Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
In-Hospital Outcomes of Heart Failure Patients with Valvular Heart Disease: Insights from Real-World Claims Data
Authors Izumi C, Matsuyama R, Yamabe K, Iwasaki K, Takeshima T, Murphy SME , Teng L, Igarashi A
Received 21 January 2023
Accepted for publication 8 May 2023
Published 18 May 2023 Volume 2023:15 Pages 349—360
DOI https://doi.org/10.2147/CEOR.S405079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samer Hamidi
Chisato Izumi,1 Rei Matsuyama,2 Kaoru Yamabe,2 Kosuke Iwasaki,3 Tomomi Takeshima,3 Shannon ME Murphy,4 Lida Teng,5 Ataru Igarashi5,6
1Division of Heart Failure, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, Osaka, Japan; 2Market Access, Edwards Lifesciences Limited, Tokyo, Japan; 3Milliman Inc., Tokyo, Japan; 4Edwards Lifesciences Corporation, Irvine, CA, USA; 5Department of Health Economic and Outcomes Research, Graduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo, Japan; 6Unit of Public Health and Preventive Medicine Yokohama City University School of Medicine, Kanagawa, Japan
Correspondence: Ataru Igarashi, Department of Health Economic and Outcomes Research, Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan, Tel +81 3 5841 4828, Email [email protected] Kaoru Yamabe, Market Access, Edwards Lifesciences Limited, Nittochi Nishi-shinjuku Building, 6-10-1, Nishi-shinjuku, Shinjuku-ku, Tokyo, 160-0023, Japan, Tel +81 80 4442 0883, Fax +81 3 6894 0034, Email [email protected]
Purpose: Heart failure (HF) is a serious public health burden that is rapidly increasing in the aging population. Valvular heart disease (VHD) is a known etiology of heart failure (HF); however, the impact of VHD on outcomes of patients with HF has not been well-studied in Japan. This study aimed to determine the rates of VHD in Japanese patients admitted for HF and explore associations of VHD with in-hospital outcomes through a claim-based analysis.
Patients and methods: We analyzed claims data from 86,763 HF hospitalizations (January 2017 through December 2019) from the Medical Data Vision database. Common etiologies of HF were examined, then hospitalizations were categorized into those with VHD and those without. Covariate-adjusted models were used to explore the association of VHD with in-hospital mortality, length of stay, and medical cost.
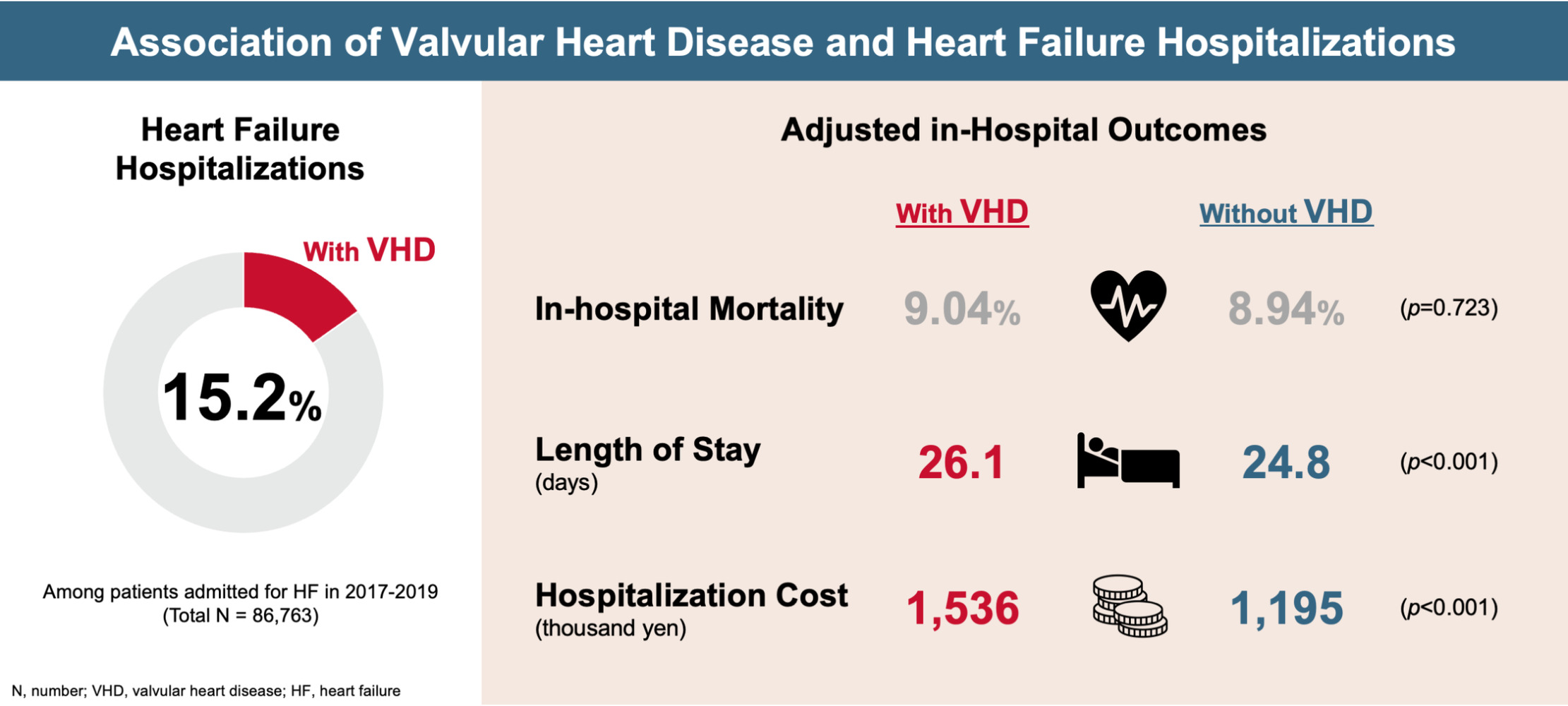
Results: Of 86,763 hospitalizations for HF, 13,183 had VHD and 73,580 did not. VHD was the second most frequent etiology of HF (15.2%). The most frequent type of VHD was mitral regurgitation (36.4% of all hospitalizations with VHD), followed by aortic stenosis (33.7%) and aortic regurgitation (16.4%). There was no significant difference in in-hospital mortality between hospitalizations with VHD vs those without (9.0% vs 8.9%; odds ratio [95% CI]: 1.01 [0.95– 1.08]; p=0.723). Hospitalizations with VHD were associated with significantly longer length of stay (26.1 vs 24.8 days; incident rate ratio [95% CI]: 1.05 [1.03– 1.07]; p< 0.001) and higher medical costs (1536 vs 1195 thousand yen; rate ratio [95% CI]: 1.29 [1.25– 1.32]; p< 0.001).
Conclusion: VHD was a frequent etiology of HF that was associated with significant medical resource use. Future studies are needed to investigate whether timely VHD treatment could reduce HF progression and its associated healthcare resource utilization.
