Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Improvement of Quality of Life in Dialysis and Diabetic Patients by Skin Dryness and Pruritus Management with an Ecobiological Dermo-Cosmetic Product
Authors Polena H, Chavagnac-Bonneville M , Sayag M
Received 20 May 2022
Accepted for publication 31 August 2022
Published 6 October 2022 Volume 2022:15 Pages 2143—2152
DOI https://doi.org/10.2147/CCID.S375472
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Helena Polena,1 Marlène Chavagnac-Bonneville,1,2 Michèle Sayag1
1NAOS Group, Research and Development Department, Aix-en-Provence, France; 2NAOS Institute of Life Science, Aix-en-Provence, France
Correspondence: Helena Polena, NAOS Group, 355 rue Pierre Simon Laplace, Cedex 03, Aix-en-Provence, 13593, France, Tel +33 4 72 11 37 19, Fax +33 4 78 53 82 54, Email [email protected]
Purpose: Xerosis and pruritus are common chronic dermatological disorders among dialysis and diabetic patients that are frequently underdiagnosed or neglected, which can impact the quality of life of these patients. This study aimed to evaluate the efficacy and safety of a specific dermo-cosmetic product in the treatment of dry skin and pruritus associated with dialysis and diabetes.
Patients and Methods: Twenty-nine dialysis patients (mean age 62 years) and 40 diabetic patients (mean age 57 years, 88% type 2) were included in two different single-center open-label uncontrolled clinical trials. All patients presented skin dryness according to the Scaling Roughness Redness and Cracks (SRRC) scale, and pruritus and/or insomnia. They applied the dermo-cosmetic product Medi-Secure Atoderm Xereane (NAOS, Laboratoire Bioderma) once or twice a day. The clinical efficacy (SRRC, pruritus, and insomnia), the skin-related quality of life (Dermatological Life Quality Index, DLQI), and the subjective efficacy were assessed at the inclusion visit and after 28 days of product application, as well as the safety.
Results: After 28 days of application, the product significantly reduced the SRRC global score of 83% (0.9± 0.8 vs 5.1± 1.2) and 66% (1.4± 1.2 vs 4.2± 0.5), pruritus intensity of 76% (1.1± 1.3 vs 4.6± 2.1) and 78% (0.9± 1.7 vs 4.2± 2.6), and insomnia intensity of 61% (0.9± 1.3 vs 2.4± 2.3) and 82% (0.9± 1.7 vs 4.8± 2.7) in dialysis and diabetic patients, respectively. Furthermore, the product’s application led to an improvement of the skin-related quality of life of 50% (5.4 vs 2.7; p< 0.0001) in dialysis patients and 71% (6.6 vs 1.9; p< 0.0001) in diabetic patients at D28. In addition, the product was greatly appreciated by all patients for its soothing, comforting, repairing, nourishing, and hydrating effects and was very well tolerated by the entire panels.
Conclusion: This specific dermo-cosmetic product significantly reduces skin dryness, pruritus, and insomnia in dialysis and diabetic patients, thereby greatly improves their skin-related quality of life. By managing and avoiding bothersome symptoms associated with their disease or treatment, this ecobiological dermo-cosmetic can prevent serious complications that constitute a substantial burden on their daily life.
