Back to Journals » Drug Design, Development and Therapy » Volume 13
In Silico, Cytotoxic and Antioxidant Potential of Novel Ester, 3-hydroxyoctyl -5- trans-docosenoate Isolated from Anchusa arvensis (L.) M.Bieb. Against HepG-2 Cancer Cells
Authors Hussain S, Ullah F, Ayaz M , Ali Shah SA , Ali Shah AH, Shah SM, Wadood A, Aman W, Ullah R, Shahat AA , Nasr FA
Received 29 August 2019
Accepted for publication 13 November 2019
Published 10 December 2019 Volume 2019:13 Pages 4195—4205
DOI https://doi.org/10.2147/DDDT.S228971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Sajid Hussain,1,2 Farhat Ullah,1 Muhammad Ayaz,1 Syed Adnan Ali Shah,3 Azhar-ul-Haq Ali Shah,4 Syed Majid Shah,1,2 Abdul Wadood,5 Waqar Aman,2 Riaz Ullah,6 Abdelaaty A Shahat,6,7 Fahd A Nasr6
1Department of Pharmacy, University of Malakand, Malakand, Pakistan; 2Department of Pharmacy, Kohat University of Science & Technology, Kohat, Pakistan; 3Research Institute of Natural Products for Drug Discovery (RiND), Faculty of Pharmacy, Universiti Teknologi MARA (UiTM), Shah Alam, Malaysia; 4Department of Chemistry, Kohat University of Science & Technology, Kohat, Pakistan; 5Department of Biochemistry, Abdul Wali Khan University Mardan, Mardan, Pakistan; 6Department of Pharmacognosy (Medicinal Aromatic and Poisonous Plants Research Center), College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; 7Chemistry of Medicinal Plants Department, National Research Centre, Dokki, Giza, Egypt
Correspondence: Sajid Hussain; Farhat Ullah
Department of Pharmacy, University of Malakand, Dir (L), Chakdara 18000, KPK, Pakistan
Tel +92 3339178183; +92 3339361513
Email [email protected]; [email protected]
Background: Cancer is one of the chronic health conditions worldwide. Various therapeutically active compounds from medicinal plants were the current focus of this research in order to uncover a treatment regimen for cancer. Anchusa arvensis (A. anchusa) (L.) M.Bieb. contains many biologically active compounds.
Methods: In the current study, new ester 3-hydroxyoctyl -5- trans-docosenoate (compound-1) was isolated from the chloroform soluble fraction of A. anchusa using column chromatography. Using MTT assay, the anticancer effect of the compound was determined in human hepatocellular carcinoma cells (HepG-2) compared with normal epithelial cell line (Vero). DPPH and ABTS radical scavenging assays were performed to assess the antioxidant potential. The Molecular Operating Environment (MOE-2016) tool was used against tyrosine kinase.
Results: The structure of the compound was elucidated based on IR, EI, and NMR spectroscopy technique. It exhibited a considerable cytotoxic effect against HepG-2 cell lines with IC50 value of 6.50 ± 0.70 μg/mL in comparison to positive control (doxorubicin) which showed IC50 value of 1.3±0.21 μg/mL. The compound did not show a cytotoxic effect against normal epithelial cell line (Vero). The compound also exhibited significant DPHH scavenging ability with IC50 value of 12 ± 0.80 μg/mL, whereas ascorbic acid, used as positive control, demonstrated activity with IC50 = 05 ± 0.15 μg/mL. Similarly, it showed ABTS radical scavenging ability (IC50 = 130 ± 0.20 μg/mL) compared with the value obtained for ascorbic acid (06 ± 0.85 μg/mL). In docking studies using MOE-2016 tool, it was observed that compound-1 was highly bound to tyrosine kinase by having two hydrogen bonds at the hinge region. This good bonding network by the compound might be one of the reasons for showing significant activity against this enzyme.
Conclusion: Our findings led to the isolation of a new compound from A. anchusa which has significant cytotoxic activity against HepG-2 cell lines with marked antioxidant potential.
