a high prevalence of macrolide-resistant S. pneumoniae strains in some areas.90–94
Zhanel et al88 sought to replicate these findings with azithromycin using the same model described above. Azithromycin was modeled simulating a dosage of 500 mg on day 1, followed by 250 mg on day 2 orally. Macrolide-susceptible S. pneumoniae, as well as low-level (mefA) and high-level (ermB) macrolide-resistant strains, were tested. These authors discovered that clinically achievable concentrations of azithromycin in serum, ELF, and middle ear fluid eradicated macrolide-susceptible S. pneumoniae; no similar effect was seen vs macrolide-resistant S. pneumoniae of any resistance phenotype. The authors suggested that the failure of azithromycin to eradicate any of the resistant phenotypes may be associated with the increasing incidence of macrolide-resistant S. pneumoniae.
Mutant-prevention concentration (MPC)
Blondeau et al95,96 used a novel pharmacodynamic parameter known as the MPC to explore the differences between the second-generation macrolides. The MPC is based on the concept that the frequency at which mutations occur is typically in the order of 1×10−7–1×10−9, one that would not normally be detected by traditional susceptibility testing. Consequently, an isolate considered to be susceptible might nevertheless contain an undetected subpopulation of resistant cells that would require a higher drug concentration to restrict growth. In some respiratory tract infections, such as CAP, bacteria present at the site of infection may well exceed the 105 CFU concentration used in traditional laboratory MIC testing. Using this approach, Blondeau et al95,96 inferred that the elevated prevalence of azithromycin resistance in some regions of Canada was likely due to its unfavorable MPC value.
Animal modeling
Hoffman et al97 extended these studies to examine if the conclusions of Zhanel et al88,89 were borne out in vivo. They evaluated the activities of clarithromycin and azithromycin against 19 isolates of S. pneumoniae using a neutropenic lung infection model in mice. They included susceptible (clarithromycin and azithromycin MICs ≤0.12 µg/mL), mefA-mediated resistant (clarithromycin and azithromycin MICs 0.5–32 µg/mL), and highly macrolide-resistant (clarithromycin and azithromycin MICs ≥64 µg/mL) strains in their study.
Infected mice were treated with clarithromycin (4, 40, or 200 mg/kg body weight twice daily, or 200 mg/kg once daily) or azithromycin (4, 40, or 200 mg/kg once daily, or 40 mg/kg twice daily) by oral gavage for 72 hours. Mortality/survival was assessed over 10 days and to that of saline-treated controls.
Animals infected with susceptible isolates and then treated with either agent at doses of ≥40 mg/kg demonstrated significant improvements in survival vs controls. Neither treatment improved survival in animals infected with highly macrolide-resistant isolates. However, among mice infected with strains expressing low-level resistance (mefA), a significant (P<0.05) improvement in survival was noted among animals treated with clarithromycin at 40 mg/kg/day (7/9 isolates) and 200 mg/kg twice daily (9/9 isolates). Corresponding survival rates with azithromycin 40 or 200 mg/kg once daily or 40 mg/kg twice a day were significantly (P<0.05) better than in the control groups but clearly lower than those for clarithromycin (2/9, 4/9, and 1/9 isolates, respectively). These findings correlated well with predictions based on the in vitro pharmacodynamic models of Zhanel et al88 and Noreddin et al89 outlined above.
Epidemiological data
Davidson et al98 examined macrolide resistance across the Canadian provinces over a 7-year period and correlated their findings with macrolide consumption in the same regions. They discovered that macrolide resistance in S. pneumoniae isolates had increased dramatically in some regions of Canada, but not in others. When macrolide resistance was compared with overall macrolide consumption, it was discovered that azithromycin was the most commonly prescribed macrolide in areas with the highest rates of macrolide resistance, whereas macrolide resistance was relatively low in areas where clarithromycin was prescribed preferentially. A striking feature of this study was the high correlation between the predictions made by both the pharmacodynamic modeling of Zhanel et al88,89 and the MPC studies of Blondeau et al.95
Further epidemiological evidence that azithromycin may be driving macrolide resistance was observed by Kuster et al.99 These researchers showed that, in patients previously exposed to antibiotics who subsequently developed pneumococcal disease, the time lapse from the last treatment course is of considerable value in predicting antimicrobial resistance. They reported that repeated exposure to either fluoroquinolone or macrolide antibiotics within a 90-day period increased a patient’s likelihood of harboring a resistant strain of S. pneumoniae. This finding was true for exposure to all fluoroquinolones and macrolides. However, while both erythromycin and clarithromycin were implicated in this phenomenon, the contribution of azithromycin was significant: after exposure to it, resistance rates decreased more slowly. This might be explained by the longer plasma half-life of azithromycin leading to prolonged sub-therapeutic concentrations, and the resultant selection of resistant bacterial strains for >3 weeks after treatment.