Keywords: length of stay, medical costs, mortality, etiology, hospital claims, medical data vision
Graphical Abstract:
Introduction
Heart failure is a major clinical and public health burden affecting more than 26 million adults worldwide.1,2 The incidence and prevalence of heart failure increases with age, occurring in approximately 2% of adults aged over 60 years and doubling in prevalence with each decade of life.3,4 Elderly heart failure patients tend to demonstrate complex comorbidity profiles that increase their risk for worse outcomes.5
Japan has the highest proportion of elderly patients in the world, with 27.7% of the population over the age of 65.6 The number of patients with heart failure in Japan is rapidly increasing as the population ages and is estimated to exceed 1.3 million by 2030.7,8 Japanese patients with acute heart failure who are elderly (75–84 years) and super-elderly (>84 years) have a higher all-cause mortality risk than non-elderly patients.9 The economic burden of heart failure in Japan is high, as heart failure patients tend to be hospitalized repeatedly.10,11
Valvular heart disease (VHD) is one of the causative diseases of heart failure,12,13 accounting for the second most common etiology in Japan (11.8–28.1%) following ischemic heart disease (26.7–56.5%).14 Recent observational studies in Europe15 and Canada16 suggest that patients who present with both heart failure and VHD have significantly worse outcomes compared to patients with heart failure and no VHD. However, there are limited Japanese data on the frequency and types of VHD,14,17 although it is likely increasing due to the rapidly aging population.
The prognosis of severe symptomatic VHD is poor if left untreated18,19 and the progression of VHD can lead to additional healthcare costs.20 A variety of treatment options exist for VHD, including medical management, percutaneous dilation, and valve replacement or repair.19 Less invasive surgeries and transcatheter treatments, which have shown good outcomes in heart failure patients with VHD,21,22 have recently become available in Japan.23 However, patients with VHD often remain undiagnosed due to a latent and asymptomatic period and the majority of patients do not undergo treatment.24–26
Early diagnosis and treatment of VHD could potentially decrease the number and severity of heart failure hospitalizations, and/or associated healthcare resource utilization, by preventing the progression of heart failure. Yet, there are few previous reports describing in-hospital outcomes for patients admitted for heart failure with or without VHD in Japan.14 There is an urgent need for analysis of real-world data to develop insight into the frequency of VHD in Japan and the association between VHD and outcomes for patients with heart failure. Such population-level observations could identify novel potential strategies to slow or prevent heart failure progression.
Our objective was to conduct a claims-based analysis in Japan to determine the rates of VHD in patients admitted for heart failure, explore associations of VHD with in-hospital outcomes and healthcare resource utilization, and identify potential opportunities to optimize patient care and resource use.
