Back to Journals » Infection and Drug Resistance » Volume 10
In situ detection of Chlamydia pneumoniae, C. trachomatis, and cytokines among cardiovascular diseased patients from the Amazon region of Brazil
Authors Freitas LS, Almeida NCC, Freitas Queiroz MA , Zaninotto MM, Fuzii HT, Ribeiro-Silva A, Vallinoto ACR , Ishak MOG, Quaresma JAS , Ishak R
Received 4 October 2016
Accepted for publication 20 December 2016
Published 10 April 2017 Volume 2017:10 Pages 109—114
DOI https://doi.org/10.2147/IDR.S123801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Larissa S Freitas,1 Núbia Caroline C Almeida,1 Maria Alice Freitas Queiroz,1 Marcelo M Zaninotto,2 Hellen T Fuzii,3 Alfredo Ribeiro-Silva,4 Antonio CR Vallinoto,1 Marluísa OG Ishak,1 Juarez AS Quaresma,3 Ricardo Ishak1
1Virus Laboratory, Institute of Biological Sciences, Federal University of Para, 2Hospital of Clinic Gaspar Viana, 3Laboratory of Immunopathology, Nucleus of Tropical Medicine, Federal University of Para, Belém, 4Departament of Pathology, Medical School of Ribeirão Preto, University of Sao Paulo, São Paulo, Brazil
Background: Chronic coronary artery disease has been associated, as a consequence of the local inflammatory reaction with previous or persistent infection with Chlamydia pneumoniae, which led to the investigation of the association of cardiovascular disease and previous infection with C. trachomatis and the role of cytokine profile (in situ) markers in the vascular system tissues.
Methods: Sixty-nine biopsies were collected for immunohistochemical analysis for the presence of IL-6, IL-8, TNF-α, IFN-γ, TGF-β, and IL-10, in 16 fragments from atheromatous plaques, 32 aorta fragments, and 21 valve fragments, using a tissue microarray technique for paraffin embedded tissues.
Results: Most patients undergoing revascularization surgery were men >50 years, while those undergoing valve replacement were mostly women <50 years. TNF-α was the most prevalent marker, detected in 91.7% (55/60) of the samples. The mean percent area stained was greater in patients infected with C. pneumoniae (3.81% vs 1.92%; p=0.0115) and specifically in the aorta (4.83% vs 2.25%; p=0.0025); C. trachomatis infection was higher in valves, and C. pneumoniae in plaques, both without statistical significance. There was no significant difference in the cytokine staining profile between patients previously infected with both species and uninfected patients.
Conclusion: Although there was no difference in the cytokine profile between patients previously infected with both species of Chlamydia, and uninfected patients, the presence of the bacteria antigens in the three biological specimens indicates it is important to focus on the role of C. trachomatis. It is necessary to improve the understanding of the natural history of chronic coronary artery disease and the clinical history of the patients and cytokine dynamics in cardiac disease in the presence or absence of infectious agents.
Keywords: Chlamydia, C. pneumoniae, C. trachomatis, atherosclerosis, cytokines
Background
Coronary artery disease (CAD) is considered to be an inflammatory disease, which has its roots in childhood and progresses slowly until adulthood when clinical manifestations will be observed, thus demonstrating the existence of a long asymptomatic period.1,2
Atherosclerosis and related diseases, particularly myocardial infarction and coronary disease, are a leading cause of morbidity and mortality in the world.3 Several infectious agents have been indicated as possible causes of vascular injury and inflammation that lead to the development of CAD and peripheral vascular disease.4,5 Studies have shown that minor bacterial infections such as respiratory infections can increase the risk of stroke.6
Chlamydia pneumoniae has been detected in coronary atherosclerotic plaques by different methods, including immunocytochemistry, transmission electron microscopy,7 and molecular biology techniques.8 On the basis of the hypothesis that inflammation associated with Chlamydia infection plays an important role in atherosclerotic plaque instability, causing fissure or rupture of the plaque, it has been suggested that in patients with unstable angina lymphocytes react with chlamydial structures in a cell-mediated immune response. The alteration in the inflammatory response observed in patients with symptomatic CAD may be associated with the combination of a humoral and cell-mediated response against C. pneumoniae.9
The presence of C. pneumoniae and C. trachomatis, using immunohistochemistry, was investigated in biological specimens of patients undergoing myocardial revascularization and aortic or mitral valve replacement, and assessed the cytokine profile of patients in the presence or absence of previous Chlamydia infection.