Conclusion
There is an abundance of literature demonstrating the utility of using macrolides to manage patients with respiratory tract disease. As a result, macrolides feature prominently in many guidelines as first- and second-line therapy. They have also been shown to have utility in managing some patients with chronic inflammatory diseases and are considered the standard of care for treating conditions such as diffuse panbronchiolitis and advanced COPD.
There is also, however, ample evidence to suggest that the second-generation macrolides differ in their propensity to select for resistance. Mean serum and tissue concentrations of clarithromycin far exceed those of azithromycin. Pharmacodynamic studies designed to study these differences have clearly shown that azithromycin is more likely to select for resistance than clarithromycin. Animal modeling data and clinical epidemiological data support this conclusion. Because the macrolides are an important therapeutic option for treating patients with a myriad of respiratory tract diseases, physicians should be aware of the differences in these molecules and choose accordingly when treating their patients.
Acknowledgment
Hughes associates, Oxford, UK, provided editorial services in the preparation of this review.
Disclosure
Dr Davidson has received honoraria for services to advisory boards and a speakers’ bureau of Abbott Products Operations AG, Allschwil, Switzerland. Dr Davidson has also served as a consultant and on speakers’ bureau for Abbott Laboratories and Bayer Pharmaceuticals. The author reports no other conflicts of interest in this work.
References
Sood SK. Macrolides: clarithromycin and azithromycin. Semin Pediatr Infect Dis. 1999;10(1):23–30. | ||
Peters DH, Clissold SP. Clarithromycin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic potential. Drugs. 1992;44(1):117–164. | ||
Dinos GP. The macrolide antibiotic renaissance. Br J Pharmacol. 2017;174(18):2967–2983. | ||
Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):1279–1282. | ||
van Driel ML, De Sutter AI, Habraken H, Thorning S, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst Rev. 2016;9:CD004406. | ||
Chiappini E, Regoli M, Bonsignori F, et al. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther. 2011;33(1):48–58. | ||
Sela S, Barzilai A. Why do we fail with penicillin in the treatment of group A Streptococcus infections? Ann Med. 1999;31(5):303–307. | ||
Savini V, Catavitello C, Talia M, et al. Beta-lactam failure in treatment of two group G Streptococcus dysgalactiae subsp. equisimilis pharyngitis patients. J Clin Microbiol. 2008;46(2):814–816. | ||
Gooch WM, 3rd, Gan VN, Corder WT, Khurana CM, Andrews WP. Clarithromycin and cefaclor suspensions in the treatment of acute otitis media in children. Pediatr Infect Dis J. 1993;12(12 Suppl 3):S128–S133. | ||
Aspin MM, Hoberman A, McCarty J, et al. Comparative study of the safety and efficacy of clarithromycin and amoxicillin-clavulanate in the treatment of acute otitis media in children. J Pediatr. 1994;125(1):136–141. | ||
McCarty JM, Phillips A, Wiisanen R. Comparative safety and efficacy of clarithromycin and amoxicillin/clavulanate in the treatment of acute otitis media in children. Pediatr Infect Dis J. 1993;12(12 Suppl 3): S122–S127. | ||
Coles SJ, Addlestone MB, Kamdar MK, Macklin JL. A comparative study of clarithromycin and amoxycillin suspensions in the treatment of pediatric patients with acute otitis media. Infection. 1993;21(4):272–278. | ||
Aronovitz G. A multicenter, open label trial of azithromycin vs. amoxicillin/clavulanate for the management of acute otitis media in children. Pediatr Infect Dis J. 1996;15(9 Suppl):15–19. | ||
Dagan R, Johnson CE, McLinn S, et al. Bacteriologic and clinical efficacy of amoxicillin/clavulanate vs. azithromycin in acute otitis media. Pediatr Infect Dis J. 2000;19(2):95–104. | ||
Anti-infective Review Panel. Anti-infective guidelines for community acquired infections. Toronto, ON: MUMS Guideline Clearinghouse; 2013. | ||
