Back to Journals » Drug Design, Development and Therapy » Volume 14
Improvement of Pain Relief of Fentanyl Citrate Drug Encapsulated in Nanostructured Lipid Carrier: Drug Formulation, Parameter Optimization, in vitro and in vivo Studies
Authors Bahrami MA, Farhadian N , Karimi M, Forouzan A , Masoumi K
Received 30 October 2019
Accepted for publication 29 April 2020
Published 25 May 2020 Volume 2020:14 Pages 2033—2045
DOI https://doi.org/10.2147/DDDT.S235474
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Mohammad Amin Bahrami,1 Nafiseh Farhadian,1 Mohammad Karimi,2,3 Arash Forouzan,3 Kambiz Masoumi3
1Chemical Engineering Department, Faculty of Engineering, Ferdowsi University of Mashhad, Mashhad, Iran; 2Cardiovascular Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; 3Emergency Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Correspondence: Nafiseh Farhadian Tel +985138805140
Fax +985138816841
Email [email protected]
Introduction: In this study, the encapsulation of fentanyl citrate as an opioid drug with hydrophobic nature in the nanostructured lipid carrier (NLC) is performed.
Methods: For encapsulation of fentanyl citrate drug, hot homogenization method is used. The pharmacokinetics of encapsulated fentanyl citrate for pain relief of rats are investigated. The influence of important variables such as the ratio of liquid lipid to the total amount of lipids, surfactant type and concentration on the particle size is investigated using response surface method.
Results: Results show that the optimal NLC size is about 90 nm with PDI value around 0.2 and zeta potential of − 25± 4.01 mV. Characterization analysis of optimal nanostructure shows successful encapsulation of the drug in nanostructure with a spherical morphology of the NLC structure. Results of drug release from commercial fentanyl citrate ampoule and NLC form indicate a control drug release from the NLC within 72 hours in comparison to the commercial ampoule. In vivo studies show that fentanyl citrate-loaded NLC not only has the potential to relieve pain in doses equal to commercial drug but also it can reduce the dose of the drug about 50%.
Conclusion: In conclusion, NLC form of fentanyl citrate can increase the efficacy of the drug by appropriate drug distribution in the body and can reduce the risks of overdose.
Keywords: fentanyl citrate, nanostructured lipid carrier, drug control release, pain relief, formalin test
Introduction
Fentanyl is a synthesized drug that is first synthesized by Paul Johnson at Jansen Company in Belgium in December 1960.1 This drug is known as a potent analgesic and is used for acute and chronic pain.2 Fentanyl is an opioid drug that stimulates µ-opioid receptors.3 Fentanyl can medically use in several forms like patch,4 intranasal,5 sublingual,6 lozenge,7 injectable, etc. The intravenous fentanyl injection is generally used for anesthesia and pain relief. Fentanyl citrate (fentanyl salts produced from the combination with citric acid 1:1) is also used for intravenous and intramuscular injection.8 Fentanyl citrate (FC) is also very potent as fentanyl.9 The presence of about 0.2–1.2 ng/mL fentanyl in blood can result in severe anesthesia for people who do not have the experience of using opioids.2 In recent years, the number of people killed due to excessive use of this drug has increased significantly. In 2016, about 20,000 people in the United States have died due to excessive use of this drug.10 The triple symptoms of excessive use of opioids include reduced awareness, pupil shrinkage and shortness of breath.11 This drug exerts an influence on the brain and part of the central nervous system (CNS). During overdoses, it induces respiratory depression, which may lead to death. Identifying and producing a smart carrier for loading fentanyl may improve its therapeutic efficiency, reduce its side effects and necessary dose, and increase drug release time in the body that prolongs the pain relief. In addition, applying smart carriers can reduce drug dosage and price of the product.