Methods
Data Source
This was a retrospective study using a Japanese administrative claims database provided by Medical Data Vision (MDV) Co. Ltd. (Tokyo, Japan). The MDV database is derived from hospitals accredited as providing acute care services in Japan and participating in the Diagnostic Procedure Combination / Per-Diem bundled payment system (termed DPC hospitals). As of April 1, 2019, the MDV database included anonymized data from 380 contracted hospitals, representing 22% of the 1727 DPC hospitals in Japan.27 The database includes records of patient demographics, diagnoses, procedures, prescriptions, inpatient and outpatient visits, clinical indicators, reimbursements, and discharge summary data. It utilizes patient identifiers that are unique to each hospital, which prevents tracking of patients between hospitals. This study was approved by the ethics committee of the University of Tokyo.
Study Population
The study population for the main analysis included all adult (≥20 years) heart failure hospitalizations with admission dates from January 2017 through December 2019. A heart failure hospitalization was defined via International Classification of Diseases (ICD)-10 code I50 (heart failure) recorded as the primary diagnosis, the diagnosis with the largest medical expense, or the diagnosis leading to the hospitalization. For patients admitted for heart failure multiple times, all hospitalizations during the study period were analyzed.
Hospitalizations with cardiac arrest or end stage renal disease at admission were excluded because they have a very poor prognosis, and they are relatively rare. Hospitalizations were also excluded if they had missing data. All diagnoses used in this study were confirmed diagnoses and based on diagnosis codes, provided in Supplemental Table 1. After exclusions, hospitalizations were examined for three of the most common etiologies of heart failure (VHD, ischemic heart disease, and cardiomyopathy) and results were reported for each. If a hospitalization had multiple etiologies, it was counted under each type.
Patient Classification
The study population was categorized into those with VHD at admission and those without. VHD at admission was defined as any hospitalization with a comorbid diagnosis of VHD, except if VHD was the secondary diagnosis or a diagnosis acquired after admission. VHD comorbidities were further categorized into 8 subsets based on the 4 types of valves (aortic, mitral, tricuspid, or pulmonary) and the 2 types of disease (regurgitation or stenosis), as identified by Standard Diseases Names. Rates and types of invasive treatment for VHD (surgical or transcatheter) were described using procedure codes, provided in Supplemental Table 2.
Outcomes and Covariates
The primary outcomes were in-hospital mortality, length of stay (LOS), and medical cost of the hospitalization. In-hospital mortality was defined as death listed as the discharge status. Medical cost was defined as the hospital payment amount based upon the national fee schedule in DPC hospitals.
All outcome models were adjusted using 15 covariates, which were included because they are common cardiac risk factors: etiology of heart failure (ie, VHD, ischemic heart disease, and/or cardiomyopathy), age, sex, BMI, smoking index (Brinkman Index; cigarettes per day times smoking years), activities of daily living (ADL) at admission (maximum score is 20), emergency hospitalization, hypertension, diabetes, CKD, dyslipidemia, atrial fibrillation, and hospital size (ie, small size [≤199 beds], mid-size [200–499 beds], or large size [≥500 beds]).
Statistical Analysis
Patient demographic and risk characteristics at baseline, reported for patients with and without VHD, were used to assess baseline differences between groups. Baseline differences were evaluated using chi-squared tests for binary and categorical data and Wilcoxon rank-sum tests after examining normality for continuous data.
For the subset of hospitalizations with VHD, descriptive statistics were used to report the prevalence of the other heart failure etiologies, the types of VHD, and the rates of VHD treatment.
A generalized estimating equation (GEE) model was used to evaluate the association of VHD and patient and hospital characteristics with each of the outcomes, as it allows for adjustment for repeat patient hospitalizations. The probability of death was modeled assuming a Bernoulli distribution and the logit link function and reported as an odds ratio (OR). For the utilization outcomes, the models assumed a Poisson distribution for the log of LOS and a gamma distribution for the log of cost and were reported as incident rate ratios (IRR) and rate ratios (RR), respectively. Additionally, for each outcome, means and 95% confidence intervals (CI) were plotted based on both unadjusted and covariate-adjusted model results.
A sensitivity analysis was conducted using the same GEE models as the main analysis to assess the effect of using clinical imputation rather than exclusion to manage missing values. In the sensitivity analysis, median values were imputed for missing values at admission of ADL, height, weight, or smoking index.
Statistical significance was considered at p<0.05 for all testing.
Results
Patient Characteristics
A total of 133,936 heart failure hospitalizations were identified from January 2017 through December 2019 (Figure 1). After exclusions, the final cohort included 86,763 hospitalizations, of which 13,183 (15.2%) had VHD at admission and 73,580 (84.8%) did not.