Keywords: cancer, Anchusa arvensis, 3-hydroxyoctyl-5, trans-docosenoate, cytotoxicity against HepG-2 cell lines, antioxidant
Introduction
Cancer is a major public health problem worldwide and is the second leading cause of death after cardiovascular disorders.1–3 Hepatocellular carcinoma is one of the various types of cancer that leads to more than 600,000 deaths worldwide on an annual basis.4,5
Plants have a long history of use in cancer therapy6–8 and several plant-derived anti-cancer drugs are still in clinical use including vincristine, vinblastine, etoposide, camptothecin derivatives, topotecan, irinotecan and paclitaxel, all isolated from plants.9,10 However, there is a dire need for development of new and novel cytotoxic drugs, drug combinations, and chemotherapy strategies, by exploration of a pool of synthetic, biological, and natural products.11
Tyrosine kinases (TKs) are beneficial for the study of drug targeting molecules which are used for different types of cancer.12,13 TKs play an important role in the variety of growth factor signalling.14 TKs are vital for different biological procedures like apoptosis, growth and migration, differentiation, and metabolism for both external and internal stimuli. TKs are enzymes which initiate the process of phosphorylation by utilizing ATP for specific tyrosine residues. The covalent and enzymatic modification of protein following biosynthesis is an important integral part for the maintenance of normal cellular communication and homeostasis.15
A. arvensis which belongs to the family Boraginaceae, has traditionally been used for the treatment of arthritis, rheumatism, gout, cancer, gastroprotection, hypotension and hypoglycemic effects.16–19 A. arvensis contains phytochemical constituents like enantiomeric pigments of naphthoquinone of alkannins and group of shikonins. The enantiomeric alkannins, shikonins and their derivatives comprise a new class of drugs, exhibiting antimicrobial, antitumor, and anti-inflammatory activities.20 Herein we report the chromatographic isolation of a new aliphatic ester from A. arvensis which was evaluated against HepG2 cell lines. The results revealed that compound-1 is an antioxidant agent due to its DPHH and ABTS radical scavenging. The in-vitro effect was further evaluated through molecular docking while targeting tyrosine kinase as target enzyme.
Materials and Methods
Chemicals and Equipment
Methanol, n-hexane, chloroform, and ethyl acetate were purchased from Merck, Germany. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemicals Co. (St. Louis, USA). Silica gel 60 F254 card (Merck) was used for thin layer chromatography (TLC) while column chromatography was carried out on silica gel (70–230 mesh, Scharlau). 2,2-diphenyl-1-picrylhydrazyl (Sigma- Aldrich, USA, CAS 1898-66-4). ABTS (Sigma-Aldrich, USA, CAS 30931-67-0). All chemicals used in experiments were of analytical grade. ELISA reader (ELx800 BioTek) was used for measuring absorbance. The extracts of A. arvensis were concentrated by using Rotary evaporator (Rota vapour 210). Nuclear magnetic resonance (NMR) spectra were recorded on Bruker AVANCE-600 MHz NMR spectrometer with 5 mm BBO probe and microcoil-based CapNMR probe.
Extraction and Isolation
The plant material was obtained from district Karak, Pakistan in April 2016. The botanical identity of the plant was determined by the plant taxonomist, Dr. Waheed Murad, Department of Botany, Kohat University of Science and Technology. A voucher specimen with catalog no KUH-1001 was submitted to the herbarium of Department of Botany, Kohat University of Science and Technology. The air-dried plant was chopped and ground to coarse powder and macerated in methanol at room temperature for 14 days.21,22 The methanol soluble residue was filtered off and concentrated under vacuum rotary evaporator at 40°C. A crude methanol extract (600 gm) was obtained from the filtrates. The crude extract (500 gm) was suspended in distilled water and sequentially fractionated with n-hexane (3 x 500 mL), chloroform (3 x 500 mL), and ethyl acetate (3 x 500 mL), yielding n-hexane (140 gm), chloroform (160 gm), ethyl acetate (90 gm), and aqueous (60 gm) fractions respectively.23,24 The chloroform extract was subjected to column chromatography over silica gel (70–230 mesh). Elution was started with n-hexane/ethyl acetate in increasing order of polarity (95:5, 90:10, 85:15, 75:25, 60:40) resulting in isolation of compound-1. The structure of the compound was determined on the basis of IR, EI, NMR spectroscopy including 2D NMR techniques and chemical methods.25
Cell Culture
Human hepatocellular carcinoma (HepG-2) and normal Vero cell lines were obtained from National Centre of Excellence in Molecular Biology (CEMB), University of the Punjab, Lahore, Pakistan. The cell lines were cultured and preserved in the Department of Biotechnology, Quaid-i-Azam University, Islamabad, Pakistan until they were used in experiments. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), HEPES, 3% glutamine, sodium bicarbonate and antibiotic (streptomycin/penicillin). The cultured cell lines were maintained in a humidified CO2 incubator at 37°C.