There are limited studies that design sustained release vehicles for FC and other opioid drugs. In 1992, Bernards et al, encapsulated alfentanil as a highly diffusible 4-anilopiperidine opioid drug based on liposome for intrathecal administration. They observed that liposome encapsulation reduce systemic side effects and increase the duration of action for a given dose of the drug.12 In 1994, Wallace et al investigated the spinal antinociceptive and supraspinally mediated side effects of intrathecal alfentanil encapsulated in liposomes in rats. Supraspinal side effects of the drug were tested by measuring catalepsy and the eye blink evoked by touching the cornea.13 After that, in 1995, Isacson et al encapsulated alfentanil in a liposome and investigated its antinociception, side effects, and algogenic behavior for the spinal administration.14 In both mentioned studies, results confirmed that applying liposome encapsulation can totally decrease the side effects of the drug or the side effects can be observed at the highest alfentanil dose.
Since fentanyl citrate is a lipophilic drug with extensive use in recent years and enormous side effects, it can be a suitable case for study. However, lipid nanoparticles are one of the most suitable carriers for loading and improving the function of this drug in the body. Lipid nanoparticles are easily dissolved in the body, resulting in no side effects.15 Economically, it is very cost-effective and can be synthesized in a short time. The process of lipid nanoparticles formation is scalable and the final product has commercialization capability. Lipid nanocarriers protect sensitive molecules against the external environment. There is no obligation to use chemical and toxic solvents in the lipid nanostructure manufacturing. In addition, biodegradable fats are used in lipid carriers. So, the risk of toxicants from chemicals is minimized.16
Nanostructured lipid carriers (NLCs) derive from oil-based nano-emulsions. Like nanoemulsions and microemulsions, fat, surface-active agents and water are the main ingredients for the preparation of NLCs. Drug loading in the NLC structure may increase its efficacy and reduce side effects.15,17,18
In this study, the encapsulation of fentanyl citrate drug in a nanostructured lipid carrier is performed. Hot homogenization method is used to synthesize nanoparticles. Optimization of the effective parameters on the size is performed by applying statistical analysis using the response surface method. In addition, drug release profile is investigated and compared with commercial fentanyl citrate ampoule. At last, the performance of the NLC form of drug as well as commercial fentanyl ampoule is examined on the pain relief of rats.
Materials and Methods
Materials
Glycerol monostearate (GMS) powder and Tween 80 were purchased from Merck in commercial grade. Pumpkin seed oil was purchased locally (Mashhad, Iran). The poloxamer 407 was purchased from Sigma Aldrich (Steinheim, Germany). Fentanyl citrate was purchased from Tofigh Daru Research & Engineering Company (Tehran, Iran) as drug. Commercial fentanyl citrate ampoule was purchased from Darou Pakhsh Pharmaceutical Mfg Co. Sodium chloride, potassium chloride, potassium dihydrogen phosphate, disodium hydrogen phosphate and HCl were purchased from Merck for preparation of PBS saline buffer adjusted in pH=7.4. Deionized water was used throughout the experimental pathway.
Animals
In order to evaluate the in vivo performance of fentanyl citrate loaded in NLC, 36 male Sprague Dawley rats with a weight range of 300–500 g were purchased from the Razi vaccine and serum research institute. The rats were kept in appropriate laboratory conditions at a controlled temperature of 23 ± 2 °C, an optical period of 12 hours and relative humidity in the range of 40–60%.
Preparation of NLC
Encapsulated fentanyl citrate drug in NLCs (FC-NLC) was prepared by hot homogenization method.19 Using natural oils in the structure of NLCs can improve the efficacy of the carrier.20,21 Pumpkin seed oil was selected for this aim. Two milligrams of fentanyl citrate was dissolved in the mixture of 40 mg liquid oil (pumpkin seed oil) and 120 mg melted solid lipid (GMS) at the temperature of 85°C. Then, 8mL of the hot aqueous surfactant solution (poloxamer 407 or Tween 80) with the concentration of 9 mg/mL at the temperature equalto lipid phase was added slowly into the lipid phase under ultra-sonication. The sonicate power was fixed at 20W all through the work and the sonication time was about 1 minute. Finally, the NLC was formed by cooling down the temperature of the mixture by adding water at the temperature of 5°C.