The mean age of patients was 81 years and 51% were male (Table 1). The heterogeneity between the VHD and no VHD groups was evident, as all demographics and risk characteristics were significantly different between groups. The VHD group had significantly higher mean age and ADL, as well as lower mean BMI and smoking index. The VHD group also had a lower proportion of males and lower rates of ischemic heart disease, cardiomyopathy, hypertension, diabetes, CKD, and dyslipidemia, but significantly higher rates of emergency hospitalization and atrial fibrillation. Hospital size was significantly different between groups, with more small hospitals (≤199 beds) in the group without VHD and more mid-size hospitals (200–499 beds) in the group with VHD.
 |
Table 1 Baseline Characteristics of Heart Failure Hospitalizations Overall and Stratified by the Presence or Absence of Valvular Heart Disease |
Etiologies of Heart Failure and Types of VHD
Of the three common etiologies of heart failure that we examined, the most frequently observed was ischemic heart disease (25.6%), followed by VHD (15.2%), and cardiomyopathy (4.5%) (Figure 2A).
Of all patients admitted for heart failure with VHD, examination of the common heart failure etiologies showed that most patients (77.3%) had only VHD (Figure 2B). VHD was less frequently observed with ischemic heart disease (19.2%) or with cardiomyopathy (3.0%). A small proportion of hospitalizations (0.4%) had all three etiologies (ie, VHD, ischemic heart disease, and cardiomyopathy).
The most common type of VHD was mitral regurgitation (36.4% of all hospitalizations with VHD), followed by aortic stenosis (33.7%) (Figure 3). Other types of VHD were aortic regurgitation (16.4%), tricuspid regurgitation (7.3%), and mitral stenosis (2.5%). Pulmonary stenosis, pulmonary regurgitation, and tricuspid stenosis occurred at very low rates (≤0.1%).
 |
Figure 3 Types of VHD in patients admitted for heart failure. Patients with more than one type of VHD were counted under each type. Abbreviation: VHD, valvular heart disease. |
Invasive treatment for VHD (surgical or transcatheter) during the same episode of heart failure hospitalization was provided to less than 10% of patients admitted for heart failure with VHD. For the two most observed types of VHD, mitral regurgitation and aortic stenosis, invasive treatment was delivered to 5.4% and 6.7% of hospitalizations, respectively (Supplemental Table 3).
Association of Heart Failure Etiology with in-Hospital Outcomes
There was no significant difference in in-hospital mortality between hospitalizations with and without VHD (OR 1.01; 95% CI 0.95–1.08; p = 0.723) (Table 2, Figure 4A and Table 3). LOS was significantly longer for hospitalizations with VHD (26.1 days) compared to without (24.8 days; IRR 1.05; 95% CI 1.03–1.07; p < 0.001) (Figure 4B). Mean medical cost was higher in hospitalizations with VHD (1536 thousand yen) compared to without VHD (1195 thousand yen; RR 1.29; 95% CI 1.25–1.32; p < 0.001) (Figure 4C).
 |
Table 2 Unadjusted and Adjusted in-Hospital Outcomes During Heart Failure Hospitalizations in Patients Stratified by the Presence or Absence of VHD |
 |
Table 3 Multivariate Analysis for in-Hospital Mortality, LOS, and Medical Cost During Heart Failure Hospitalizations in Patients Stratified by the Presence or Absence of VHD |
Of the other main etiologies of heart failure, ischemic heart disease was associated with a significant decrease in in-hospital mortality (OR 0.78; 95% CI 0.73–0.83; p < 0.001), was not associated with LOS (IRR 0.99; 95% CI 0.98–1.01; p=0.28) and was associated with a significant increase in medical cost (RR 1.16; 95% CI 1.14–1.19; p < 0.001) (Table 3). Cardiomyopathy was associated with significant increases in in-hospital mortality (OR 1.37; 95% CI 1.20–1.57; p < 0.001), LOS (IRR 1.10; 95% CI 1.06–1.14; p < 0.001), and medical cost (RR 1.15; 95% CI 1.11–1.20; p < 0.001). VHD was most associated with increased medical cost among the three etiologies of heart failure.
Sensitivity Analysis
In the sensitivity analysis, clinical imputation was used for missing values, such that the study population in the sensitivity analysis (N=118,664) was larger than in the main analysis (N=86,763) (Supplemental Figure 1). There were 18,014 hospitalizations with VHD and 100,650 without VHD. Similar to the main analysis, the mean age was 81 years and 51% were male, with significant differences between groups in all baseline characteristics (p < 0.05) (Supplemental Table 4). The results from the models in the sensitivity analysis were comparable to the main analysis. There was no significant association of VHD with in-hospital mortality (OR 0.99; 95% CI 0.94–1.05; p = 0.749). VHD was significantly associated with increased LOS (IRR 1.05; 95% CI 1.03–1.07; p < 0.001) and medical cost (RR 1.27; 95% CI 1.24–1.29; p < 0.001) (Supplemental Tables 5 and 6, Supplemental Figure 2).
Discussion
This large-scale claims database study investigated the association between VHD and in-hospital outcomes of heart failure hospitalizations in Japan. In the dataset of 86,763 hospitalizations, VHD was the second most common comorbidity of those known to cause heart failure (15.2%), following ischemic heart disease. VHD was associated with statistically significant increases in medical cost and LOS, but not in in-hospital mortality.