Keywords: xerosis, itch, uremic, diabetes mellitus, insomnia, emollient
Introduction
Xerosis and pruritus are common chronic dermatological disorders among patients undergoing maintenance renal dialysis1,2 and in diabetic patients.3 In patients with end-stage renal disease, dry skin is observed in 50% to 90%, persisting or even worsening despite dialysis where it affects up to 80% of the patients. In the majority of cases, it disappears after renal transplantation.4 Uremic pruritus, for which the etiopathogenesis remains unclear, is also reported in 12% to 90% of these patients and is more severe in patients with dry skin than in those with normal skin.2,5,6 Several underlying causes have been proposed, including skin dryness, hypervitaminosis A, mast cell accumulation (increase in histamine release), disturbance in tryptase and chymase activity (pH increase in the stratum corneum), uremic toxins, allergic sensitization related to dialysis, imbalance in divalent ions, peripheral neuropathy, and opioid system involvement.7–9 Xerosis, called uremic xerosis in these patients, is not the primary cause of pruritus. But in the presence of pruritus, dry skin may have an exacerbating effect by reducing the threshold for itch.4 Furthermore uremic pruritus is a predictor of poor sleep10 and profoundly impacts the quality of life.9,11,12 Sleep quality or insomnia concerns approximately half of the patients with advanced chronic kidney diseases13 and especially caused by anxiety, depression, pain, and disorder in circadian rhythm.14
Concerning diabetes mellitus, 30% to 70% of patients have associated skin disorders.15 Xerosis is one of the commonly observed cutaneous manifestations of diabetic patients, with a prevalence higher than 40%.16–19 Approximately 3% to 49% of diabetic patients have itching, considerably impairing their quality of life.20,21 Management of diabetes mellitus-related skin conditions, in addition to improving patient quality of life, can avoid serious complications, such as diabetic foot development.3 Moreover, their quality of life is impacted by a poor sleep quality22 which prevalence and severity are higher in diabetic patients, but the causal relationship remains to be clarified.23 Diabetic xerosis results from impairment of the skin barrier due to reduction of epidermal proliferation and differentiation (an abnormal persistent cohesion between corneocytes, with secondary thickening of the tidy stratum corneum) associated with decreased hydration and sebaceous gland activity,15,16 and appears to be correlated with microangiopathy.24 It results in a higher risk of chronic wounds and infection.15,21 Xerosis in diabetes mellitus is often associated with pruritus, mostly localized to the scalp, ankles, feet, trunk, or genitalia.15 The pathogenesis of diabetic pruritus may involve polyneuropathy, linked with sweating dysfunction due to sympathetic nervous system impairment.20 It appears most commonly on the feet in diabetic patients, with at times intense pain associated with slight to severe scaling, cracks, fissures, and erythema.15
Therefore, management of these skin conditions in dialysis and diabetic patients is important for patient skin-related quality of life as it allows bothersome symptoms and even serious complications to be avoided.3,11,12 Unfortunately, these conditions are frequently underdiagnosed and usually neglected,3,12,19 although dermo-cosmetic management can be beneficial.3,25 Indeed, skincare can be able to overcome and even prevent skin alterations in dialysis and diabetes mellitus, especially by improving skin hydration.
Moreover, in this context, the ecobiological approach is relevant since this original approach considers the patients globally. It also takes into account the skin in relation with its environment, as an ever-evolving ecosystem, whose natural resources and mechanisms must be preserved.26,27 Following this principle of ecobiology to have a positive footprint on the skin ecosystem, we investigate the efficacy and safety of a specific dermo-cosmetic product (Medi-Secure Atoderm Xereane, Laboratoire Bioderma, NAOS, France) on dry skin and pruritus associated with dialysis and diabetes in two single-center open-label uncontrolled clinical trials. This nourishing balm is formulated with D-panthenol, glycerine, vegetal waxes and shea oil, all skin biomimetic ingredients, and known to improve skin hydration and to restore skin barrier, in association with a kyotorphin-like structure dipeptide, Antalgicine®, with anti-itching properties.
Patients and Methods
Patients and Study Design
Two clinical studies were undertaken as single-center open-label uncontrolled trials. One of these studies was carried out in a clinical center in Poland (Eurofins, Dermscan Poland) involving dialysis patients, and it was approved (#KB – 818/2019/10.09.2019) by the Bioethics Committee of the Regional Chamber of Physicians in Gdansk (Poland). The second study, which involved diabetic patients, took place in a clinical center in Mauritius (Insight Research, Mauritius), where no approval from local ethics committees was required according to the local regulatory guidelines. Both studies complied with the Declaration of Helsinki, Good Clinical Practice Guidelines, and local laws and regulations. All participants provided written informed consent prior to study participation.
These two studies included both male and female patients, aged 18–85 years and undergoing dialysis due to renal failure for at least 3 years, or with type 1 or type 2 diabetes mellitus. All patients had a xerosis score between grade 4 and 12 (according to the Scaling, Roughness, Redness, and Cracks [SRRC] score, see below), and pruritus and/or insomnia ≥ 1 (according to a 10-point Visual Analogue Scale [VAS], see below) related to the treatment or the pathology. The standard exclusion criteria were as follows: pregnant or breastfeeding women or women who intended to become pregnant, the use of topical or systemic treatment in prior weeks liable to interfere with the assessment of the cutaneous acceptability of the study product (except antidiabetics and immunosuppressors for diabetic patients), excessive exposure to sunlight in the month preceding the study, and subjects enrolled in another clinical study during the study period. They applied the product Medi-Secure Atoderm Xereane (NAOS, Laboratoire Bioderma, Aix-en-Provence, France; ingredients list in Table 1) for 28 days once or twice daily to the face and body previously cleansed with gentle standardized hygiene products.