Methods
Study design and target population
A cross-sectional, analytical study was conducted on patients with coronary insufficiency submitted to myocardial revascularization and on patients with valve insufficiency submitted to valve implantation between February 2012 and February 2013. Patients admitted as a first-time surgical indication for myocardial revascularization or valve (aortic or mitral) replacement, who did not use antibiotics, were included in the study. Patients from whom no biological specimens were collected, or with an indication for surgery other than the procedures of interest, and those who refused to sign the informed consent form were excluded.
Sample collection
Sixty-nine biological samples (one per patient) were collected at three hospitals in the metropolitan region of Belém: Fundação Hospital das Clínicas Gaspar Viana (n=10), Hospital da Ordem Terceira (n=8), and Hospital Beneficente Portuguesa (n=51). Fragments from atheromatous plaques (n=16), ascending aorta (n=32), mitral valve (n=15), and aortic valve (n=6) were obtained during the surgical procedures, stored in microtubes containing 10% formalin and were sent to the Laboratory of Immunopathology, Center of Tropical Medicine, Federal University of Pará (Núcleo de Medicina Tropical da Universidade Federal do Pará – NMT-UFPA), where they were embedded in paraffin blocks for histopathological and immunohistochemical analysis.
Tissue microarray
The tissue microarray (TMA) technique has been improved by other investigators,10 and further developed at the end of the 1990.11 It consists of the construction of paraffin blocks containing cylindrical fragments of tissue samples extracted from paraffin donor blocks. These cylinders are transferred to the recipient block following a predetermined order.
The TMA blocks were produced at the Laboratory of Oncopathology, Department of Pathology and Forensic Medicine, School of Medicine of Ribeirão Preto, University of São Paulo (Faculdade de Medicina de Ribeirão Preto – Universidade de São Paulo – FMRP-USP). The Tissue MicroArray Builder Kit (TMA builder ab1802, Abcam, Cambridge, UK) was used for construction of the microarrays. A maximum of three cylindrical fragments were removed from the donor block of the biopsy of each patient and embedded in the TMA block.
The samples were grouped in the blocks according to the type of biopsy. Thus, seven TMA blocks were produced as follows: block 1 (B1) consisted of aortic and mitral valve fragments, block 2 (B2) consisted of mitral valve fragments, block 3 (B3) and block 4 (B4) consisted of aortas, block 5 (B5) consisted of aortas and atheromatous plaques, block 6 (B6) consisted of atheromatous plaques, and block 7 (B7) consisted of atheromatous plaques and aortic and mitral valves.
Staining for cytokines, C. pneumoniae, and C. trachomatis
TMA blocks were cut into 3 µm histological sections in a rotary microtome, and the sections were mounted on slides for immunohistochemistry.
The sections were deparaffinized in xylene and dehydrated in ethyl alcohol. Endogenous peroxidase was blocked by three incubations of 15 minutes each in 3% hydrogen peroxide in a dark chamber. Antigen retrieval was performed by incubating the sections in citrate buffer, pH 6, at 95ºC in a Pascal chamber for 20 minutes. The sections were then incubated in skim milk to block nonspecific proteins. Next, the sections were incubated overnight with the following primary monoclonal antibodies diluted in 1% bovine serum albumin at 4–8ºC in a moist chamber: anti-IL-6 (Abcam 6672; diluted 1:400); anti-IL-8 (Abcam 7747; diluted 1:100); anti-TNF-α (Abcam 9739; diluted 1:100); anti-IFN-γ (R&D Systems 285-IF; diluted 1:125); anti-TGF-β (Abcam 66043; diluted 1:100); anti-IL-10 (Abcam 34843; diluted 1:400), anti-C. pneumoniae (Fitzgerald 10-C27B; diluted 1:50 [Fitzgerald, Acton, MA, USA]), and anti-C. trachomatis (Novus Biologicals K14.67; diluted 1:50 [Novus Biologicals, Littleton CO, USA]).
The slides were washed in phosphate buffered saline (PBS) for subsequent incubation with the biotinylated secondary antibody for 30 minutes at 37ºC. After a new wash in PBS, the slides were incubated with streptavidin for 30 minutes at 37ºC. After washing, the reaction was developed with a chromogen solution consisting of diaminobenzidine and hydrogen peroxide according to the manufacturer’s recommendations. The slides were counterstained with hematoxylin and hydrated in alcohol.