In Japan, there has been a lack of information regarding the importance of VHD in heart failure hospitalizations. Previous studies have evaluated the overall characteristics14 and hospitalization costs10 of patients with heart failure in Japan, but none have examined the association of VHD with these outcomes.
According to the heart failure guidelines published by the Japanese Circulation Society, VHD is classified as Stage B (risk stage with organic heart disease) of the four stages of heart failure (Stage A - Stage D), and the treatment goals for Stage B include prevention of the progression of organic heart disease and the onset of heart failure.28 Treatment of VHD in Japan has made remarkable progress over the last five years with the introduction of transcatheter treatments and the advancements in surgical and valve technology.23 The results of the present study suggest that there is likely a subset of Japanese patients with heart failure that can potentially benefit from these treatments and that timely interventions could potentially prevent disease progression and reduce the cost burden of heart failure on the healthcare system.
Prevalence and Types of Valvular Heart Disease
To our knowledge, this is the largest Japanese registry study of VHD in heart failure hospitalizations reported to date, including 86,638 heart failure hospitalizations. This is more than six times the size of the largest prior study, which included 13,238 patients with acute heart failure from the Japanese Registry of Acute Decompensated Heart Failure (JROADHF) database.14
The prevalence of VHD in this study (15.2% of 86,638 heart failure hospitalizations) was marginally lower than in previous works, which have reported VHD rates of 18.5–28.1% in heart failure patients.14,29–31 This difference may be explained by the fact that these prior studies were limited to patients with acute and/or worsening heart failure; whereas, our study used a more inclusive definition of heart failure that may have resulted in a larger but healthier population. The lower prevalence rate of VHD may also be explained by recent advancements in treatment that have reduced the rate of patients presenting with VHD. Alternatively, our use of diagnosis codes to identify etiologies of heart failure may have underestimated the rate of VHD.
These data are the first time that the distribution of types of VHD comorbidities have been described in patients with heart failure in Japan. The most common type of VHD was mitral regurgitation (36.4% of all hospitalizations with VHD), while aortic valve disease was the highest VHD by valve (aortic stenosis [33.7%] and aortic regurgitation [16.4%]). This is slightly different than what has been observed in developed nations worldwide, where aortic stenosis was the most common valve pathology followed by mitral regurgitation.32 However, global data suggest that mitral regurgitation is the most frequent VHD in elderly patients (≥75 years), followed by aortic stenosis,33 explaining why we would see the highest prevalence of mitral regurgitation in the elderly population of Japan. This finding also agrees with a 2021 Japanese study of the JROADHF database that found mitral regurgitation to be the most common type of VHD in patients with heart failure.14
In-Hospital Mortality
The rate of in-hospital mortality observed here (9.0% with VHD and 8.9% without VHD) was on the higher end of what has been observed in previous Japanese heart failure registry studies based on data prior to 2015 (4.7–10.0%).10,14,30,31 This is potentially because the mean age in our cohort (81 years) was high compared to the previous studies (73–81 years), which reflects the aging population of Japan.
We did not observe a statistically significant difference in in-hospital mortality between heart failure patients with and without VHD. This is likely because the MDV database does not contain information on the severity of VHD, which is a major determinant of outcomes.33 We also did not examine the association of VHD and mortality by type of VHD or by primary versus secondary VHD. For example, previous studies have shown that mitral valve disease has the highest short-term mortality rate in super-elderly patients.34 Future studies should investigate the impact of severity, type, and etiology of VHD on its association with mortality in heart failure patients.
Healthcare Resource Utilization
In this study, we observed that VHD in heart failure hospitalization was associated with increased medical resource use. VHD was associated with significantly longer LOS versus no VHD (26 versus 25 days). LOS was longer than previously reported values (ranging from 14 days to 21 days) in Japanese patients with heart failure.10,14,30,31,35 Similar to above, this may be explained by the higher mean age of our cohort compared to previous studies,10 as age has been shown to be significantly associated with length of hospitalization in heart failure patients.35
VHD was also associated with approximately 300 thousand yen of additional medical cost compared to without VHD and added more cost than ischemic heart disease or cardiomyopathy. The higher cost with VHD is likely explained by the additional costs of the longer LOS,36 as well as the cost of invasive treatment for VHD (surgical or transcatheter),37–39 which occurred in a minority of patients (Supplemental Table 3).