 |
Table 1 Ingredients of the Dermo-Cosmetic Product (Medi-Secure Atoderm Xereane) |
Efficacy and Safety Assessments
The SRRC score was used in accordance with the EEMCO guidelines for assessment of dry skin guidelines.28 The SRRC score uses a 5-point scale (from 0=absent to 4=extreme) to evaluate scaling, roughness, redness, and cracks, separately and as the sum of each evaluated sign. SRRC was assessed at baseline (Day 0, D0) and after four weeks of product application (D28). The pruritus and insomnia severity scores were assessed by patient questioning using a VAS from 0 (no pruritus/no insomnia) to 10 (severe pruritus/severe insomnia). The overall efficacy was also evaluated by the dermatologist after 28 days, and pictures were taken of selected skin areas with standardized indirect lighting.
The skin-related quality of life was assessed by the patients using the Dermatological Life Quality Index (DLQI) questionnaire.29 The self-assessment of the product efficacy was evaluated by the patients at D28 using a standard questionnaire with a 5-point scale (strongly agree, agree, undecided, disagree, or strongly disagree), where strongly agree and agree were considered to be positive answers.
Safety was evaluated by the patients as reported adverse events from D0 to D28 using a 5-point scale (0=none, 1=very mild, 2=mild, 3=moderate, or 4=severe), and by the dermatologist at D28 using a 4-point rating scale (very good, good, average, or poor).
Statistical Analysis
Statistical analysis was performed using the paired t-test when the data followed a normal distribution (assumption with a Shapiro–Wilk test), otherwise with the Wilcoxon test. p < 0.05 values were considered statistically significant. Correlation analysis between continuous variables and DLQI scores were performed using Spearman's correlation test.
Results
Patient Characteristics
In total, 33 dialysis patients with dry skin were included, of whom 29 completed the study and adhered to the protocol (28 for the DLQI). The mean age was 62 years, with 16 men and 13 women (Table 2), all suffering from pruritus at D0. Concerning the enrollment of diabetic patients with dry skin, 43 were included and 40, including 5 with type 1 and 35 with type 2 diabetes, were analyzed (34 women and 6 men, mean age 57 years), of whom 37 (92.5%) suffered from pruritus at D0.
 |
Table 2 Characteristics of the Dialysis and Diabetic Patients Included in the Clinical Studies |
Clinical Assessments
After using the product for 28 days, all clinical signs evaluated on the dialysis patients as well as the SRRC total score decreased in a significant manner at D28 compared to D0: −91% for scaling (0.2±0.4 vs 1.9±0.5), −73% for roughness (0.6±0.5 vs 2.1±0.5) and redness (0.1±0.3 vs 0.4±0.6), −100% for cracks (0.0±0.0 vs 0.6±0; 7) (Figure 1A), and −83% (0.9±0.8 vs 5.1±1.2) for the SRRC total score (Figure 1B). In diabetic patients, scaling and roughness had decreased in a significant manner at D28 compared to D0, −71% (0.8±0.8 vs 2.9±0.5) and −61% (0.4±0.5 vs 1.1±0.3), respectively (Figure 1C), as well as the mean overall SRRC score, with −66% decrease (1.4±1.2 vs 4.2±0.5) (Figure 1D).
At D0, all the dialysis patients suffered from pruritus and 22 suffered from insomnia, while at D28 there were only 16 and 14, respectively, corresponding to a decrease of −49% and −36%, respectively (data not shown). Similarly, at D0, 37 diabetic patients suffered from pruritus and 17 from insomnia (n=20), and at D28 there were only 11 and 6, respectively, corresponding to a reduction of −70% and −67%, respectively (data not shown).
In terms of the intensity, pruritus and insomnia were significantly reduced in the dialysis patients by −76% (1.1±1.3 vs 4.6±2.1) and −61% (0.9±1.3 vs 2.4±2.3), respectively (Figure 2A), and in the diabetic patients by −78% (0.9±1.7 vs 4.2±2.6) and −82% (0.9±1.7 vs 4.8±2.7), respectively (Figure 2B).
