Back to Journals » Cancer Management and Research » Volume 15
Improvements in Survival and Early Retirement Rates – Real-World Evidence on Danish Breast Cancer Patients 2004–2018
Authors Khan H, Rudolfsen JH, Olsen J, Borgquist S, Poulsen PB
Received 10 October 2022
Accepted for publication 28 December 2022
Published 13 January 2023 Volume 2023:15 Pages 43—53
DOI https://doi.org/10.2147/CMAR.S392440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Humma Khan,1 Jan Håkon Rudolfsen,2 Jens Olsen,2 Signe Borgquist,3 Peter Bo Poulsen1
1Pfizer Denmark, Ballerup, Denmark; 2Incentive, Holte, Denmark; 3Department of Oncology, Aarhus University Hospital and Aarhus University, Aarhus, Denmark
Correspondence: Peter Bo Poulsen, Pfizer Denmark, Lautrupvang 8, Ballerup, 2750, Denmark, Tel +45 2920 3211, Email [email protected]
Background: Historically, Denmark has had poor survival for cancer patients relative to other western countries with comparable health-care systems. In this study, we examine the long-term cancer impact of a nationwide reform addressing all cancer diagnostics, implemented in 2006. The analyses include patients diagnosed with breast cancer and their spouses (informal caregivers). Patients and their spouses diagnosed before and after the reform were compared. Focus is on the potential impact on overall survival, early retirement, sick leave, unemployment as well as earnings (income).
Methods: In a nationwide retrospective cohort study utilizing the Danish National Patient Register we identified 77,474 breast cancer patients between 1st January 2002 and 31st December 2018. Data was merged with the National Cancer Register, the Central Person Register, the Education Register, the DREAM Register and the Income Register using citizens’ personal identification number. Spouses of cancer patients were identified through the Central Person Register. Propensity score matching was applied to match populations before and after the reform. Analyses on matched as well as unmatched populations were performed.
Results: In a matched sample, risk of mortality was reduced by 15% for breast cancer patients diagnosed after the reform. Moreover, there was a 15% reduced risk of early retirement. The patients diagnosed after the reform had reduced income three to five years after diagnosis relative to those diagnosed before the reform, likely due to survival bias and labor market conditions. In an unmatched sample of patients diagnosed two years before or after the reform, mortality was reduced by 7%.
Conclusion: Implementation of the nationwide cancer reform together with advancement in new cancer treatments had a positive impact on survival and reduced risk of early retirement. The results from this study are reassuring that relevant health-care reforms improve cancer outcome.
Keywords: breast cancer, survival, early retirement, register data
Introduction
Breast cancer (BC) is the most common cancer in women worldwide.1 BC is one of the primary causes of disability adjusted life expectancy, independent of disease classification.2 However, early detection, increased accuracy of diagnosis and new treatments have resulted in a 5-year survival rate of more than 95%.3 Treatments tailored for individual patients have improved tolerability, reduced toxicities and hence have improved the quality of life (QoL) of patients receiving treatment.4
In Denmark, breast cancer is also the most frequent cancer in women, with approximately 4800 new cases per year with 38% of patients being younger than 61 years at the time of diagnosis. In ultimo 2017, 68,269 women (23.63 per 100 000 women) were living with breast cancer.
For years, Denmark has had poor outcomes in terms of survival after cancer treatment compared to other developed health-care systems.5 As part of improving cancer treatment outcomes, the Danish Parliament and Danish health authorities have implemented several policy changes since 2000. From January 1st 2006, the National Cancer Plan II (In Danish: Kræftplan II) was implemented.6 The overall aim of this political reform was to prevent cancer cases and increase screening of cancer, improve cancer diagnostics, optimize and standardize treatment pathways, improve the treatment of cancer, secure patient involvement and selfcare, educate health-care personnel as well as improve data quality and data monitoring and research. Prevention in the National Cancer Plan II focused on initiatives to reduce smoking and for screening recommendations were provided for programs to screen for cervical, mammae and colon cancer, as well as initiatives towards the public and general practitioners to highlight the importance of early awareness of symptoms caused by cancer. Standardized treatment pathways aimed at in a shared care flow to secure that all cancer patients undergo the same diagnostic tests and investigations, get the same treatments, etc. With respect to cancer treatment, the plan focused on surgery, medical treatment, and radiation therapy. For cancer surgery focus was on requirement for a minimum number of operations performed per surgeon and department to improve learning and thereby quality, and for medical cancer treatment focus was on the integration of newly developed cancer medicines as standard care based on national recommendations and monitoring of their effects and side-effects, whereas focus on radiation therapy dealt with reducing waiting times.