Optimization of FC-NLC Formulation
Statistical test was performed in order to optimize the input variables. A randomized central composite design–response surface methodology with two numerical and one categorical factors was used to systemically investigate the influence of variables on the particle size of the prepared NLCs. The details of the design are listed in Table 1. The range of each factor was selected on the basis of the results of preliminary experiments and the feasibility of preparing the NLC at the extreme values. All the formulations in these experiments were prepared in duplicate. Finally, the best sample in terms of size was selected for further characterization. The amount of drug used in all the samples was constant.
 |
Table 1 Range of Variables Applied in Statistical Analysis |
Characterization
Particle Size
The hydrodynamic average diameter of particles and polydispersity index (PDI) was determined by particle size analyzer (Cordouan Technologies – VASCO NP size analyzer) using the well-known technique of Dynamic Light Scattering (DLS), at the temperature of 25°C.
Zeta Potential
In order to investigate the stability and the surface charge of NLCs, zeta potential analysis was used. The zeta potential was measured using a zeta potential analyzer (CAD instrument, France). The samples were allowed to dilute with deionized water prior to zeta potential calculation.
Transmission Electron Microscopy (TEM) Analysis
The morphological observation of NLCs was performed by transmission electron microscopy (Leo 912 AB, Zeiss, Germany) with the negative staining method using 2% uranyl acetate. The sample demanded was prepared by placing a drop of formulation which was diluted twofold with deionized water onto a 300-mesh copper grid coated with carbon film and air-dried for 3h.
Differential Scanning Calorimetry (DSC) Analysis
Differential scanning calorimetry is a thermal test that measures the physical properties of the sample in terms of temperature. This analysis is considered as one of the main analyses in determining the characteristics of a nanostructured lipid carrier. Enthalpy and melting point are some of the information that can be obtained from this analysis.22 DSC analysis was performed using a DSC-60 differential scanning calorimeter (Shimadzu, Japan).
The samples weighed accurately were placed in aluminum pans and sealed with a lid. In the scanning process, a heating rate of 5°C was applied in the temperature range from 20°C to 180°C with a nitrogen purge of 0.2 mL/min.
X-Ray Diffraction (XRD) Analysis
X-ray diffraction (XRD) was used to identify the crystal form of FC dispersed in the matrix of lipid.23 XRD studies were performed by X-ray diffractometer (Explorer, GNR, Italy) using Cu-Ka radiation at λ= 1.54Å. The samples were scanned over a range of 5–50 ◦C at a scan current of 30 mA.
Entrapment Efficiency (EE)
Entrapment efficiency evaluates the amount of drug as a percentage that is successfully entrapped or adsorbed into the lipid nanoparticles (Equation 1).
(1)
To obtain the amount of free drug in supernatants, 5mL of each sample were centrifuged at 16,000 rpm for 20 min in 4°C. Then, the obtained suspension was allowed to filter through 0.22 membrane filters. Finally, the resulting solution was analyzed using HPLC (Agilent 1260) equipped with a UV visible detector. The detection procedure was extracted from Anderson et al study.24 Drug detection was set up at λ=210 nm using UV-vis detector and the injection volume was 5µL. Column was C-18 (100mm×4.6 mm). The mobile phase consisted of 65% aqueous buffer containing 0.1% triethylamine adjusted to pH= 2.5 with phosphoric acid, and 35% acetonitrile. Figure S1 shows the HPLC curve of a sample containing drug with special concentration (see Figure S1). As Figure S1 shows, the retention time of fentanyl citrate is 1.479 min. Calibration curve is shown in Figure S1-b.
Drug Leakage
A solid powder sample obtained from the freeze drying stage was stored for 3 months in a refrigerator at 4 °C, in order to determine the amount of drug leakage from the carrier structure. Then, 10 mg of sample was dissolved in 20 mL of distilled water and exposed to a magnetic stirrer for 2 minutes, according to the method provided by Pongpeerapat et al.25 Then, the solution was filtered and analyzed by HPLC to measure the amount of leaked drug from the structure over a period of 3 months.