In-vitro Cytotoxicity Assay
We performed MTT assay to evaluate the cytotoxicity of the isolated compound.26,27 HepG2 and normal Vero cell lines were seeded in 200 μL culture medium at a density of 5×103 cells/well in 96-well plates. After incubation for 24 h, the HepG2 and normal cell lines were treated with different concentrations (200, 100, 50, 10, 5 µg/mL) of the compound and incubated for a further 24 h. Following washing and incubation with 4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (20 μL of 5 mg/mL) at 37ºC for 3 h, cells were lyzed with dimethyl sulfoxide (DMSO). Doxorubicin and DMSO were used as positive and negative controls respectively. The MTT dye was reduced by succinic dehydrogenase in the mitochondria of viable cells to formazan purple crystals. Absorbance (OD) was recorded at 570 nm using a microplate reader. The percentage of cytotoxicity compared to the untreated cells was determined using the following equation:
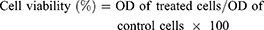
The results were generated from three independent experiments; each experiment was performed in triplicate. The IC50 values were calculated from dose-response curve.
DPPH Radical Scavenging Assay
In the present study we determined the antioxidant ability of the compound by using DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging method.28 Various concentrations (1000, 500, 250,125, 62.5, 31 and 15.5 μg/mL) of compound-1 (0.1 mL) were added to methanolic solution (0.004%) of DPPH. Absorbance of samples was measured at 517 nm wavelength using UV spectrophotometer after of 30 mins interval. Ascorbic acid was used as positive control; percent scavenging activity of samples was calculated by using the equation,
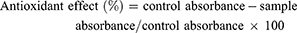
Each experiment was done in triplicate and inhibition graphs were constructed using the GraphPad prism program (GraphPad, San Diego, California, USA) and IC50 values (concentration required for 50% reduction of DPPH free radicals) were determined.
ABTS Free Radical Scavenging Assay
The antioxidant potential of compound-1 was determined using 2, 2-azinobis [3-ethylbenzthiazoline]-6-sulfonic acid (ABTS) method.29 The ABTS free radical scavenging assay is based on the ability of antioxidants to scavenge ABTS radical cation causing a reduction in absorbance. Briefly, solutions of potassium persulphate (K2S2O4) 2.45 mM and ABTS 7 mM were prepared and mixed well. The resultant mixture was kept in the dark at room temperature to get dark colored solution containing radical cations. The antioxidant ability of compound-1 was determined by mixing different concentrations (1000, 500, 250,125, 62.5, 31 and 15.5 μg/mL) of compound-1 (300 μL) with ABTS solution (3.0 mL). The reduction in absorbance was measured using UV spectrophotometer after one minute of mixing the solutions and continued for six minutes. Ascorbic acid was used as positive control and percentage inhibition was calculated using the equation:

Each experiment was repeated in triplicate. The antioxidant effect was expressed in terms of percent inhibition and IC50 (concentration required for 50% reduction of ABTS radicals) was determined.
Molecular Docking
The three dimensional (3D) structure of the newly isolated compound was modeled by Molecular Operating Environment (MOE-2016) software.30 The hydrogen atoms were added to compound-1 by 3D protonation followed by energy minimization by using MOE. The crystal structure of the tyrosine kinase was retrieved from the protein databank (PDB id: 1M17) [www.rcsb.org/pdb]. Prior to molecular docking, all water molecules were removed from the retrieved crystal structure using the Molecular Operating Environment software (www.chemcomp.com). The 3D protonation and energy minimization of the retrieved protein molecule was carried out by using MOE software with the default parameters. Compound-1 was docked into the active site of prepared tyrosine kinase using MOE by using the default parameters i.e., Placement: Triangle Matcher, Rescoring: London dG. For each ligand ten conformations were generated. The top-ranked conformation of each compound was used for further analysis.