Since the reform, new innovative treatments have become available for BC patients. One would expect that the reform as well as the introduction of innovative treatments have led to improvements in the treatment of Danish BC patients with an impact upon important outcomes such as survival, sick leave, retirement, and productivity. This is also for their spouses, who as informal caregivers are expected to be affected as well.
Using patient-level data from Danish national registers, this study investigated the effects of the 2006 policy change with the national reform program as well as developments in breast cancer treatment in general for patients diagnosed with breast cancer in the period 2002–2018. Outcomes of interest in this comparison before and after 2006 were overall survival, incidence of early retirement, unemployment, and productivity as proxied by earnings. Furthermore, we considered how the policy changes and developments in cancer treatment affected spouses as informal caregivers. Similarly, for spouses, outcomes considered were productivity as proxied by earnings, early retirement, but also long-term sick leave as being a relative to a BC patient with cancer may have impacted both their somatic and mental health.
Materials and Methods
The study was designed as a retrospective cohort study utilizing the national Danish registers covering the entire Danish population (5.7 million). From these national registers, the population of BC patients were identified as women with at least two hospital contacts (admissions or outpatient visits) in which the International Classification of Diseases (ICD)-10 code DC50 (BC diagnosis in the ICD-10 system, including sub-levels) was registered as the primary or secondary diagnosis.
Data Sources
All cancer patients with residency in Denmark are registered in the National Patient Register (NPR)7 and other registers with a 10-digit unique personal identification number. This personal identification number allowed for person-level data to be merged across all public registers in Denmark.
From the Danish Cancer Register, we verified and cross-checked the population identified in the NPR, and we collected TNM (Tumour, Node, Metastasis) classification codes used to determine cancer stage.8 We collected Systemized Nomenclature of Medicine (SNOMED) codes used to determine pathology of the cancer from the Danish Pathology Register.9 From the Central Person Register,10 we collected date of birth, marital status, vital status, date of death and region of residency. Data on income was collected from the Income Statistics Register.11 It was only income in the form of earnings, ie, salary from work, that was included. Transfer payments in the form of pensions or social benefits were not part of this study, as this is not normally included talking about productivity. The highest achieved education level before diagnosis was collected from the Population Education Register.12 Labor market affiliation data such as early retirement, unemployment and sick leave were collected from the DREAM database, which collects data on all public transfer payments in Denmark.13
Additionally, in the NPR for specialized care and in the National Health Service Register for primary care,14 we gathered number of contacts, health-care costs and treatments for spouses.
Study Populations
The study population consisted of individuals who were diagnosed with BC in the period from January 1st, 2002, to December 31st, 2018, as identified in the NPR and The Danish Cancer Register. With the implementation of the National Cancer Plan II on January 1st, 2006, we defined patients diagnosed prior to this date as “Before” patients, while those diagnosed after this date were defined as “After” patients for purpose of comparison.
Within the population, several subgroups were created. Using TNM codes from the Cancer Register, patients were classified either as Stage 1, Stage 2, Stage 3a, Stage 3b, Stage 4 or “Unknown” (registered as unknown metastases, M variable in TNM). During the study period, three different versions of the TNM classification system were applied. To ensure that Before and After patients were staged on the same criteria, cancer stage was defined using an algorithm which is not sensitive to the TNM code migration from the 6th version to the 7th and 8th version. A table describing the algorithm can be found in Appendix Table 1A.
In addition, subgroups were defined based on pathology using SNOMED codes from the Danish National Pathology Register registered 30 days before or after date of diagnosis. The criteria are presented in Table 1. If a patient was registered with the SNOMED code T04 (indicating breast) in combination with F29603 (HER2 (Human Epidermal growth factor Receptor 2) oncogene overexpression) or FE13B5 (HER2 gene amplification), the patient was classified as HER2-positive. For a patient with T04 in combination with F29601 (normal oncogene expression) or FE13B1 (HER2 without gene amplification), the patient was classified as HER2-negative. A SNOMED code T04 in combination with F29521 (estrogenic receptor-positive) was used to classify an ER (estrogen receptor)-positive patient, while T04 in combination with F29525 was used to classify an ER-negative patient. Triple-negative patients were classified if they had T04 in combination with either SNOMED code F29601 and F29525, or FE13B1 and F29525.
 |
Table 1 Criteria for Pathology Subgroup |
To analyze the impact of BC disease burden on spouses as informal caregivers, we identified spouses of BC patients through the Danish Central Person Register. A random draw of individuals from the general population not associated with BC were extracted from the Central Person Register as control group for the spouses.