In vitro Drug Release Study
In vitro drug release was estimated using a dialysis bag with a molecular weight cutoff of 12 kDa. A dialysis bag was prepared according to the protocol provided by Sigma and then soaked in the release medium over 72 hr. The release medium was 1 mM PBS (pH 7.4). Four milliliters of sample and pure drug were placed separately in dialysis bags that float in 40 mL of buffer in 2 different beakers. Beakers were placed in the shaker incubator that was adjusted in 37°C. And, 0.5 mL sample was collected from the buffer at the specific time interval and immediately the same pure buffer was replaced in the system. Samples were sent to HPLC analysis to detect the amounts of released drug.
Formalin Test
Analgesic effect in animals was evaluated using formalin test based on Dubuisson and Dennis.26 The rats tested in this study were divided into 6 equal groups. The volume of injected drug to each rat in all groups was 0.2 mL. Injection of the drug into each rat was performed only once. The groups included in this study were
Group 1: Rats receiving the physiologic serum (control group).
Group 2: Rats receiving FC-NLC at the dose of 0.005 mg/kg body weight.
Group 3: Rats receiving FC-NLC at the dose of 0.01 mg/kg body weight.
Group 4: Rats receiving FC-NLC at the dose of 0.03 mg/kg body weight.
Group 5: Rats receiving FC-NLC at the dose of 0.06 mg/kg body weight.
Group 6: Rats receiving the fentanyl citrate ampoule at the dose of 0.06 mg/kg body weight.
Formalin test was performed 15 minutes after injection. The amount of 50 μL of 5% formalin was injected into the rats. The pain score was recorded in the first and second stages of pain in 0–5 minutes and 15–45 minutes after injection of formalin, respectively. Pain response was scored as follows:
Score 0: Both paws are placed on the floor, the weight of the animal is evenly spaced. During the move, there is no option for using the opposite paw.
Score 1: The injected paw is placed lightly on the floor and applied a little pressure on it. The animal lags clearly during the movement.
Score 2: The injected paw is elevated, and not in contact with any surface. The uninjected paw is placed firmly on the floor. Attempts to sleep by curling up with only the injected paw off the floor, even when it is tucked under the body, are given this rating.
Score 3: The injected paw is licked, bitten, or shaken, while the uninjected paw is not. This behavior is quite distinct from normal grooming (rating l), although transitions between the two are common.
It should be noted that all animal experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EC) and national committee of laboratory animal rights (91-01-159-18022) to minimize damage to the animals. In addition, this study was approved by Ahvaz Jundishapur University of Medical Sciences animal care and use committee.
Results and Discussion
Experimental Design
At this stage, according to the initial model provided by Design Expert software, 22 different tests were performed to discover the consistent equation on the process of making FC-NLC and finally identifying the optimal carrier. The relevant information is shown in Table 2.
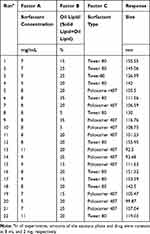 |
Table 2 Experiments Designed by Software and Their Responses |
One of the important tools for distinguishing the importance of parameters separately and their interactions together is analysis of variance (ANOVA) table. The significance of each of the parameters presented in this table can be obtained from the corresponding P value. The more this value is less than 0.05, the more important.27 Table 3 shows the results of the modified ANOVA for the important parameters affecting the particle size.
 |
Table 3 Modified Analysis of Variance Table |
As shown above, among the three main input parameters, parameters A and C, which are related to the surfactant concentration and type, are significant, and parameter B, which is the oil to total lipid ratio, is not significant. But since parameters AB, BC, B2, B2C and A2B are considered important parameters, parameter B cannot be deleted from the final equation.