The slides were observed and analyzed under a Zeiss microscope (model 456006) equipped with a 40× A-plan objective. Five photomicrographs were acquired from each cylindrical fragment per patient, with an average of 15 images per patient. The photomicrographs were analyzed using the ImageJ 1.43g program (National Institutes of Health, Bethesda, MD, USA). After determination of the percent area stained per image (15 images/patient/marker), one mean was calculated and defined as the gross value of stained area to each patient.
Statistical analysis
The results were stored in Excel spreadsheets, analyzed using the GraphPad Prism 5.0 program, and were presented in tables and/or figures. Continuous variables were reported as measures of central tendency (mean) and variability (standard deviation). The Mann–Whitney test and a binomial test were used for comparative analysis between independent samples, adopting an alpha level (α) of 0.05 (5%) for a 95% confidence interval.
Ethics
The project was approved by the Ethics Committee of the Hemotherapy and Hematology Center of Para, Fundacao HEMOPA (No. 0011.0.324.000-09). All participating patients signed an informed consent form.
Results
Thirty of the 69 samples collected were obtained from females and 39 from males. The age of the patients ranged from 22 to 77 years (mean of 55.8 years). Among the patients submitted to revascularization, 18 were females and 30 were males, with a mean age of 59.4 years, which yielded 32 samples of aorta and 16 plaques. The valve replacement group included 12 females and nine males, with a mean age of 48.04 years, which yielded six biological samples from aortic valve and 15 from mitral valves (Table 1).
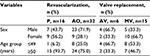  | Table 1 Frequency distribution of histological sections by sex and age group, according to the surgical procedure Abbreviations: P, atheromatous plaque; AO, aorta; AV, aortic valve; MV, mitral valve. |
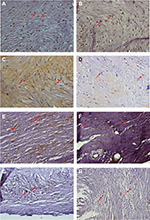
All histological sections were tested for all markers. Positive staining for each marker is illustrated in Figure 1. Regarding the overall prevalence of the markers, TNF-α was the most frequent (91.7%), followed by IL-6 (90.6%), IL-8 (60.6%), IL-10 (54.7%), TGF-β (27,5%), and IFN-γ (20.8%). Markers of C. pneumoniae were detected in 70.7% and in 65.4% of C. trachomatis.
A significant difference was observed in the overall percent area stained, which was almost twice of that for C. pneumoniae compared to C. trachomatis (3.814 vs 1.9818; Figure 2A). The presence of C. trachomatis was higher in the valves (2.474 vs 1.800) and greater for C. pneumoniae in the plaques (3.783 vs 0.702), but without statistical significance in both situations. The aorta fragments, however, showed a significantly higher difference in the stained areas for C. pneumoniae (4.830 vs 2.247; Figure 2B).
The percentage of the area stained with each cytokine, in the presence or absence of infection with one or both species of Chlamydia, was higher for TNF-α and IL-6 in C. pneumoniae and C. trachomatis infected biological specimens than in non-infected material, but in both there was no statistical significance (Table 2). All the other cytokines were present mostly in equal levels between the infected and non-infected specimens.
Discussion
Although the aorta fragments did not exhibit plaque formation, they were obtained from patients with an indication for revascularization surgery. With regard to the patients with aortic and mitral valvulopathies who had an indication for valve replacement, it was not possible to obtain information about other cardiovascular diseases, such as the formation of atheromatous plaques, therefore it cannot be ruled out the chance that these patients did not suffer from such illness.
Most patients undergoing revascularization surgery were men. Although atherosclerosis continues to be a disease that affects mainly men;12 presently, the different roles played by women in society may hamper access to resources that could reduce exposure to risk factors.13 The clinical presentation of the disease varies in women, a fact that leads to inadequate diagnostic and treatment interventions.14
The risk of developing cardiovascular diseases increases with age, as the natural process of cellular aging results in vascular changes.15 The number of stem cells and their different functional aspects decreases with aging, compromising the vascular reparative capacity and leading to the progression of atherosclerosis.16 In the present study, the patients undergoing revascularization surgery had a mean age of more than 50 years which is in agreement with the increase of the thickness of the intima with aging.17
Most of the patients undergoing valve replacement were women younger than 50 years, which was also found before.18 Developing countries present a major chance of socioeconomic losses since valvulopathies usually affect young age individuals in their full working abilities.19 The possible causes of valvulopathies (eg, rheumatic fever and congenital malformations) were not included as the main focus of the study.