Outcomes
Long-term survival was one outcome in the current study for the patients treated before and after January 1st 2006, where the national cancer plan was implemented. Date of diagnosis as noted in the Cancer Register was used as index date. The date of death was collected from the Central Person Register as end date and occurring before December 31st 2019.
An additional outcome was the incidence of early retirement. Early retirement is granted on municipality level, and only if a person is evaluated to be in a health state where their ability to work is permanently reduced. As there was a substantial social reform in Denmark altering the criteria for early retirement in 2013, we censored cases on January 1st, 2013, to exclude any impact from this reform.
Furthermore, the number of weeks of unemployment and patients’ productivity, measured by earnings (active income, adjusted to 2018 price level15), were compared between the groups in the five years following diagnosis.
In the spouse analyses, early retirement and earnings were considered in the same way as for the BC patients. Additionally, use of health services was also compared in the form of health-care costs associated with primary sector or hospital sector care and cost of prescription drugs. Costs were adjusted to 2018 price level, using exchange of 1 USD = 6.317 DKK. Additionally, the incidence of long-term sick leave was considered. Long-term sick leave was defined as a person being on sick leave from work for more than four weeks. All incidences of sick leave lasting shorter than four weeks were not considered in the analyses.
Propensity Score Matching
Propensity score matching was used to balance the Before and After populations in the analyses. The groups were matched based on age, highest level of education obtained before diagnosis, cancer stage, income one year before diagnosis as well as region of residence. Our criteria for successful matching were: 1) a variance ratio <15%, 2) standardized difference in mean ±0.10 for all covariates, and 3) retaining at least 80% of the smallest group. The study allowed Before patients to be controls for multiple After patients in order to retain as much data as possible in the analysis. We aimed for a 3:1 ratio of Before and After patients, but this was not always achieved due to a criterion of exact matching for some covariates. In order to obtain an acceptable match for income, we had to truncate income for each observation at 1583 USD (DKK 10,000).
When matching the subsets, matching specification was adapted across the different subsets (eg, reduced number of variables or changed distance measure), in order to fulfil the criteria for successful matching. Besides the propensity score matched analyses, an analysis was also carried out on unmatched Before-After samples for exploratory reasons.
Statistical Analysis
For mortality and early retirement, Cox Proportional Hazard Regression was used to determine whether the risk of death or early retirement differed between the groups. In the Cox models, age, cancer stage, education, and region of residence were adjusted for. Adjustments in the Cox models were done to reduce residual imbalance not accounted for during matching.16
For mortality, cases were censored if they migrated to other countries. For early retirement, patients were not considered at risk if they had exited the labour market before time of diagnosis or were 65 years or older at time of diagnosis. We censored for migration, and when patients turned 65 years old. The retirement age in Denmark was increased from 65 to 67 years of age by the Danish Parliament in 2008. Censoring at 65 years of age was therefore used to obtain accurate risk estimates for early retirement for the Before and After samples.
For earnings, unemployment, sick leave and healthcare service utilization, we used ordinary least squares with interaction between the treatment variable and year relative to diagnosis. We included the year before diagnosis (t-1) and all years until five years after diagnosis (t5).
Statistical significance was determined from 95% confidence intervals. All analyses were conducted using R, version 4.1.0 (www.r-project.org).
Results
Figure 1 shows a flowchart of the merging of the datasets and an overview of the excluded patients. A total of 77,474 BC patients were identified between 2002 and 2018. Of these patients, 69,403 patients were included in the analysis. The remaining 8072 patients were excluded, if they were not identified in The Central Person Register (362 patients), uncertain region of residence (4 patients), or had otherwise missing information for any of the matching variables (7706 patients). Furthermore, patients diagnosed in 2002 and 2003 were excluded from the analysis, because TNM classification was not applied until 2004, which is why the cancer stage could not be determined before this year.
 |
Figure 1 Flowchart from identification of cases to matching of main datasets. |
Table 2 presents summary statistics of the BC patients and their spouses. The median age for BC patients was 64 years (IQR (interquartile range) = 54–73) in the year before diagnosis. In the After period, 61,557 BC patients were identified, while 11,677 patients were diagnosed before January 1st, 2006. The median active income (earnings) in the patient population was 0, meaning that more than half of the BC patients were outside the working force due to retirement (early or age-based).