According to the dermatologists, the product was very effective for all dialysis and diabetic patients, as illustrated in Figure 3, and helped overcome the skin discomfort associated with the illness for the diabetic patients.
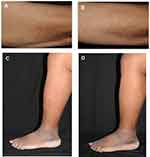 |
Figure 3 Illustrative pictures of dialysis patients at D0 (A) and D28 (B) and of diabetic patients at D0 (C) and D28 (D). |
Quality of Life Assessment
The quality of life of the dialysis and diabetic patients were also improved from D0 to D28, with a significant decrease in the DLQI of −50% (2.7±4.2 vs 5.4±4.6) and −71% (1.9±0.5 vs 6.6±0.7), respectively (Figure 4). Interestingly, the DLQI scores between 0 to 5 that correspond to normal to slight impairment of quality of life (according to Hongbo et al30), concerning 54% of the dialysis patients at D0, increased to 79% at D28, and for the diabetic patients, 53% at D0 increased to 98% at D28 (Table 3). This improvement was particularly noticeable for the items: itchy, sore, painful, or stinging skin, embarrassed or self-conscious due to the appearance of the skin, and in terms of the impact at work or studies. At baseline, a significant correlation was only demonstrated between the pruritus and the DLQI (r=0.686 for dialysis p < 0.001, r=0.348 for diabetes p < 0.05).
 |
Table 3 Evaluation of Skin-Related Quality of Life Using the DLQI in Dialysis and Diabetic Patients at D0 and D28 (Interpretation According to Hongbo et al30) |
 |
Figure 4 Evaluation of skin-related quality of life using the DLQI at D0 and D28 in dialysis (n=28) (A) and diabetic patients (n=40) (B) (***p<0.0001). |
To be noticed, some questions in the DLQI were answered “non-applicable” especially in dialysis patients (n=28) on the question 6 concerning the impact on doing sport over the last week (n=13). Indeed, some patients may not practice sport over the last week. Similarly, it was also the case for the questions 7 (n=10) and 9 (n=11) concerning working/studying and sexual difficulties over the last week, respectively. And in diabetic patients (n=40), on questions 8 (n=11), 9 (n=11) and 10 (n=9 at D0 and n=10 at D28) some patients were not concerned by the impact on partner/friends/relatives, sexual difficulties and on the treatment, respectively.
Subjective Efficacy Assessment
After 28 days of product application, the dialysis patients deemed their skin to be less reactive (77% of them), more comfortable (83% of them), repaired (80% of them), nourished and hydrated (90% of them) (Figure 5A). Concerning the diabetic patients, all of them deemed the product to be efficient for the same items (Figure 5B). Furthermore, the cosmetic acceptability of the product was good for all the patients (data not shown).
 |
Figure 5 Self-assessment of the product efficacy at D28 by dialysis (n=29) (A) and by diabetic patients (n=40) (B). |
Safety Assessment
During the study, the safety of the product was rated as “good” to “very good” by 93% of the dialysis patients and by 100% of the diabetic patients. The dermatologist rated the safety as “very good” for all of the dialysis and diabetic patients.
Discussion
This study at two trial sites demonstrated that application of the dermo-cosmetic product for 28 days reduced skin dryness, pruritus, and insomnia, and improved the skin-related quality of life in dialysis and diabetic patients. Some studies have already described that some emollients can counteract the alterations of dry skin and pruritus due to dialysis or diabetes, but to our knowledge, this is the first clinical study to evaluate the same dermo-cosmetic product on both types of patients.