Results and Discussion
The current study revealed the isolation of a new aliphatic ester from the chloroform soluble fraction of A. arvensis. The newly isolated compound exhibited marked antioxidant and anticancer potential in in-vitro assay that was strongly supported by molecular docking studies.
Compound-1 was obtained as colorless amorphous powder and the structure of the compound was established by IR, EI, NMR spectroscopy including 2D NMR techniques and chemical methods. The molecular formula was determined based on HREIMS and 13CNMR spectra. In HREIMS, the molecular ion peak was found at 466.7778 (calculated for C30H58O3, 466.7785) suggesting the molecular formula C30H58O3. 1HNMR spectrum displayed a triplet-like peak at δ 0.89 and a broad singlet at δ 1.27 typical of a straight chain hydrocarbon. Other prominent peaks appeared at δ 5.37, 5.28, 4.32, 4.16 that could be assigned to the protons of oxymethine, olefinic bond, and oxymethylene group respectively based on their HSQC spectrum. The 13CNMR spectrum (BB and DEPT) showed signals at δ 172.3, 130 and 129.4 that could be assigned to the carbonyl and olefinic carbons respectively (Table 1 and Figures 1–6). The oxygenated methine and oxygenated methylene resonated at δ 68.9 and 62.1 respectively. The signals observed between 29.7−29.1 revealed the presence of a long chain. The absorption band at 966 cm−1 in the IR spectrum showed the stereochemistry of the double to be trans. The 13CNMR spectrum showed allylic carbon in the chain at δ 31.8 typical of methylene adjacent to a trans double bond.31 The position of the double bond was confirmed by oxidative cleavage with osmium tetraoxide which produced heptadecanal along with other products. The position of the hydroxyl and double was also confirmed from HMBC correlations as shown in Figure 7. Thus, the structure of compound-1 was elucidated as 3-hydroxyoctyl -5- trans-docosenoate.
 |
Table 1 1HNMR Data (in CDCl3, 600 MHz) and 13CNMR Data (in CDCl3, 125 MHz) of Compound-1 |
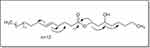 |
Figure 1 1HNMR of isolated compound, 3-hydroxyoctyl -5- trans-docosenoate. |
 |
Figure 2 1HNMR of isolated compound, 3-hydroxyoctyl -5- trans-docosenoate. |
 |
Figure 3 13CNMR of isolated compound, 3-hydroxyoctyl -5- trans-docosenoate. |
 |
Figure 4 HMBC-NMR of isolated compound, 3-hydroxyoctyl -5- trans-docosenoate. |
 |
Figure 5 HSQC-NMR of isolated compound, 3-hydroxyoctyl -5- trans-docosenoate. |
 |
Figure 6 COSY-NMR of isolated compound, 3-hydroxyoctyl -5- trans-docosenoate. |
 |
Figure 7 Chemical structure and HMBC correlations of isolated compound. |
Cytotoxicity of the compound isolated from A. arvensis was assessed against HepG2 cells by performing MTT assay (Figure 8). This assay is frequently used to investigate the cytotoxicity of test compounds.32,33 This study demonstrated that the isolated compound showed promising activity against HepG-2 cell lines with IC50 value of 6.50 ± 0.70 µg/mL in comparison to doxorubicin, a positive cytotoxic drug (IC50 value = 1.3 ± 0.21 µg/mL). DMSO was used as negative control. The compound did not show a cytotoxic effect against normal epithelial cell line (Vero). The other species belonging to the Boraginaceae family including Arnebia euchroma (Royle) Jonst,34 Alkanna cappadocica,35 Heliotropium indicum,36 Heliotropium bacciferum,37 Glandora rosmarinifolia,38 have already been reported for their anti-cancer activity.