Forgie S, Zhanel G, Robinson J. Management of acute otitis media. Paediatr Child Health. 2009;14(7):457–464. | ||
Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999. | ||
National Institute for Health and Care Excellence. Guideline: otitis media (acute): antimicrobial prescribing; 2017. Available from: https://www.nice.org.uk/guidance/gid-apg10001/documents/draft-guideline. Accessed July 11, 2018. | ||
Dubois J, Saint-Pierre C, Tremblay C. Efficacy of clarithromycin vs. amoxicillin/clavulanate in the treatment of acute maxillary sinusitis. Ear Nose Throat J. 1993;72(12):804–810. | ||
Adelglass J, Jones TM, Ruoff G, et al. A multicenter, investigator-blinded, randomized comparison of oral levofloxacin and oral clarithromycin in the treatment of acute bacterial sinusitis. Pharmacotherapy. 1998;18(6):1255–1263. | ||
Riffer E, Spiller J, Palmer R, Shortridge V, Busman TA, Valdes J. Once daily clarithromycin extended-release vs twice-daily amoxicillin/clavulanate in patients with acute bacterial sinusitis: a randomized, investigator-blinded study. Curr Med Res Opin. 2005;21(1):61–70. | ||
Casiano RR. Azithromycin and amoxicillin in the treatment of acute maxillary sinusitis. Am J Med. 1991;91(3A):27S–30S. | ||
Gutiérrez-Castrellón P, Mayorga-Buitron JL, Bosch-Canto V, Solomon-Santibañez G, de Colsa-Ranero A. Efficacy and safety of clarithromycin in pediatric patients with upper respiratory infections: a systematic review with meta-analysis. Rev Invest Clin. 2012;64(2):126–135. | ||
Aw C, Benninger MS, Brook I, et al. Executive summary: IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:1041–1045. | ||
Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of rhinosinusitis and nasal polyposis. Clin Exp Allergy. 2008;38(2):260–275. | ||
Oakley GM, Harvey RJ, Lund VJ. The role of macrolides in chronic rhinosinusitis (CRSsNP and CRSwNP). Curr Allergy Asthma Rep. 2017;17(5):30. | ||
Bishai WR. Macrolide immunomodulatory effects and symptom resolution in acute exacerbation of chronic bronchitis and acute maxillary sinusitis: a focus on clarithromycin. Expert Rev Anti Infect Ther. 2006;4(3):405–416. | ||
Cervin A, Wallwork B, Mackay-Sim A, Coman WB, Greiff L. Effects of long-term clarithromycin treatment on lavage-fluid markers of inflammation in chronic rhinosinusitis. Clin Physiol Funct Imaging. 2009;29(2):136–142. | ||
Banerjee D, Honeybourne D, Khair OA. The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: a randomized controlled trial. Treat Respir Med. 2004;3(1):59–65. | ||
Anderson R, Steel HC, Cockeran R, et al. Clarithromycin alone and in combination with ceftriaxone inhibits the production of pneumolysin by both macrolide-susceptible and macrolide-resistant strains of Streptococcus pneumoniae. J Antimicrob Chemother. 2007;59(2):224–229. | ||
Chien SM, Pichotta P, Siepman N, Chan CK. Treatment of community-acquired pneumonia: a multi-center, double-blind, randomized study comparing clarithromycin with erythromycin. Chest. 1993;103(3):697–701. | ||
Anderson G, Esmonde TS, Coles S, Macklin J, Carnegie C. A comparative safety and efficacy study of clarithromycin and erythromycin stearate in community-acquired pneumonia. J Antimicrob Chemother. 1991;27(Suppl A):117–124. | ||
O’Doherty B, Muller O. Randomized, multicentre study of the efficacy and tolerance of azithromycin versus clarithromycin in the treatment of adults with mild to moderate community-acquired pneumonia. Azithromycin Study Group. Eur J Clin Microbiol Infect Dis. 1998;17(12):828–833. | ||
Macklin JL, James I, Kearsley NJ, Coles SJ. A single-blind, randomised, comparative study of clarithromycin and amoxycillin suspensions in the treatment of children with lower respiratory tract infections. J Chemother. 1993;5(3):174–180. | ||
Gotfried MH, Dattani D, Riffer E, et al. A controlled, double-blind, multicenter study comparing clarithromycin extended-release tablets and levofloxacin tablets in the treatment of community-acquired pneumonia. Clin Ther. 2002;24(5):736–751. | ||