Concerning dialysis, the baseline scores for dryness, pruritus, insomnia, and DLQI are close to the data in the literature.11,12 Four previous studies demonstrated the efficacy of an emollient in xerosis and uremic pruritus,7,8,31,32 but only one evaluated the skin-related quality of life.32 Interestingly, uremic pruritus affected the quality of life of patients with end-stage renal disease,11,12 but not xerosis (evaluated by the El Gammal index), corresponding more to a prognostic factor for worsening of the skin-related quality of life.12 In the current study, similarly only a correlation between pruritus with DLQI was observed. Therefore, evaluation of both clinical signs and the skin-related quality of life is important to assess the overall efficacy of the product, as these are complementary by associating clinical and psychological aspects. Indeed, quality of life measurement is an important outcome, especially in chronic diseases, and better treatment compliance can be expected when it is improved.33,34 The objective is to place the patient in the center of the self-management, where the health-related quality of life (HRQL) can be relevant.35 Several studies have evaluated the effect of an emollient in diabetic patients, but most of them focused on xerosis in diabetic foot.3,36,37 Indeed, management of dry skin in diabetic foot is important as there is an increased risk of ulceration, which is a major and common complication in diabetes mellitus that can lead to amputation. One study investigated the efficacy of an emollient on the arms and legs of 40 diabetic patients, the majority of which were type 1 (32 patients) after one month of application and showed an improvement in terms of dryness and pruritus.17 However, to our knowledge, the current study is the first to also evaluate the impact of applying a dermo-cosmetic product on the skin-related quality of life of these patients. In diabetic foot xerosis, urea-based formulations are commonly used to prevent callus removal, which plays a crucial role by reducing focal plantar pressures. However, this type of skincare is not always suitable for management of dry skin on other parts of the body that do not present callus.
An ecological balance of the skin is essential in dermatology, and this can be considered as the ultimate aim of ecobiology. This original approach considers the skin, in relation with its environment, as an ever-evolving ecosystem, whose natural resources and mechanisms must be preserved.26,27 This approach applies to adapted skincare products, which play a fundamental role in dermatological treatment for healthy as well as diseased skin. Following the principle of ecobiology to have a positive footprint on the skin ecosystem, the investigative product is a nourishing balm formulated with D-panthenol, a natural skin precursor of fatty acids and sphingolipids synthesis,38 known to improve skin hydration and repair the skin barrier through regeneration.39,40 In addition, it also contains glycerine, which plays a role in skin hydration, cutaneous elasticity, and epidermal barrier repair,41 as well as lipids, such as vegetal waxes and shea oil, that are structurally similar to the lipids that occur naturally in skin. These biomimetic ingredients are known to enhance hydration and restore the skin barrier. This moisturizing effect keeps the skin well hydrated, thereby exerting a soothing effect and preventing itching. The anti-itching effect is also due to the presence of Antalgicine®, which is a dipeptide with a structure similar to kyotorphin that exerts lasting analgesic effects.42 Kyotorphin has an analgesic effect by stimulation of the release of the opioid neuromodulator met-enkephalin in nerve cells.43 Therefore, this formula had both moisturizing and anti-itching properties that are suitable for application to all parts of the body.
The two main limitations of the study are that both studies were pilot trials including a small sample size, and the lack of comparison with a reference product (since the absence of a placebo or non-treated area would be unethical). In addition, another limitation of this study could be directly linked to the DLQI questionnaire. A non-applicable answer might constitute a false-negative answer potentially leading to an underestimation of the disease impact (21% of the answers of dialysis patients and 11% of diabetic patients).
Further controlled randomized studies with another known product or local practices are warranted to confirm the efficacy. The results obtained are however promising and suggest a relevant degree of efficacy of this dermo-cosmetic product in this clinical setting. In conclusion, this study showed that topical application of an ecobiological dermo-cosmetic product can improve their skin-related quality of life, which was correlated to the presence of pruritus at baseline. This customized skincare warrants being used more frequently in these types of patients and could also contribute to preventing further complications.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Eurofins/Dermscan (Villeurbanne, France) for managing the two clinical trials, Benoit Cadars and Félix Giraud (NAOS ILS, Aix-en-Provence, France) for their support regarding the correlation analysis, and Eric Perrier (NAOS ILS, Aix-en-Provence, France) for his proofreading.
Disclosure
This study was funded by NAOS (Laboratoire Bioderma - Institut Esthederm - Etat Pur, Aix-en-Provence, France). H. Polena, M. Chavagnac-Bonneville, and M. Sayag are employees of NAOS Group, Aix-en-Provence, France. The authors report no other conflicts of interest in this work.