 |
Figure 8 Cytotoxic effect of compound-1 against HepG2 cell line. |
The antioxidant activity was determined to evaluate the antioxidant potential of the compound. Results of DPHH and ABTS radical scavenging assays of compound-1 are given in Figures 9 and 10. The compound was effective in a concentration-dependant manner. The isolated compound exhibited significant DPHH scavenging ability with IC50 value of 12 ± 0.80 µg/mL whereas ascorbic acid, used as positive control, exhibited antioxidant activity with IC50 = 05 ± 0.15 µg/mL. The compound exhibited ABTS radical scavenging ability (IC50 = 130 ± 0.20 µg/mL) compared with the value obtained for ascorbic acid (06 ± 0.85 µg/mL). Oxygen is a vital element of life but under certain circumstances it can influence our health by formation of free radicals (reactive oxygen species) leading to some dangerous diseases, like coronary heart disease, diabetes, atherosclerosis, immune-suppression, and cancer.39,40 Consequently, agents with free radical scavenging abilities are useful for the prevention and therapy of several dangerous diseases including cancer.41
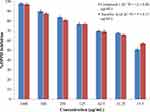 |
Figure 9 DPHH scavenging ability of compound-1. |
 |
Figure 10 ABTS scavenging ability of compound-1. |
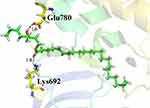
To assess the interactions of the newly isolated compound in the active site of tyrosine kinase, molecular docking, using Molecular Operating Environment (MOE-2016) software, was carried out. From the docking study, it was observed that the top ranked conformation of the isolated compound fit well and showed good interactions and docking score (−10.0745) with active site residues of tyrosine kinase. The compound showed two polar and several hydrophobic interactions with active site residues of the enzyme. The hydroxyl oxygen of compound-1 formed hydrogen bond with the carbonyl oxygen atom of active site residue Glu780. Whereas the carbonyl oxygen of the compound showed hydrogen bond with nitrogen atom of active site residue Lys692 (Figure 11). Both hydrogen bonds formed by compound-1 with active site residues were strong hydrogen bonds in terms of distance and energy (Table 2). As in both hydrogen bonds, the bonding atoms became very close to each other and that was the reason why the energy of both bonds was very small. Furthermore, there were hydrophobic interactions between compound-1 and active site residues of tyrosine kinase (Figure 11). This good bonding network shown by compound-1 might be one of the reasons why this compound showed good activity against this enzyme.42
 |
Table 2 Interaction of Ligand with the Active Site Residues |
Conclusion
In conclusion, for the first time a new compound, 3-hydroxyoctyl -5- trans-docosenoate, was isolated from the chloroform soluble fraction of A. arvensis. Our test compound exhibited significant cytotoxicity against human hepatocellular carcinoma cells (HepG-2) with IC50 value of 6.50 ±0.70 µg/mL. Also, data from the present results revealed that compound-1 is an antioxidant agent due to its DPHH and ABTS radical scavenging. In docking studies against tyrosine kinase enzyme, our compound showed top ranked conformation of our compound fit well and showed good interactions and docking score (−10.0745) with active site residues of tyrosine kinase. Further studies are underway in our Laboratory.
Abbreviations
MTT, 3-(4,5-Dimethylthiazol-yl)-diphenyl tetrazolium bromide; A. arvensis, Anchusa arvensis; TKs, tyrosine kinases; DMSO, dimethyl sulfoxide.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no RGP-262. The authors are also thankful to the Department of Pharmacy, University of Malakand, for provision of a laboratory.
Author Contributions
SH, FU, WA, and RU performed experimental work and data collection. Data were evaluated by MA, AAS, and SAAS. Computational studies were performed by AW. MA, SH and SMS were involved in literature search and manuscript preparation. MA, SH and refined the manuscript for publication. AAS, RU, and FAN financially supported the research work. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ahmad S, Ullah F, Ayaz M, Zeb A, Ullah F, Sadiq A. Antitumor and anti-angiogenic potentials of isolated crude saponins and various fractions of Rumex hastatus D. Don. Biol Res. 2016;49(1):18. doi:10.1186/s40659-016-0079-2
2. Amin A, Bajbouj K, Koch A, Gandesiri M, Schneider-Stock R. Defective autophagosome formation in p53-null colorectal cancer reinforces crocin-induced apoptosis. Int J Mol Sci. 2015;16(1):1544–1561. doi:10.3390/ijms16011544
3. Hamza AA, Ahmed MM, Elwey HM, Amin A. Melissa officinalis protects against doxorubicin-induced cardiotoxicity in rats and potentiates its anticancer activity on MCF-7 cells. PLoS One. 2016;11(11):e0167049. doi:10.1371/journal.pone.0167049
4. A Saad Zaghloul M, H Abadi A, I Abdelaziz A. Functional evaluation of imatinib mesylate in hepatocellular carcinoma cells. Recent Pat Biomark. 2013;3(1):65–71. doi:10.2174/2210309011303010065
5. Chaiboonchoe A, Khraiwesh B, Murali C, et al. Safranal induces DNA double-strand breakage and ER-stress-mediated cell death in hepatocellular carcinoma cells. Sci Rep. 2018;8(1):16951. doi:10.1038/s41598-018-34855-0
6. Baig B, Hilal-Alnaqbi A, Amin A. Cancer and biotechnology: a matchup that should never slowdown. In: Malik S, editor. Biotechnology and Production of Anti-Cancer Compounds. Springer; 2017:73–97.