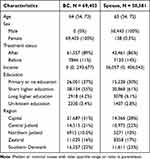 |
Table 2 Summary Statistics, Breast Cancer Patients and Their Spouses |
We identified 50,581 spouses of BC patients. Spouses of BC patients diagnosed between 2003 and 2018 were included in the analysis. Of these, 43,461 were spouses of After patients, while 7120 were spouses of Before patients. They had a median age of 63 years (IQR = 54–72) and a median income (earnings) of USD 5708 (DKK 36,057) in the year before their spouse was diagnosed with cancer.
We present the frequency of diagnosis by year, and the number of patients categorized by stage, and pathological subgroup (Appendix Table 2A).
The focus of our study was the matched sample of BC patients treated before or after January 1st, 2006. Table 3 presents summary statistics for this matched dataset, with summary of the Before sample, the After sample, and the overall of these two samples. We were able to identify matches for 7554 out of 7846 Before patients (96.3%). These were compared with 21,904 of 61,557 After patients (35.6%). The median age in the matched sample is two years lower than in the full population of BC patients (see Table 2). This is due to the Before sample being younger than the full population.
 |
Table 3 Summary Statistics, Matched Breast Cancer Sample Before/After |
Table 4 shows the output from the Cox proportional hazard regression model for all analyses. The matched Before/After analysis shows that patients belonging to the After group (BC patients treated after January 1st, 2006) have a 14% reduced risk of death compared to the patients before the reform (p < 0.001).
 |
Table 4 Hazard Ratios for Overall Mortality |
For all subgroup analyses, with exception of those with stage 4 cancer, it was seen that the After patients had a significantly reduced risk of death. The BC patients with HER2-positive cancer experienced the highest mortality reduction, with a 28% reduced risk of death (p < 0.001). Stage 1–3 patients had 16% reduced risk of death (p < 0.001), ER-positive/HER2-negative had 12% reduced risk of death (p < 0.001). Interestingly, the unmatched Before/After analysis including the whole population without matching showed a reduced risk of death of 18% for the After patients, while the unmatched sample of patients diagnosed two years before or after implementation of the National Cancer Plan II (January 1st, 2006, patients diagnosed 2004–2007), on the other hand, only had 8% reduction in the risk of death (Table 4, bottom).
During the study period, no new medications or treatments have become available for triple negative BC cases. When matching Before and After BC patients with triple negative patients (independent of diagnosis), we found Before patients to have 29% reduced risk of death, while After patients had 38% reduced risk of death (both p < 0.001). From the confidence intervals of the hazard rates, it is clear that the After population had significant reduced risk of death compared to the Before sample in this model as well.
Compared to the Before patients, the risk of early retirement was reduced for the After patients by 15% in the matched analysis (Table 5). While none of the subgroups of BC patients had a significant reduction in risk of early retirement, a clear pattern was seen for all hazard ratios being smaller than 1. In the unmatched analysis of the population, the risk of early retirement was found to be reduced by 16% reduction for After patients.
 |
Table 5 Hazard Ratios for Early Retirement |
Results for income and unemployment are not shown, as we did not find any significant differences, with the exemption of After patients in the matched analysis having a lower income in year 3–5 after diagnosis. Similarly, spouses of After patients had a significantly higher income in year 3–5 after diagnosis compared to spouses of Before patients. We did not identify differences in cost of health-care services or prescription drugs that can be attributed to the implementation of the national cancer plan in 2006.
Discussion
Our analysis shows that the implementation of the National Cancer Plan II as well as other improvements since 2006, eg, developments and innovations in breast cancer treatment, have reduced mortality in BC patients. In addition, the results from the matched analysis with matched Before/After patients, the After group (from January 1st, 2006) resulted in a 14% reduced risk of death. Similarly, the results showed that the After patients had a 15% reduced risk of experiencing early retirement. As early retirement is granted based on health state, it indicated that patients in the After population may have had an improved health status which could be reflected in a higher health-related quality of life compared to the Before patients.