References
1. Tajalli F, Mirahmadi SMS, Mozafarpoor S, Goodarzi A, Nasiri Partovi M, Lakestani D. Mucocutaneous manifestations of patients with chronic kidney disease under hemodialysis: a cross-sectional study of 49 patients. Dermatol Ther. 2021;34(4). doi:10.1111/dth.15015
2. Sommer R, Ständer S, Augustin M. Skin lesions, skin care, and characteristics of pruritus in patients undergoing haemodialysis. Skin Pharmacol Physiol. 2022;35(2):87–93. doi:10.1159/000519367
3. De Macedo GMC, Nunes S, Barreto T. Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetol Metab Syndr. 2016;8(1):63. doi:10.1186/s13098-016-0176-y
4. Szepietowski JC, Reich A, Schwartz RA. Uraemic xerosis. Nephrol Dial Transplant. 2004;19(11):2709–2712. doi:10.1093/ndt/gfh480
5. Van De Velde-Kossmann KM. Skin examination: an important diagnostic tool in renal failure patients. Blood Purif. 2018;45:1–3. doi:10.1159/000478972
6. Falodun O, Ogunbiyi A, Salako B, George AK. Skin changes in patients with chronic renal failure. Saudi J Kidney Dis Transpl. 2011;22(2):268–272.
7. Okada K, Matsumoto K. Effect of skin care with an emollient containing a high water content on mild uremic pruritus. Ther Apher Dial. 2004;8(5):419–422. doi:10.1111/j.1526-0968.2004.00175.x
8. Castello M, Milani M. Efficacy of topical hydrating and emollient lotion containing 10% urea ISDIN® plus dexpanthenol (Ureadin Rx 10) in the treatment of skin xerosis and pruritus in hemodialyzed patients: an open prospective pilot trial. G Ital di Dermatologia e Venereol. 2011;146(5):321–325.
9. Lanot A, Kottler D, Béchade C. Pruritus associated chronic kidney disease. Nephrol Ther. 2021;17(7):488–495. doi:10.1016/j.nephro.2021.07.002
10. Kosmadakis GC, Papakonstantinou S, Theodoros C, Emmanouel P, Demetrios V, Nicolas Z. Characteristics of uremic pruritus in hemodialysis patients: data from a single center. Kidney Int. 2008;74(7):962–963. doi:10.1038/ki.2008.359
11. Suseł J, Batycka-Baran A, Reich A, Szepietowski JC. Uraemic pruritus markedly affects the quality of life and depressive symptoms in haemodialysis patients with end-stage renal disease. Acta Derm Venereol. 2014;94(3):276–281. doi:10.2340/00015555-1749
12. Szepietowski JC, Balaskas E, Taube KM, Taberly A, Dupuy P. Quality of life in patients with uraemic xerosis and pruritus. Acta Derm Venereol. 2011;91(3):313–317. doi:10.2340/00015555-1075
13. Tan L-H, Chen PS, Chiang H-Y, et al. Insomnia and poor sleep in CKD: a systematic review and meta-analysis. Kidney Med. 2022;4(5):1–12. doi:10.1016/j.xkme.2022.100458
14. Diaz S, Abad K, Patel SR, Unruh ML. Emerging treatments for insomnia, sleep apnea, and restless leg syndrome among dialysis patients. Semin Nephrol. 2021;41(6):526–533. doi:10.1016/j.semnephrol.2021.10.005
15. Kirsner RS, Yosipovitch G, Hu S, et al. Diabetic skin changes can benefit from moisturizer and cleanser use: a review. J Drugs Dermatol. 2019;18(12):1211–1217.
16. Piérard GE, Seité S, Hermanns-Lê T, Delvenne P, Scheen A, Piérard-Franchimont C. The skin landscape in diabetes mellitus. Focus on dermocosmetic management. Clin Cosmet Investig Dermatol. 2013;6:127–135. doi:10.2147/CCID.S43141
17. Seité S, Khemis A, Rougier A, Ortonne JP. Importance of treatment of skin xerosis in diabetes. J Eur Acad Dermatology Venereol. 2011;25(5):607–609. doi:10.1111/j.1468-3083.2010.03807.x
18. Goyal A, Raina S, Kaushal S, Mahajan V, Sharma N. Pattern of cutaneous manifestations in diabetes mellitus. Indian J Dermatol. 2010;55(1):39–41. doi:10.4103/0019-5154.60349
19. Stingeni L, Tramontana M, Cordera L, Castello M, Parodi A. Xerosis in patients with Type 2 diabetes: an Italian multicentre study. Acta Derm Venereol. 2021;101(10):adv00577. doi:10.2340/actadv.v101.263
20. Lima AL, Illing T, Schliemann S, Elsner P. Cutaneous manifestations of diabetes mellitus: a review. Am J Clin Dermatol. 2017;18(4):541–553. doi:10.1007/s40257-017-0275-z
21. Stefaniak AA, Krajewski PK, Bednarska-Chabowska D, Bolanowski M, Mazur G, Szepietowski JC. Itch in adult population with type 2 diabetes mellitus: clinical profile, pathogenesis and disease-related burden in a cross-sectional study. Biology. 2021;10(12). doi:10.3390/biology10121332
22. Dong D, Lou P, Wang J, et al. Interaction of sleep quality and anxiety on quality of life in individuals with type 2 diabetes mellitus. Health Qual Life Outcomes. 2020;18(1). doi:10.1186/s12955-020-01406-z
23. Ogilvie RP, Patel SR. The epidemiology of sleep and diabetes. Curr Diab Rep. 2018;18(10). doi:10.1007/s11892-018-1055-8
24. Flagothier C, Quatresooz P, Bourguignon R. Cutaneous stigmata of diabetes mellitus | stigmates cutan?s du diab?te. Rev Med Liege. 2005;60(5–6):553–559.