The Lack of Fit value indicates whether the proposed model is appropriate or not, due to the total effect of the deleted and non-existent parameters. Since the necessary corrections have been made in Table 3 and the non-important parameters have been removed, the value of P for the Lack of Fit parameter in the table is 0.9969. This value is not significant and indicates that the information in the table is appropriate and no more corrections are needed to be implemented. The quantitative parameters required for a more accurate examination of the accuracy of the ANOVA table are shown in Table 4.
 |
Table 4 Quantitative Parameters Needed to Verify the Accuracy of the ANOVA Table Parameters |
The accuracy of the model can be verified by the values of the R-Squared and Adj R-Squared. The closer the value of these two parameters to 1 is the higher the validity of the model and the better it can predict the results.28 According to Table 4, the values of R-Squared and Adj R-Squared are 0.99 and 0.97, respectively, which are acceptable values. The Adeq Precision parameter represents the signal-to-noise ratio in the system. This ratio is acceptable for values higher than 4.29 The value obtained from the above model is 24.294, which confirms the acceptability of the results. Finally, the consistent equation on the size of the nanoparticles produced in this study is as follows:
(2)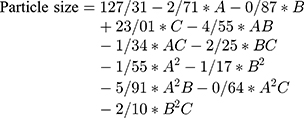
With respect to the coefficients of the above equation, parameter C has the greatest effect on the particle size. It is worth noting that the above equation is valid only in the range of parameters provided to the software and cannot be expressed with confidence in relation to external points.
Figure 1A and B illustrates the three- and two-dimensional diagrams of the interaction between the parameters A and B, respectively. Figure 1C and D shows the effect of the interactions between variables A–C and B–C on the particle size, respectively. The severe effect of the type of surfactant on the particle size is clear on figures.
 |
Figure 1 Effect of interactions between (A) variables A and B as 3D diagram, (B) variables A and B as 2D diagram, (C) variables A–C and (D) variables B and C on the size of nanostructured carriers. |
The optimal conditions for constructing nanostructured carriers occur when the most favorable responses are received from the system. In this study, the smaller the particle size is more desirable. The optimal conditions provided by the software occurred when the surfactant concentration was equal to 9.003% and the oil phase percentage was 27.7%. In these conditions, the final particle size provided by the software was 87.96 nm. To confirm the results of the software in optimal conditions, a retest was performed. The particle size obtained at this step was 90.71 nm, which had an acceptable proximity with software outcomes. The optimized sample was then characterized by different analyses.
Characterization Results
Zeta Potential
The amount of optimized NLC surface charge was about −25.05 ± 4.01 mV. This numerical value is due to the presence of negative charge functional groups on the surface of the nanoparticles. The negative charge causes electrostatic repulsion between the particles and prevents particles from agglomeration.
Drug Entrapment Efficiency and Leakage
According to the results of unloaded drug in the optimized nanocarrier, which was obtained by HPLC analysis, the drug entrapment efficiency was calculated to be 82%, which is an acceptable value. Drug leakage was calculated less than 1% after 3 months storage.
TEM Analysis
Figure 2 shows the structure of optimized NLC in different magnifications of TEM analysis. As shown in Figure 2, the optimized NLCs have a spherical structure. Lighter parts within the spherical structure probably represent rigid or solid portions of the NLC, and darker sections represent the oil phase inside the structure. According to the images, the average particle size is 80 nm. The particle size obtained from TEM images is about 10 nm smaller than the particle size obtained by the DLS analysis. The DLS analysis measures the hydrodynamic diameter of the nanoparticles in an aqueous medium. Therefore, the actual particle size, which is measured by the TEM analysis, is expected to be less than that obtained from the DLS analysis.
 |
Figure 2 Transmission electron microscopy images of optimized FC-NLC in different magnifications. |
FTIR Analysis
Figure 3 shows the results of FTIR analysis of fentanyl citrate, glycerol monostearate, and optimized NLC. As shown in Figure 3, the FTIR spectrum obtained from optimized NLC is similar to that of glycerol monostearate. This result indicates the successful encapsulation of the drug in the lipid structure. The C-H group peak of the benzene ring is visible at 837 cm−1, in the structure of FC-NLC. Corresponding to this peak, the same benzene ring peak presented at 842 cm−1 in the drug spectrum, because the lipid structure does not have this functional group. The C-N functional group is seen in the fentanyl citrate chemical structure. A new peak is observed on the nanocarrier structure at 1343 cm−1. It can be concluded that the corresponding peak in the nanostructure is related to fentanyl citrate, because glycerol monostearate lacks this functional group.