The After patients had lower earnings compared to the Before patients, three to five years after diagnosis. This could indicate reduced productivity in the After patients. However, it must be seen in the context of survival. The average age at the time of death was 74.76 and 75.36 for Before and After patients, respectively. With After patients surviving, likely as retirees, the mean income will be subject to survival bias and therefore artificially low. The higher income for spouses in the 3–5 years following diagnosis could have been to compensate for their partner's income loss.17
As the After period spans several years, other factors than the political cancer reform in 2006 may and should be considered as alternative or additional explanatory factors. Survival rates in breast cancer have increased steadily in recent decades.18 New innovative medicine, nationwide screening programmes, new surgical techniques and other policy and structural changes may all be likely to have had an impact on the result. It is therefore worth considering the subgroup analysis of patients diagnosed during the years 2004–2007. In this After subgroup (diagnosed 2006–2007), there were fewer alternative explanations for the differences in survival rates. New innovative medications became available for this After subgroup, and a national screening program was implemented at the end of 2007. However, it is unlikely that these two factors are responsible for the full 8% reduced risk of death in this After subgroup. In other words, it is likely that the National Cancer Plan II had a positive effect on BC patients’ survival.
The reform was evaluated by the Danish Health Authority in 2007,19 where they stated that standardized treatment paths for BC patients were created. Updated best practice guidelines and increased cooperation between treatment facilities were in place, and additional resources were located to address patients’ psychosocial needs (additional nurses, psychologists and physiotherapists). However, they did not consider explicit patient outcomes based on national register data like mortality, early retirement and earnings, as done here. Studies on one year survival for all cancers20 and five-year survival of colorectal cancer21 both found positive effects of the reform. Probst et al (2012) found median waiting times to start of treatment to decrease steadily from 2006 onwards for 10 different cancers, which is likely to affect outcomes.22 For breast cancer, however, the waiting times remained constant. A likely reason for the stagnant waiting times could be the implementation of national breast cancer screening in the end of 2007. The screening program increased the incidence of breast cancer through improved detection, while the treatment capacity was not equally expanded.
The results found in this study highlight the importance of knowledge exchange across treatment facilities. Going back to the intentions of the reform; reduce the use of tobacco smoking, optimize and standardize treatment pathways, improve the quality of surgical treatment and improve the quality of data collection. Data collection is separate from clinical outcomes, and reduced tobacco smoking has a delayed effect. Hence, the effects seen here must be a result of improved surgical quality and standardized treatment paths, as well as developments and innovations in BC treatment. Factors that include interaction across treatment facilities.
Strengths and Limitations
The present study has both strengths and limitations. Danish national registers are of high quality and have a high degree of completeness. Hence, one of the strengths of this study is that it was based on exhaustive, national retrospective register data covering an entire population and with data coming from national registers. This nationwide analysis did also eliminate any selection bias in the patients included in the analysis. Given the longitudinal history of the national registers, we were also able to have a long follow-up period in our analyses making the data foundation for the comparison in before and after periods rather robust. In the Before/After analysis, each patient was observed on average for 8.08 years (total of 238,102 patient years) after diagnosis. This enabled us to provide accurate estimates for unmatched samples, and it provided the foundation for accurate matching of the two groups. Similarly, the validity of these national register data is generally regarded as being high.
The current study excluded patients diagnosed in 2002 and 2003. This was due to the TNM classification coding was not implemented before 2004. It is unfortunate that we had to exclude these patients, as they would have improved the power of our analyses. However, including them with unknown cancer stage would potentially have led to bias in the analysis.
Finally, missing data and misclassifications may occur, also in national registers. A larger proportion of the After cases were also dropped in the matching process, due to the big difference in initial number of observations in the two groups. The large study population though reduced the risk of random variation in the estimates.
Conclusion
The BC patients diagnosed after the implementation of the national cancer reform in 2006 lived longer and had reduced risk of early retirement. With early retirement being a result of permanently reduced health status, it implied that their health status on average had been improved. Some of the improvements were likely due to later reforms and access to new treatments, etc. resulting in a general positive effect on the survival of BC patients. However, when only including patients diagnosed two years before or after the reform, we also did find a positive effect on survival. These groups were less sensitive to alternative structural changes and only one new treatment was introduced during this period. We can therefore conclude that the reform seems to have had a positive effect on BC patients’ survival. On the other hand, no differences were found in the analyses of spouses before and after the implementation of the national cancer reform.
Abbreviations
BC, Breast Cancer; ER, Estrogen Receptor; HER2, Human Epidermal growth Factor 2; ICD-10, International Classification of Diseases, 10th version; IQR, Interquartile Range; NPR, National Patient Register; QoL, Quality of life; SNOMED, Systemized Nomenclature of Medicine.