Sokol WN Jr, Sullivan JG, Acampora MD, Busman TA, Notario GF. A prospective, double-blind, multicenter study comparing clarithromycin extended-release with trovafloxacin in patients with community-acquired pneumonia. Clin Ther. 2002;24(4):605–615. | ||
Querol-Ribelles JM, Tenías JM, Querol-Borrás JM, et al. Levofloxacin versus ceftriaxone plus clarithromycin in the treatment of adults with community-acquired pneumonia requiring hospitalization. Int J Antimicrob Agents. 2005;25(1):75–83. | ||
Lin TY, Lin SM, Chen HC, et al. An open-label, randomized comparison of levofloxacin and amoxicillin/clavulanate plus clarithromycin for the treatment of hospitalized patients with community-acquired pneumonia. Chang Gung Med J. 2007;30(4):321–332. | ||
Hardy DJ, Swanson RN, Rode RA, Marsh K, Shipkowitz NL, Clement JJ. Enhancement of the in vitro and in vivo activities of clarithromycin against Haemophilus influenzae by 14-hydroxy-clarithromycin, its major metabolite in humans. Antimicrob Agents Chemother. 1990;34(7):1407–1413. | ||
Pakhale S, Mulpuru S, Verheij TJ, Kochen MM, Rohde GG, Bjerre LM. Antibiotics for community-acquired pneumonia in adult outpatients. Cochrane Database Syst Rev. 2014;10:CD002109. | ||
Sligl WI, Asadi L, Eurich DT, Tjosvold L, Marrie TJ, Majumdar SR. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med. 2014;42(2):420–432. | ||
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus Guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. | ||
Levy ML, Le Jeune I, Woodhead MA, Macfarlane JT, Lim WS; British Thoracic Society Community Acquired Pneumonia in Adults Guideline Group. Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK. Prim Care Respir J. 2010;19(1):21–27. | ||
Bradley JS, Byington CL, Shah SS, et al. Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):617–630. | ||
Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1–ii23. | ||
Bradbury F. Comparison of azithromycin versus clarithromycin in the treatment of patients with lower respiratory tract infection. J Antimicrob Chemother. 1993;31(Suppl E):153–162. | ||
Swanson RN, Lainez-Ventosilla A, De Salvo MC, Dunne MW, Amsden GW. Once-daily azithromycin for 3 days compared with clarithromycin for 10 days for acute exacerbation of chronic bronchitis: a multicenter, double-blind, randomized study. Treat Respir Med. 2005;4:31–39. | ||
Siempos II, Dimopoulos G, Korbila IP, Manta K, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Respir J. 2007;29(6):1127–1137. | ||
Donath E, Chaudhry A, Hernandez-Aya LF, Lit L. A meta-analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with chronic obstructive pulmonary disease. Respir Med. 2013;107(9):1385–1392. | ||
Wenzel RP, Fowler AA, Edmond MB. Antibiotic prevention of acute exacerbations of COPD. N Engl J Med. 2012;367(4):340–347. | ||
Gotfried MH, DeAbate CA, Fogarty C, Mathew CP, Sokol WN. Comparison of 5-day, short-course gatifloxacin therapy with 7-day gatifloxacin therapy and 10-day clarithromycin therapy for acute exacerbation of chronic bronchitis. Clin Ther. 2001;23(1):97–107. | ||
Wilson R, Schentag JJ, Ball P, Mandell L; 068 Study Group. A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long-term clinical outcomes. Clin Ther. 2002;24(4):639–652. | ||
Dunn CJ, Barradell LB. Azithromycin. A review of its pharmacological properties and use as 3-day therapy in respiratory tract infections. Drugs. 1996;51(3):483–505. | ||
Fernandes PB, Bailer R, Swanson R, et al. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob Agents Chemother. 1986;30(6):865–873. | ||
Hardy DJ, Guay DR, Jones RN. Clarithromycin, a unique macrolide. A pharmacokinetic, microbiological, and clinical overview. Diagn Microbiol Infect Dis. 1992;15(1):39–53. | ||
Langtry HD, Brogden RN. Clarithromycin. A review of its efficacy in the treatment of respiratory tract infections in immunocompetent patients. Drugs. 1997;53(6):973–1004. | ||
Retsema J, Girard A, Schelkly W, et al. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against Gram-negative organisms. Antimicrob Agents Chemother. 1987;31(12):1939–1947. | ||
Whitman MS, Tunkel AR. Azithromycin and clarithromycin: overview and comparison with erythromycin. Infect Control Hosp Epidemiol. 1992;13(6):357–368. | ||