 |
Figure 3 FTIR analysis of fentanyl citrate, glycerol monostearate and optimized FC-NLC. |
XRD Analysis
Figure 4 shows the X-ray diffraction pattern of the entanyl citrate drug, glycerol monostearate, optimized NLC without drug and the optimized FC-NLC. As shown in Figure 4, the pure solid lipid has an amorphous structure. According to patterns C and D, the lipid crystallinity has slightly improved after transformation into nanostructured form. At the angle of 13.85 and 14.47, small peaks appeared in the nanocarrier structure containing the drug (Figure 4D). These peaks are probably due to the presence of the drug in the carrier structure, because, at this angle, a corresponding peak is observed in the structure of the fentanyl citrate drug (Figure 4A). These peaks can indicate very low leakage of the drug from the structure.
 |
Figure 4 X-ray diffraction pattern of (A) fentanyl citrate, (B) glycerol monostearate, (C) optimized NLC and (D) optimized FC-NLC. |
DSC Analysis
Figure 5 illustrates the DSC graph for pure fentanyl citrate, glycerol monostearate, and optimized FC-NLC. As shown in Figure 5, glycerol monostearate is melted at about 59 °C. The optimized NLC is also melted at the same temperature during the heating process. In the temperature range of about 152 °C, the pure fentanyl citrate has a sharp peak. A new peak emerged the same temperature range in the optimized FC-NLC, indicating the presence of fentanyl citrate in the nanostructure.
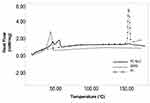 |
Figure 5 DSC graph for glycerol monostearate, fentanyl citrate, and optimized FC-NLC. |
In vitro Drug Release
Figure 6 shows the results of drug release for both optimized FC-NLC and commercial fentanyl citrate ampoule at in vitro environment. As figure shows FC-NLC displays a controllable drug release pattern during time. About 20% drug is released from the FC-NLC form after 4 h and a sustained drug release is observed until 72 h. While commercial ampoule has an explosive discharge in which about 60% drug is released during 4 h and completely release after almost 8 h. This indicates control release of drug from the nanostructure and confirms the accuracy of the proposed nanostructured function. This control release function may prolong the duration of drug analgesic action that will be checked at in vivo study in the next section. This observation is in agreement with Bernards et al’s study. They observed a prolonged action and continued release of alfentanil based on plasma concentration data from the liposome into spinal opioid receptor.12
 |
Figure 6 Cumulative drug release from optimized FC-NLC and commercial fentanyl citrate ampoule. |
Formalin Test Results
After performing formalin test and assessing the behavior of the rats against pain, the results of the pain score of the various groups in the primary and secondary phases of pain were recorded in Figure 7. Statistical analysis was performed using SPSS 24 software to evaluate the effectiveness rate of different groups. Initially, the appropriate model was determined for the statistical analysis. One-way ANOVA was selected since the data of all groups were normally distributed and their standard deviation was in a close range (see Table S1). According to the results of ANOVA, all parameters were significantly different between groups (P <0.05). Given this significant difference, it is possible to compare the mean values of the groups for each parameter. For this purpose, the homogeneity of variance test was examined initially. Since the variances were homogeneous in all cases (P> 0.05),Tukey’s test was used to compare the groups (Table 5). The results of statistical analysis can be discussed as follows:
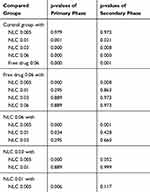 |
Table 5 Statistical Analysis Results by Applying Tukey’s Test |