7. Khattak S, Khan H. Anti-cancer potential of phyto-alkaloids: a prospective review. Curr Canc Ther Rev. 2016;12(1):66–75. doi:10.2174/1573394712666160617081638
8. Ahmad S, Ullah F, Zeb A, Ayaz M, Ullah F, Sadiq A. Evaluation of Rumex hastatus D. Don for cytotoxic potential against HeLa and NIH/3T3 cell lines: chemical characterization of chloroform fraction and identification of bioactive compounds. BMC Complement Altern Med. 2016;16(1):308. doi:10.1186/s12906-016-1302-y
9. Moudi M, Go R, Yien CYS, Nazre M. Vinca alkaloids. Int J Prev Med. 2013;4(11):1231.
10. Basili S, Moro S. Novel camptothecin derivatives as topoisomerase I inhibitors. Expert Opin Ther Pat. 2009;19(5):555–574. doi:10.1517/13543770902773437
11. Ashktorab H, Soleimani A, Singh G, et al. Saffron: the golden spice with therapeutic properties on digestive diseases. Nutrients. 2019;11(5):943. doi:10.3390/nu11050943
12. Vlahovic G, Crawford J. Activation of tyrosine kinases in cancer. Oncologist. 2003;8(6):531–538. doi:10.1634/theoncologist.8-6-531
13. Zohra T, Ovais M, Khalil AT, Qasim M, Ayaz M, Shinwari ZK. Extraction optimization, total phenolic, flavonoid contents, HPLC-DAD analysis and diverse pharmacological evaluations of Dysphania ambrosioides (L.) Mosyakin & Clemants. Nat Prod Res. 2018;33:1–7.
14. Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315(3):971–979. doi:10.1124/jpet.105.084145
15. Paul MK, Mukhopadhyay AK. Tyrosine kinase–role and significance in cancer. Int J Med Sci. 2004;1(2):101. doi:10.7150/ijms.1.101
16. Dafni A, Yaniv Z, Palevitch D. Ethnobotanical survey of medicinal plants in northern Israel. J Ethnopharmacol. 1984;10(3):295–310. doi:10.1016/0378-8741(84)90017-5
17. Ali-Shtayeh MS, Yaniv Z, Mahajna J. Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. J Ethnopharmacol. 2000;73(1):221–232. doi:10.1016/S0378-8741(00)00316-0
18. Nelly A, Annick -D-D, Frederic D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J Ethnopharmacol. 2008;120(3):315–334. doi:10.1016/j.jep.2008.08.024
19. Al-Snafi A. The pharmacology of Anchusa italica and Anchusa strigosa–A review. Int J Pharm Pharm Sci. 2014;6(4):7–10.
20. Tsermentseli S, Assimopoulou A, Gianovits-Argyriadou N, Kanaze F, Papageorgiou V. Phytochemical analysis of Anchusa arvensis roots. Planta Med. 2008;74(09):PC53. doi:10.1055/s-0028-1088319
21. Muhammad N, Saeed M, Adhikari A, Khan KM, Khan H. Isolation of a new bioactive cinnamic acid derivative from the whole plant of Viola betonicifolia. J Enzyme Inhib Med Chem. 2013;28(5):997–1001. doi:10.3109/14756366.2012.702344
22. Zohra T, Ovais M, Khalil AT, et al. Bio-guided profiling and HPLC-DAD finger printing of Atriplex lasiantha Boiss. BMC Complement Altern Med. 2019;19:4. doi:10.1186/s12906-018-2416-1
23. Ayaz M, Junaid M, Ullah F, et al. GC-MS analysis and gastroprotective evaluations of crude extracts, isolated saponins, and essential oil from Polygonum hydropiper L. Front Chem. 2017;5:58. doi:10.3389/fchem.2017.00058
24. Ovais M, Ayaz M, Khalil AT, et al. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Complement Altern Med. 2018;18(1):1. doi:10.1186/s12906-017-2057-9
25. Ayaz M, Junaid M, Ullah F, et al. Anti-Alzheimer’s studies on β-Sitosterol isolated from Polygonum hydropiper L. Front Pharmacol. 2017;8:697.