Ednie LM, Visalli MA, Jacobs MR, Appelbaum PC. Comparative activities of clarithromycin, erythromycin, and azithromycin against penicillin-susceptible and penicillin-resistant pneumococci. Antimicrob Agents Chemother. 1996;40(8):1950–1952. | ||
Barry AL, Pfaller MA, Fuchs PC, Packer RR. In vitro activities of 12 orally administered antimicrobial agents against four species of bacterial respiratory pathogens from U.S. medical centers in 1992 and 1993. Antimicrob Agents Chemother. 1994;38(10):2419–2425. | ||
Appelbaum PC. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15(1):77–83. | ||
Kays MB, Denys GA. In vitro activity and pharmacodynamics of azithromycin and clarithromycin against Streptococcus pneumoniae based on serum and intrapulmonary pharmacokinetics. Clin Ther. 2001;23(3):413–424. | ||
Goto H. Multicenter surveillance of adult atypical pneumonia in Japan: its clinical features, and efficacy and safety of clarithromycin. J Infect Chemother. 2011;17(1):97–104. | ||
Hamedani P, Ali J, Hafeez S, et al. The safety and efficacy of clarithromycin in patients with Legionella pneumonia. Chest. 1991;100(6):1503–1506. | ||
Loza E, Martínez Beltrán J, Baquero F, et al. Comparative in vitro activity of clarithromycin. Eur J Clin Microbiol Infect Dis. 1992;11(9):856–866. | ||
Unal D, Fenercioglu A, Ozbay L, Ozkirim B, Erol D. The effect of hydroxy metabolites of clarithromycin to the pharmacokinetic parameters, and determination of hydroxy metabolites ratio of clarithromycin. Eur J Drug Metab Pharmacokinet. 2009;34(1):27–30. | ||
Martin SJ, Garvin CG, McBurney CR, Sahloff EG. The activity of 14-hydroxy clarithromycin, alone and in combination with clarithromycin, against penicillin- and erythromycin-resistant Streptococcus pneumoniae. J Antimicrob Chemother. 2001;47(5):581–587. | ||
Walker KJ, Larsson AJ, Zabinski RA, Rotschafer JC. Evaluation of antimicrobial activities of clarithromycin and 14-hydroxyclarithromycin against three strains of Haemophilus influenzae by using an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1994;38(9):2003–2007. | ||
Bergeron MG, Bernier M, L’Ecuyer J. In vitro activity of clarithromycin and its 14-hydroxy-metabolite against 203 strains of Haemophilus influenzae. Infection. 1992;20(3):164–167. | ||
Hoover WW, Barrett MS, Jones RN. Clarithromycin in vitro activity enhanced by its major metabolite, 14-hydroxyclarithromycin. Diagn Microbiol Infect Dis. 1992;15(3):259–266. | ||
Bergman KL, Olsen KM, Peddicord TE, Fey PD, Rupp ME. Antimicrobial activities and postantibiotic effects of clarithromycin, 14-hydroxy-clarithromycin, and azithromycin in epithelial cell lining fluid against clinical isolates of Haemophilus influenzae and Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43(5):1291–1293. | ||
Eisenberg F, Barza M. Azithromycin and clarithromycin. Curr Clin Topics Infect Dis. 1994;14:52–79. | ||
Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25(Suppl A):73–82. | ||
Neu HC. The development of macrolides: clarithromycin in perspective. J Antimicrob Chemother. 1991;27(Suppl A):1–9. | ||
Piscitelli SC, Danziger LH, Rodvold KA. Clarithromycin and azithromycin: new macrolide antibiotics. Clin Pharm. 1992;11(2):137–152. | ||
Gotfried MH, Danziger LH, Rodvold KA. Steady-state plasma and bronchopulmonary characteristics of clarithromycin extended-release tablets in normal healthy adult subjects. J Antimicrob Chemother. 2003;52(3):450–456. | ||
Rodvold KA, Gotfried MH, Danziger LH, Servi RJ. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob Agents Chemother. 1997;41(6):1399–1402. | ||
Patel KB, Xuan D, Tessier PR, Russomanno JH, Quintiliani R, Nightingale CH. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob Agents Chemother. 1996;40(10):2375–2379. | ||
Nightingale CH. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr Infect Dis J. 1997;16(4):438–443. | ||
Conte JE, Jr, Golden J, Duncan S, McKenna E, Lin E, Zurlinden E. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob Agents Chemother. 1996;40(7):1617–1622. | ||