Appropriate and timely treatment of VHD may present an opportunity for long-term benefits to patient care, as it has the potential to reduce the number of patients who progress to heart failure and thereby strategically manage healthcare spending.40 Patients with severe VHD may be candidates for invasive treatment,41 though VHD treatment decisions depend on the type, severity, symptoms, and surgical risk of patients.19 The optimal timing of treatment depends on safety, efficacy, and long-term durability of available treatment options41 and can be informed by recent advances in echocardiographic techniques that include early detection of left ventricular and left atrial dysfunction.42,43 Recent studies have shown excellent outcomes and cost-effectiveness for innovative treatments of VHD in Japan, including transcatheter aortic valve implantation44 and minimally invasive mitral valve repair.39,45
Limitations
The results of this study should be interpreted in the context of several limitations. First, this was a retrospective observational study that provides associative evidence, but not causal links. Analyses of real-world data are subject to the effects of unmeasured confounding and potential inaccuracies or omissions in the source records. Similarly, claims data has limitations in its ability to answer clinical questions. For example, in this study we were limited to the use of comorbidity indicators based on diagnosis codes documented during the hospitalization to identify the etiology of heart failure. Nevertheless, real-world observations play a unique role in improving patient care as they facilitate the identification of meaningful associations in large, diverse populations, potentially generating hypotheses for future prospective studies. This is especially important in Japan, which has a high proportion of elderly individuals who are generally excluded from clinical trial protocols.
Second, the MDV database has certain limitations, including that it does not contain information on patient history or severity of disease, and it utilizes patient identifiers that are unique for each institution so that patients cannot be tracked across hospitals.46 Therefore, we were unable to determine the exact proportion of patients in our study cohort that had received VHD evaluation or treatment prior to their current heart failure hospitalization. Future studies should consider the treatment history and severity of VHD, including the timing, type, and complications of invasive treatment for VHD (surgical or transcatheter) to further assess the relationship between VHD treatment and heart failure.
Third, we excluded hospitalizations with cardiac arrest or end-stage renal disease at admission, so our analysis does not capture patients with these conditions. These are very unique cases with poor prognoses that would be expected to impact the analysis in a way that would be difficult to interpret. Lastly, MDV includes data only from contracted DPC hospitals, which could introduce a selection bias.
Conclusion
In this analysis of real-world claims data, VHD was the second-most common etiology of heart failure. VHD comorbidity in patients with heart failure was associated with significantly higher medical cost and longer length of stay, suggesting that VHD treatment could represent a potential opportunity to limit overburdening medical resources in heart failure hospitalizations. Future studies are needed to investigate whether appropriate and timely VHD treatment could reduce heart failure progression and its associated healthcare resource utilization.
Abbreviations
ADL, activities of daily living; CKD, chronic kidney disease; CI, confidence intervals; DPC, Diagnostic Procedure Combination; GEE, generalized estimating equation; HF, heart failure; IRR, incident rate ratio; JROADHF, Japanese Registry of Acute Decompensated Heart Failure; LOS, length of stay; MDV, Medical Data Vision; OR, odds ratio; RR, rate ratio; VHD, valvular heart disease.
Data Sharing Statement
The deidentified individual data that support the findings of this study are available from Medical Data Vision Co., Ltd. (MDV; Tokyo, Japan). Restrictions apply to the availability of these data, which were used under license for this study. Researchers looking to access the data should contact MDV via their website at: https://www.mdv.co.jp/ (Japanese) or https://www.en.mdv.co.jp/ (English).
Ethics Approval
This study was approved by the ethics committee of the University of Tokyo.
Acknowledgments
This study was funded by Edwards Lifesciences Limited. The authors are solely responsible for the design and conduct of this study, all study analyses, and the final contents of the manuscript. The authors thank Emily Farrar, Ph.D. and Tejaswi Worlikar, Ph.D. of Boston Strategic Partners, Inc. for editorial contributions and assistance with manuscript preparation, supported by Edwards Lifesciences Limited.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed upon the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
CI received consult fees from Abbott Japan LLC and Edwards Lifesciences Limited.
RM and KY are employees of Edwards Lifesciences Limited.
SM is an employee of Edwards Lifesciences Corporation and has Edwards stock.
KI and TT are employee of Milliman Inc., which has received consult fees from Edwards Lifesciences Limited.
AI received research fund from Edwards Lifesciences Limited and Boston Scientific Inc.; consult fees and lecture fees from Abbott Inc., GSK Japan Inc., Medtronic Inc., MSD Inc., Moderna Japan Inc., Pfizer Japan Inc., Sanofi-Pasteur Inc., Novartis Pharma K.K., AbbVie GK, Astellas Pharma Inc., Chugai Pharmaceutical Co. Ltd., Asahi Kasei Pharma Inc., Novo Nordisk Pharma Inc., Eisai Inc., Beckton Dickinson and Company, Maruho Co. Ltd., Ono Pharmaceutical Inc., Sato Pharmaceutical Inc., Sumitomo Dainippon Pharma Inc., and Eli Lilly Japan K.K., outside the submitted work; research grants from Taiho Pharmaceutical Co. Ltd., Janssen Pharmaceutical K.K., Beckton & Dickinson Inc., CSL Behring Japan Inc., Gilead Science K.K., Takeda Pharmaceutical Inc., Boston Scientific Japan Inc., DeSC Healthcare Inc., Otsuka Pharmaceutical K.K., Varian Medical systems and Intuitive Surgical GK., outside the submitted work.
The authors report no other conflicts of interest in this work.