There were three distinct groups according to the cytokine frequencies in situ. TNF-α and IL-6 were present in more than 90%, IL-8 and IL-10 in a little more than half of the samples, and IFN-γ and TGF-β in low frequencies.
The cytokine marker showing the highest overall frequency was TNF-α, an active cytokine in the inflammatory process and in the pathogenesis of the CAD. TNF-α is produced by activated macrophages and other cell types, including cardiac myocytes. Large amounts of TNF-α are released in response to lipopolysaccharide and other bacterial products and may induce a variety of responses involved in systemic inflammation, including the expression of surface adhesion proteins on leukocytes and endothelial cells, metabolic changes, and the secretion of platelet-derived growth factor by smooth muscle cells.20–22
IL-6 was also found in a similar high frequency and acts as both a proinflammatory cytokine and an anti-inflammatory myokine, produced by macrophages and active T lymphocytes with an important role in the acute inflammatory reaction.23,24 So far, IL-6 has not been associated to cardiac disease, but as a myokine, it is significantly elevated with exercise and muscle contraction and in response to the production of IL-10.
Although cytokines produced by vascular cells and mononuclear phagocytes have been shown to stimulate the production of additional cytokines,17–25 no difference was observed between cytokine production in patients previously infected or not with Chlamydia.
IL-8 is produced by macrophages and other cell types such as muscle cells. It induces chemotaxis and phagocytosis of neutrophils and other granulocytes, causing them to migrate toward the site of infection. IL-10 presents multiple, pleiotropic, effects in immunoregulation and inflammation. It downregulates the expression of Th1 cytokines, and enhances B cell survival, proliferation, and antibody production, possibly controlling tissue damage.26 The decrease of IL-10 regulates TNF-α levels,26 resulting in its rise and inflammation.
Live bacteria and C. pneumoniae antigens generated by sonication can induce the release of proinflammatory cytokines.27,28 These acellular components are able to induce the production of cytokines as potent as live microorganisms. The presence of dead Chlamydia in atheromatous lesions may serve as a source of antigens that trigger the paracrine and autocrine activation of cells, resulting in the maintenance of chronic inflammation in a similar fashion as observed with C. trachomatis in arthritis in which the disease may progress even when antibiotic therapy is finished.29
Antigens from both dead and live Chlamydia induce peripheral blood mononuclear cells to produce proinflammatory cytokines, including TNF-α, IL-6, and IL-8, at a proportion that was much higher than that observed for uninfected HEp2 cells.28 In the present study, although TNF-α and IL-6 were more frequently found among infected patients, no significant differences were observed when compared to uninfected persons.
The atherogenic effect of the infection of both C. pneumoniae and C. trachomatis was showed to occur in mice.30 Both species were able to establish infection in the lungs and to spread to the aorta. The mechanism by which infection accelerates the progression of atherosclerosis is still unknown. The effect could be an indirect one through lung infection and the release of proatherogenic cytokines, directed through aortic infection, or a combination of both. However, differently from C. pneumoniae, C. trachomatis does not establish persistent infection in the lungs or in the aorta and does not seem to increase lesion size in mice. In the present study, C. pneumoniae was generally more prevalent and more disseminated than C. trachomatis as seen in the figures presenting the bacteria dissemination in the cardiovascular tissue examined.
Conclusion
The present paper confirms that C. pneumoniae is present in the tissues of the cardiac and vascular system and has been long associated to disease, particularly with atherosclerosis. It is rather important to emphasize, however, that C. trachomatis a common sexually transmitted infection is, without any doubt, also capable to be present in the atheromatous plaque, the aorta and valves inside the cardiac muscle, placing the bacterium as an active factor implicated in the disease. The inflammatory process was clearly involved in the disease as pointed by the massive presence of proinflammatory cytokines, but the microenvironment shows the human host actively attempting to evade from the infection of both C. pneumoniae and C. trachomatis and from the damage of the inflammatory reaction.
Acknowledgments
This study was generously funded by a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico/ Programa de Apoio a Núcleos de Excelência/ Fundação Amazônia de Amparo à Estudos e Pesquisa (CNPq/PRONEX/FAPESPA).