Baldwin DR, Wise R, Andrews JM, Ashby JP, Honeybourne D. Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J. 1990;3(8):886–890. | ||
Kastner U, Guggenbichler JP. Influence of macrolide antibiotics on promotion of resistance in the oral flora of children. Infection. 2001;29(5):251–256. | ||
Kikuchi E, Yamazaki K, Kikuchi J, et al. Pharmacokinetics of clarithromycin in bronchial epithelial lining fluid. Respirology. 2008;13(2):221–226. | ||
Gan VN, McCarty JM, Chu SY, Carr R. Penetration of clarithromycin into middle ear fluid of children with acute otitis media. Pediatr Infect Dis J. 1997;16(1):39–43. | ||
Sundberg L, Cederberg A. Penetration of clarithromycin and its 14-hydroxy metabolite into middle ear effusion in children with secretory otitis media. J Antimicrob Chemother. 1994;33(2):299–307. | ||
Pukander J, Rautianen M. Penetration of azithromycin into middle ear effusions in acute and secretory otitis media in children. J Antimicrob Chemother. 1996;37(Suppl C):53–61. | ||
Margaritis VK, Ismailos GS, Naxakis SS, Mastronikolis NS, Goumas PD. Sinus fluid penetration of oral clarithromycin and azithromycin in patients with acute rhinosinusitis. Am J Rhinol. 2007;21(5):574–578. | ||
Zhanel GG, DeCorby M, Noreddin A, et al. Pharmacodynamic activity of azithromycin against macrolide-susceptible and -resistant Streptococcus pneumoniae simulating clinically achievable free serum, epithelial lining fluid and middle ear fluid concentrations. J Antimicrob Chemother. 2003;52(1):83–88. | ||
Noreddin AM, Roberts D, Nichol K, Wierzbowski A, Hoban DJ, Zhanel GG. Pharmacodynamic modeling of clarithromycin against macrolide-resistant [PCR-positive mef(A) or erm(B)] Streptococcus pneumoniae simulating clinically achievable serum and epithelial lining fluid free-drug concentrations. Antimicrob Agents Chemother. 2002;46(12):4029–4034. | ||
Doern GV, Heilmann KP, Huynh HK, Rhomberg PR, Coffman SL, Brueggemann AB. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999–2000, including a comparison of resistance rates since 1994–1995. Antimicrob Agents Chemother. 2001;45(6):1721–1729. | ||
Powis J, McGeer A, Green K, et al. In vitro antimicrobial susceptibilities of Streptococcus pneumoniae clinical isolates obtained in Canada in 2002. Antimicrob Agents Chemother. 2004;48(9):3305–3311. | ||
Low DE. Resistance and treatment implications: pneumococcus, Staphylococcus aureus and Gram-negative rods. Infect Dis Clin North Am. 1998;3:613–630. | ||
Hyde TB, Gay K, Stephens DS, et al. Macrolide resistance among invasive Streptococcus pneumoniae isolates. JAMA. 2001;286(15):1857–1862. | ||
Bergman M, Huikko S, Huovinen P, Paakkari P, Seppälä H; Finnish Study Group for Antimicrobial Resistance (FiRE Network). Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2006;50:3646–3650. | ||
Blondeau JM, Shebelski SD, Hesje CK. Killing of Streptococcus pneumoniae by azithromycin, clarithromycin, erythromycin, telithromycin and gemifloxacin using drug minimum inhibitory concentrations and mutant prevention concentrations. Int J Antimicrob Agents. 2015;45(6):594–599. | ||
Metzler K, Drlica K, Blondeau JM. Minimal inhibitory and mutant prevention concentrations of azithromycin, clarithromycin and erythromycin for clinical isolates of Streptococcus pneumoniae. J Antimicrob Chemother. 2013;68(3):631–635. | ||
Hoffman HL, Klepser ME, Ernst EJ, Petzold CR, Sa’Adah LM, Doern GV. Influence of macrolide susceptibility on efficacies of clarithromycin and azithromycin against Streptococcus pneumoniae in a murine lung infection model. Antimicrob Agents Chemother. 2003;47(2):739–746. | ||
Davidson RJ, Chan CCK, Doern G, Zhanel GG. Macrolide-resistant Streptococcus pneumoniae in Canada: correlation with azithromycin use. Clin Microb Infect. 2003;9:240. | ||
Kuster SP, Rudnick W, Shigayeva A, et al. Previous antibiotic exposure and antimicrobial resistance in invasive pneumococcal disease: results from prospective surveillance. Clin Infect Dis. 2014;59(7):944–952. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