References
1. Savarese G, Lund LH. Global public health burden of heart failure. Heart Fail Rev. 2017;3(1):7–11. doi:10.15420/cfr.2016:25:2
2. Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Failure. 2014;1(1):4–25. doi:10.1002/ehf2.12005
3. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. doi:10.1136/hrt.2003.025270
4. Li H, Hastings MH, Rhee J, Trager LE, Roh JD, Rosenzweig A. Targeting age-related pathways in heart failure. Circ Res. 2020;126(4):533–551. doi:10.1161/CIRCRESAHA.119.315889
5. Lazzarini V, Mentz RJ, Fiuzat M, Metra M, O’Connor CM. Heart failure in elderly patients: distinctive features and unresolved issues. Eur J Heart Fail. 2013;15(7):717–723. doi:10.1093/eurjhf/hft028
6. Nakatani H. Population aging in Japan: policy transformation, sustainable development goals, universal health coverage, and social determinates of health. Global Health is J. 2019;1(1):3–10. doi:10.35772/ghm.2019.01011
7. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17(9):884–892. doi:10.1002/ejhf.319
8. Okura Y, Ramadan MM, Ohno Y, et al. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J. 2008;72(3):489–491. doi:10.1253/circj.72.489
9. Takabayashi K, Kitaguchi S, Yamamoto T, et al. Mode of death in elderly and super-elderly patients with acute heart failure: insights from Japanese heart failure registry. Clin Cardiol. 2021;44(6):848–856. doi:10.1002/clc.23619
10. Kanaoka K, Okayama S, Nakai M, et al. Hospitalization costs for patients with acute congestive heart failure in Japan. Circ J. 2019;83(5):1025–1031. doi:10.1253/circj.CJ-18-1212
11. Sasaki N, Kunisawa S, Ikai H, Imanaka Y. Differences between determinants of in-hospital mortality and hospitalisation costs for patients with acute heart failure: a nationwide observational study from Japan. BMJ Open. 2017;7(3):e013753. doi:10.1136/bmjopen-2016-013753
12. Adamo M, Alos B, Metra M, et al. Patient with heart failure: importance to treat valvular diseases. Eur Heart J. 2020;22(Supplement_P):P38–P41. doi:10.1093/eurheartj/suaa184
13. Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2017;52(4):616–664. doi:10.1093/ejcts/ezx324
14. Ide T, Kaku H, Matsushima S, et al. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large-scale Japanese Registry Of Acute Decompensated Heart Failure (JROADHF). Circ J. 2021;85(9):1438–1450. doi:10.1253/circj.CJ-20-0947
15. Bartko PE, Heitzinger G, Pavo N, et al. Burden, treatment use, and outcome of secondary mitral regurgitation across the spectrum of heart failure: observational cohort study. BMJ. 2021;373:n1421. doi:10.1136/bmj.n1421
16. Gevaert AB, Tibebu S, Mamas MA, et al. Clinical phenogroups are more effective than left ventricular ejection fraction categories in stratifying heart failure outcomes. ESC Heart Failure. 2021;8(4):2741–2754. doi:10.1002/ehf2.13344
17. Koyama T. Trends in surgical treatment for valvular heart disease in Japan. J St Marianna Univer. 2021;12(1):19–28. doi:10.17264/stmarieng.12.19
18. Campo J, Tsoris A, Kruse J, et al. Prognosis of severe asymptomatic aortic stenosis with and without surgery. Ann Thorac Surg. 2019;108(1):74–79. doi:10.1016/j.athoracsur.2019.01.031
19. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e72–e227. doi:10.1161/CIR.0000000000000923
20. Clark MA, Arnold SV, Duhay FG, et al. Five-year clinical and economic outcomes among patients with medically managed severe aortic stenosis: results from a Medicare claims analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):697–704. doi:10.1161/circoutcomes.112.966002
21. Saia F, Loforte A, Pacini D. Innovative transcatheter procedures for the treatment of heart failure. Cardiovasc Diagn Ther. 2021;11(1):292–300. doi:10.21037/cdt-20-335
22. Davidson LJ, Davidson CJ. Transcatheter treatment of valvular heart disease: a review. JAMA. 2021;325(24):2480–2494. doi:10.1001/jama.2021.2133
23. Izumi C, Eishi K, Ashihara K, et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the management of valvular heart disease. Circ J. 2020;84(11):2037–2119. doi:10.1253/circj.CJ-20-0135
24. Chung CH, Wang YJ, Lee CY. Epidemiology of heart valve disease in Taiwan. Int Heart J. 2021;62(5):1026–1034. doi:10.1536/ihj.21-044
25. Iung B, Delgado V, Rosenhek R, et al. Contemporary presentation and management of valvular heart disease. Circulation. 2019;140(14):1156–1169. doi:10.1161/CIRCULATIONAHA.119.041080
26. Brennan JM, Lowenstern A, Sheridan P, et al. Association between patient survival and clinician variability in treatment rates for aortic valve stenosis. J Am Heart Assoc. 2021;10(16):e020490. doi:10.1161/JAHA.120.020490