Data Sharing Statement
The data that support the findings of this study are available from Statistics Denmark’s Research Service, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Ethics Approval and Consent to Participate
The study was register-based and complied with the regulations and instructions set up by Statistics Denmark. Neither informed consent from patients nor approval from an ethics committee are required by Danish law for register-based research using retrospective data only. Administrative permissions to access the raw data used in the study were acquired from Statistics Denmark. The study complies with international ethical standards including the EU general data protection regulation and the Declaration of Helsinki.
Consent for Publication
Data were previously collected. No individuals were contacted during this study. Consent for participation is not required under Danish law.
Acknowledgments
The authors would like to thank Mikkel H. Pedersen at Incentive for valuable comments and input during this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Pfizer Denmark Aps.
Disclosure
Jens Olsen and Jan Håkon Rudolfsen are employees at Incentive, which is a paid vendor of Pfizer Denmark Aps. Humma Khan and Peter Bo Poulsen are employees of Pfizer Denmark Aps. Peter Bo Poulsen own shares from Pfizer Inc. Signe Borgquist was paid by Pfizer Denmark Aps. for her work as an oncology specialist in the project group. The authors report no other conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/s1040-6736(20)30925-9
3. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288. doi:10.1001/jama.2018.19323
4. Nguyen OT. The global challenge of cancer. Nat Cancer. 2020;1(1):1–2. doi:10.1038/s43018-019-0023-9
5. Butler J, Foot C, Bomb M, et al. The International Cancer Benchmarking Partnership: an international collaboration to inform cancer policy in Australia, Canada, Denmark, Norway, Sweden and the United Kingdom. Health Policy. 2013;112(1–2):148–155. doi:10.1016/j.healthpol.2013.03.021
6. Danmark, Sundhedsstyrelsen. National Cancer Plan II - Denmark: National Board of Health Recommendations for Improving Cancer Healthcare Services. Copenhagen: Sundhedsstyrelsen; 2005.
7. Lynge E, Sandegaard JL, Rebolj M. The Danish National patient register. Scand J Public Health. 2011;39(7):30–33. doi:10.1177/1403494811401482
8. Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7):42–45. doi:10.1177/1403494810393562
9. Erichsen R, Lash TL, Hamilton-Dutoit SJ, Bjerregaard B, Vyberg M, Pedersen L. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and Data Bank. Clin Epidemiol. 2010;2:51–56. doi:10.2147/CLEP.S9908
10. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7):22–25. doi:10.1177/1403494810387965
11. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7_suppl):103–105. doi:10.1177/1403494811405098
12. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7):91–94. doi:10.1177/1403494810394715
13. Hjollund NH, Larsen FB, Andersen JH. Register-based follow-up of social benefits and other transfer payments: accuracy and degree of completeness in a Danish interdepartmental administrative database compared with a population-based survey. Scand J Public Health. 2007;35(5):497–502. doi:10.1080/14034940701271882
14. Andersen JS, Olivarius NDF, Krasnik A. The Danish National health service register. Scand J Public Health. 2011;39(7 Suppl):34–37. doi:10.1177/1403494810394718
15. Consumer price index [Internet]. Available from: https://www.dst.dk/en/Statistik/emner/oekonomi/prisindeks/forbrugerprisindeks.
16. Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15(3):199–236. doi:10.1093/pan/mpl013
17. Fadlon I, Nielsen TH. Family labor supply responses to severe health shocks: evidence from Danish administrative records. Am Econ J Appl Econ. 2021;13(3):1–30. doi:10.1257/app.20170604
18. Ahmad A. Breast cancer statistics: recent trends. In: Ahmad A, editor. Breast Cancer Metastasis and Drug Resistance [Internet]. Cham: Springer International Publishing; 2019:1–7.
19. Danmark, Sundhedsstyrelsen. Follow up on the National Cancer Plan II - Denmark (In Danish: Opfølging på Kræftplan II). Copenhagen: Sundhedsstyrelsen; 2007.
20. Storm HH, Kejs AMT, Engholm G. Improved survival of Danish cancer patients 2007–2009 compared with earlier periods. Dan Med Bull. 2011;58:12.
21. Iversen LH, Green A, Ingeholm P, Østerlind K, Gögenur I. Improved survival of colorectal cancer in Denmark during 2001–2012 – the efforts of several national initiatives. Acta Oncol. 2016;55(sup2):10–23. doi:10.3109/0284186X.2015.1131331
22. Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians–A national Danish project. Health Policy. 2012;105(1):65–70. doi:10.1016/j.healthpol.2011.11.001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