26. Khorrami S, Zarrabi A, Khaleghi M, Danaei M, Mozafari M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int J Nanomedicine. 2018;13:8013. doi:10.2147/IJN
27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4
28. Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from bauhinia tarapotensis. J Nat Prod. 2001;64(7):892–895. doi:10.1021/np0100845
29. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9):1231–1237. doi:10.1016/s0891-5849(98)00315-3
30. CCG. Molecular Operating Environment (MOE). 1010 Sherbooke St. West, Suite# 910, Montreal: Chemical Computing Group Inc; 2016.
31. Khan SB, Afza N, Malik A, Khan MTH, Shah MR, Choudhary MI. Tyrosinase-inhibitory long-chain esters from Amberboa ramosa. Chem Pharm Bull. 2005;53(1):86–89. doi:10.1248/cpb.53.86
32. Jakštys B, Ruzgys P, Tamošiūnas M, Šatkauskas S. Different cell viability assays reveal inconsistent results after bleomycin electrotransfer in vitro. J Membr Biol. 2015;248(5):857–863. doi:10.1007/s00232-015-9813-x
33. Zhang L, Zeng D, Chen Y, et al. miR-937 contributes to the lung cancer cell proliferation by targeting INPP4B. Life Sci. 2016;155:110–115. doi:10.1016/j.lfs.2016.05.014
34. Damianakos H, Kretschmer N, Sykłowska-Baranek K, Pietrosiuk A, Bauer R, Chinou I. Antimicrobial and cytotoxic isohexenylnaphthazarins from Arnebia euchroma (Royle) Jonst. (Boraginaceae) callus and cell suspension culture. Molecules. 2012;17(12):14310–14322. doi:10.3390/molecules171214310
35. Sevimli-Gur C, Akgun IH, Deliloglu-Gurhan I, Korkmaz KS, Bedir E. Cytotoxic naphthoquinones from Alkanna cappadocica. J Na Prod. 2010;73(5):860–864. doi:10.1021/np900778j
36. Chunthorng-Orn J, Dechayont B, Phuaklee P, Prajuabjinda O, Juckmeta T, Itharat A. Cytotoxic, Anti-inflammatory and Antioxidant Activities of Heliotropium indicum Extracts. J Med Assoc Thai. 2016;99:S102.
37. Aïssaoui H, Mencherini T, Esposito T, et al. Heliotropium bacciferum Forssk. (Boraginaceae) extracts: chemical constituents, antioxidant activity and cytotoxic effect in human cancer cell lines. Nat Prod Res. 2018;33:1–6.
38. Poma P, Labbozzetta M, Notarbartolo M, et al. Chemical composition, in vitro antitumor and pro-oxidant activities of Glandora rosmarinifolia (Boraginaceae) essential oil. PLoS One. 2018;13(5):e0196947. doi:10.1371/journal.pone.0196947
39. Aruoma OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res-Fund Mol M. 2003;523:9–20. doi:10.1016/S0027-5107(02)00317-2
40. Jadhav HR, Bhutani K. Antioxidant properties of Indian medicinal plants. Phytother Res. 2002;16(8):771–773. doi:10.1002/(ISSN)1099-1573
41. Saeed SA, Urfy MZS, Ali TM, Khimani F, Gilani A. Antioxidants: their role in health and disease. Int J Pharmacol. 2005;1(3):210–217.
42. Jenkins JL, Bender A, Davies JW. In silico target fishing: predicting biological targets from chemical structure. Drug Discov Today Technol. 2006;3(4):413–421. doi:10.1016/j.ddtec.2006.12.008
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.