27. Hayashida K, Murakami G, Matsuda S, Fushimi K. History and profile of diagnosis procedure combination (DPC): development of a real data collection system for acute inpatient care in Japan. J Epidemiol. 2021;31(1):1–11. doi:10.2188/jea.JE20200288
28. Tsutsui H, Ide T, Ito H, et al. JCS/JHFS 2021 Guideline focused update on diagnosis and treatment of acute and chronic heart failure. Circ J. 2021;85(12):2252–2291. doi:10.1253/circj.CJ-21-0431
29. Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, et al. Characteristics, management, and outcomes for patients during hospitalization due to worsening heart failure-A report from the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD). J Cardiol. 2013;62(2):95–101. doi:10.1016/j.jjcc.2013.03.009
30. Sato N, Kajimoto K, Keida T, et al. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J. 2013;77(4):944–951. doi:10.1253/circj.cj-13-0187
31. Shiraishi Y, Kohsaka S, Sato N, et al. 9-Year trend in the management of acute heart failure in Japan: a report from the national consortium of acute heart failure registries. J Am Heart Assoc. 2018;7(18):e008687. doi:10.1161/jaha.118.008687
32. Aluru JS, Barsouk A, Saginala K, Rawla P, Barsouk A. Valvular heart disease epidemiology. J Med Sci. 2022;10(2):32. doi:10.3390/medsci10020032
33. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi:10.1016/s0140-6736(06)69208-8
34. Kodali SK, Velagapudi P, Hahn RT, Abbott D, Leon MB. Valvular heart disease in patients ≥80 years of age. J Am Coll Cardiol. 2018;71(18):2058–2072. doi:10.1016/j.jacc.2018.03.459
35. Mitani H, Funakubo M, Sato N, et al. In-hospital resource utilization, worsening heart failure, and factors associated with length of hospital stay in patients with hospitalized heart failure: a Japanese database cohort study. J Cardiol. 2020;76(4):342–349. doi:10.1016/j.jjcc.2020.05.010
36. Ministry of Health, Labour and Welfare. FY2020 medical expenses, etc. by hospital function and system. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/iryouhoken/database/zenpan/topics_r03.html.
37. Fukuda H, Kiyohara K, Sato D, Kitamura T, Kodera S. A real-world comparison of 1-year survival and expenditures for transcatheter aortic valve replacements: SAPIEN 3 versus CoreValve versus Evolut R. Value Health. 2021;24(4):497–504. doi:10.1016/j.jval.2020.10.022
38. Kamon T, Kaneko H, Kiriyama H, et al. Transcatheter aortic valve implantation and surgical aortic valve replacement for aortic stenosis in Japan: analysis of a nationwide inpatient database. Circ Rep. 2020;2(12):753–758. doi:10.1253/circrep.CR-20-0116
39. Sakamaki H, Nakao K, Matsumoto T, Inoue S. Cost-effectiveness analysis of percutaneous mitral valve repair with the MitraClip delivery system for patients with mitral regurgitation in Japan. J Med Econ. 2019;22(12):1312–1320. doi:10.1080/13696998.2019.1668132
40. Saku K, Yokota S, Nishikawa T, Kinugawa K. Interventional heart failure therapy: a new concept fighting against heart failure. J Cardiol. 2022;80(2):101–109. doi:10.1016/j.jjcc.2021.11.018
41. Baumgartner H, Iung B, Otto CM. Timing of intervention in asymptomatic patients with valvular heart disease. Eur Heart J. 2020;41(45):4349–4356. doi:10.1093/eurheartj/ehaa485
42. Cameli M, Sciaccaluga C, Mandoli GE, D’Ascenzi F, Tsioulpas C, Mondillo S. The role of the left atrial function in the surgical management of aortic and mitral valve disease. Echocardiography. 2019;36(8):1559–1565. doi:10.1111/echo.14426
43. Sonaglioni A, Nicolosi GL, Rigamonti E, Lombardo M. Incremental prognostic role of left atrial reservoir strain in asymptomatic patients with moderate aortic stenosis. Int J Card Imaging. 2021;37(6):1913–1925. doi:10.1007/s10554-021-02175-6
44. Inoue S, Nakao K, Hanyu M, et al. Cost-effectiveness of transcatheter aortic valve implantation using a balloon-expandable valve in Japan: experience from the Japanese pilot health technology assessment. Value Health Reg Issues. 2020;21:82–90. doi:10.1016/j.vhri.2019.07.013
45. Nishi H, Miyata H, Motomura N, et al. Propensity-matched analysis of minimally invasive mitral valve repair using a nationwide surgical database. Surg Today. 2015;45(9):1144–1152. doi:10.1007/s00595-015-1210-7
46. Laurent T, Simeone J, Kuwatsuru R, et al. Context and considerations for use of two Japanese real-world databases in Japan: medical data vision and Japanese medical data center. Drugs Real World Outcomes. 2022;9(2):175–187. doi:10.1007/s40801-022-00296-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