25. Szepietowski JC, Schwartz RA. Uremic pruritus. Int J Dermatol. 1998;37(4):247–253. doi:10.1046/j.1365-4362.1998.00459.x
26. Radman M. Ecobiological approach to research regarding ageing and diseases. Eur J Dermatol. 2019;29(1):11–14.
27. Dréno B. The microbiome, a new target for ecobiology in dermatology. Eur J Dermatol. 2019;29(1):15–18.
28. Serup J. EEMCO guidance for the assessment of dry skin (xerosis) and ichthyosis: clinical scoring systems. Ski Res Technol. 1995;1(3):109–114. doi:10.1111/j.1600-0846.1995.tb00029.x
29. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
30. Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664. doi:10.1111/j.0022-202X.2005.23621.x
31. Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerologica Croat. 2005;13(2):97–103.
32. Yoshida Y, Hirama A, Hashimoto K, et al. Efficacy of a moisturizer for pruritus accompanied by xerosis in patients undergoing dialysis: a multicenter, open-label, randomized verification study. J Dermatol. 2021;48(9):1327–1335. doi:10.1111/1346-8138.15950
33. Siboni F, Alimoradi Z, Atashi V, Alipour M, Khatooni M. Quality of life in different chronic diseases and its related factors. Int J Prev Med. 2019;10(1):45.
34. Addington-Hall J, Kalra L. Who should measure quality of life? BMJ. 2001;322:7299. doi:10.1136/bmj.322.7299.1417
35. Wilson BD, Moon S, Armstrong F. Comprehensive review of ultraviolet radiation and the current status on sunscreens. J Clin Aesthet Dermatol. 2012;5(9):18–23.
36. Martini J, Huertas C, Turlier V, Saint-Martory C, Delarue A. Efficacy of an emollient cream in the treatment of xerosis in diabetic foot: a double-blind, randomized, vehicle-controlled clinical trial. J Eur Acad Dermatology Venereol. 2017;31(4):743–747. doi:10.1111/jdv.14095
37. Federici A, Federici G, Milani M. Use of a urea, arginine and carnosine cream versus a standard emollient glycerol cream for treatment of severe xerosis of the feet in patients with type 2 diabetes: a randomized, 8 month, assessor-blinded, controlled trial. Curr Med Res Opin. 2015;31(6):1063–1069. doi:10.1185/03007995.2015.1037731
38. Proksch E, Nissen HP. Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. J Dermatolog Treat. 2002;13(4):173–178. doi:10.1080/09546630212345674
39. Ebner F, Heller A, Rippke F, Tausch I. Topical use of dexpanthenol in skin disorders. Am J Clin Dermatol. 2002;3(6):427–433. doi:10.2165/00128071-200203060-00005
40. Proksch E, de Bony R, Trapp S, Boudon S. Topical use of dexpanthenol: a 70th anniversary article. J Dermatolog Treat. 2017;28(8):766–773. doi:10.1080/09546634.2017.1325310
41. Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and functions. Br J Dermatol. 2008;159(1):23–34. doi:10.1111/j.1365-2133.2008.08643.x
42. Dzambazova E, Bocheva A. The unique brain dipeptide kyotorphin - from discovery to nowadays. J Biomed Clin Res. 2010;3(1):3–11.
43. Takagi H, Shiomi H, Ueda H, Amano H. A novel analgesic dipeptide from bovine brain is a possible Met-enkephalin releaser. Nature. 1979;282(5737):410–412. doi:10.1038/282410a0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