The authors thank Dr Marco Fossa who performed the cardiac surgeries and the subjects who participated in the present study.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Noll G. Pathogenesis of atherosclerosis: a possible relation to infection. Atherosclerosis. 1998;140(1):S3–9. | ||
Personen E, Liuba P. Footprints of atherosclerotic coronary heart disease in children. Rev Port Cardiol. 2004;23(1):127–131. | ||
Nieminen MS, Mattila K, Valtonen V. Infection and inflammation as risk factors for myocardial infarction. Eur Heart J. 1993;14(Suppl K):12–16. | ||
Kuvin JT, Kimmelstiel CD. Infectious causes of atherosclerosis. Am Heart J. 1999;137(2):216–226. | ||
Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10(2):83–92. | ||
Grau AJ, Buggle F, Heindl S, et al. Recent infection as a risk factor for cerebrovascular ischemia. Stroke. 1995;26(3):373–379. | ||
Shor A, Kuo CC, Patton DL. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J. 1992;82(3):158–161. | ||
Kuo CC, Grayston JT, Campbell LA, Goo YA, Wissler RW, Benditt EP. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old). Proc Natl Acad Sci. 1995;92(15):6911–6914. | ||
Gurfinkel E, Bozovich G. Chlamydia pneumoniae: inflammation and instability of the atherosclerotic plaque. Atherosclerosis. 1998;140(1):31–35. | ||
Battifora H, Mehta P. The checkerboard tissue block, an improved multitissue control block. Lab Invest. 1990;63(5):722–724. | ||
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–847. | ||
Rollini F, Mfeukeu L, Modena MG. Assessing coronary heart disease in women. Maturitas. 2009;62(3):243–247. | ||
WHO - World Health Organization. Global Atlas on Cardiovascular Disease Prevention and Control. WHO – Regional Office for Geneva 2011. Available from http://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/. Accessed April 5, 2013. | ||
Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010;18(12):598–603. | ||
Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: Part 1 of a 2-part review. Circulation. 2011;123(15):1650–1660. | ||
Bosch-Marce M, Okuyama H, Wesley JB, et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101(12):1310–1318. | ||
Virmani R, Avolio AP, Mergner WJ, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139(5):1119–1129. | ||
Fernandes AM, Bitencourt LS, Lessa IN, et al. Impact of socio-economic profile on the prosthesis type choice used on heart surgery. Rev Bras Cir Cardiovasc. 2012;27(2):211–216. | ||
Okello E, Kakande B, Sebatta E, et al. Socioeconomic and environmental risk factors among rheumatic heart disease patients in Uganda. PLoS One. 2012;7(8):e43917. | ||
Le J, Vilcek J. Biology of disease. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987;56(3):234–248. | ||
Raines EW, Dower SK, Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGFAA. Science. 1989;243(4889):393–396. | ||
Libby P, Hansson GK. Biology of disease. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991;64(1):5–15. | ||
Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33(3):114–119. | ||
Ferguson-Smith AC, Chen YF, Newman MS, May LT, Sehgal PB, Ruddle FH. Regional localization of the interferon-beta 2/B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15-p21. Genomics. 1988;2(3):203–208. | ||
Warner SJ, Auger KR, Libby P. Interleukin-1 induces interleukin-1. II. Recombinant human interleukin-1 induces interleukin-1 production by adult human vascular endothelial cells. J Immunol. 1987;139(6):1911–1917. | ||
Jankovic D, Kullberg MC, Feng CG, et al. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204(2):273–283. | ||
Heinemann M, Susa M, Simnacher U, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64(11):4872–4875. | ||
Netea MG, Selzman CH, Kullberg BJ, et al. Acellular components of Chlamydia pneumoniae stimulate cytokine production in human blood mononuclear cells. Eur J Immunol. 2000;30(2):541–549. | ||
Meijer A, Roholl PJM, van der Vliet JA, Gielis-Proper FK, Vries A, Ossewaarde JM. Differences in detection of Chlamydia pneumoniae protein, hsp60, lipopolysaccharide and DNA in abdominal aneurysms. In Stephens RS, Byrne GI, Christiansen G, editors. Proceedings of the Ninth International Symposium on Human Chlamydial Infection; San Francisco; 1998: 191–194 | ||
Blessing E, Nagano S, Campbell LA, Rosenfeld ME, Cho-chou K. Effect of Chlamydia trachomatis on atherosclerosis in apolipoprotein E-deficient mice. Infect Immun. 2000;68(12):7195–7197. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.