As Table 5 shows there is a significant difference between the pain score of rats at both primary and secondary phases after injection FC-NLC with the dose of 0.01 mg/kg body and higher than it (Groups 3–5) in comparison to the control group. Furthermore, comparing the results of Group 5 (0.06 mg/kg body) with other FC-NLC groups shows that increasing the drug content in the nanostructured form of NLC from 0.03 mg/kg body dosage up to 0.06 mg/kg body can not change the results of pain score in both primary and secondary phases. The comparison of the results for free drug (Groups 6) and Groups 3–5 shows a significant value greater than 0.05 at both pain phases. It concluded that there is no significant difference between these groups. This suggests that, under the same conditions, lipid nanocarrier can reduce the drug dosage lower than half time. Also, for both NLC and commercial form of drug, the changes in the level of pain were similar at both phases of pains, and it can be concluded that the nanostructure form of the fentanyl behaves the same way as the commercial drug. On the other hand, both commercial and NLC forms of drug have directly affected the central nervous system.30,31 Nanostructure form of the sample can circulate easily through the bloodstream due to its hydrophilic nature in comparison to pure drug.32 Indeed, it has better performance in transferring the drug to the central nervous system. Reducing the dose of the drug can significantly reduce the side effects and can increase the use of the drug in various cases. Similar behavior was observed in another study. In 2014, Li et al30 used a formalin test to study the analgesic effect of sufentanil and sufentanil lipid nanoparticles on mice. They observed that sufentanil lipid nanoparticles can reduce the pain caused by formalin injection to the mouse to some extent compared with the time of single injection of sufentanil. However, the analyses showed that the pharmacokinetic effect of the drug was similar in single and the nanoparticle state. In another study, Isackson et al observed that encapsulation of alfentanil in liposome can significantly enhance the therapeutic ratio of a lipid-soluble opioid after spinal delivery.14 All these studies approve the appropriate function of nanocarriers in the delivery of similar opioid drugs.
 |
Figure 7 Assessment of pain score in different groups in the primary and secondary phases of pain. |
Conclusion
In this study, a nanostructured lipid carrier is prepared based on the hot homogenization method for the encapsulation of fentanyl citrate as an opioid drug. Response surface method was used to achieve the optimal combination for preparation of a structure with the lowest particle size. Results showed that the parameters of surfactant type and concentration has direct impact on the particle size while the ratio of oil to total lipid has indirect effects on the particle size due to interaction with other parameters. Optimum nanocarrier has a spherical structure with high drug entrapment efficiency. In vitro drug release investigated a controlled drug release from FC-NLC structure during 72 hours while drug release from commercial ampoule had an explosive release profile. In vivo studies using formalin test showed fentanyl citrate-loaded nanostructured lipid carrier not only has the potential to relieve pain in doses equal to commercial drug but also it can reduce the dose of the drug more than 50%. These nanoparticles may reduce the risks of overdose. Since NLC preparation is a cost-effective and scalable method, it can be a suitable candida for chaining the commercial form of lipophilic drug to nanostructure form. However, additional work will be necessary to define the therapeutic margin for FC-NLC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stanley TH, Egan TD, Van Aken H. A tribute to Dr. Paul AJ Janssen: entrepreneur extraordinaire, innovative scientist, and significant contributor to anesthesiology. Anesth Analg. 2008;106(2):451–462. doi:10.1213/ane.0b013e3181605add
2. Stanley TH. The fentanyl story. J Pain. 2014;15(12):1215–1226. doi:10.1016/j.jpain.2014.08.010
3. Bailey P, Stanley T. Opioid Action and Sensory Evoked Potentials. By Miller RD. New York: Churchill Livingstone; 1994:320.
4. Kathe K, Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J Pharm Sci. 2017;12(6):487–497. doi:10.1016/j.ajps.2017.07.004
5. Zeppetella G. An assessment of the safety, eff icacy, and acceptability of intranasal fentanyl citrate in the management of cancer-related breakthrough pain: a pilot study. J Pain Symptom Manage. 2000;20(4):253–258. doi:10.1016/S0885-3924(00)00180-9
6. Bredenberg S, Duberg M, Lennernäs B, et al. In vitro and in vivo evaluation of a new sublingual tablet system for rapid oromucosal absorption using fentanyl citrate as the active substance. Eur J Hosp Pharm. 2003;20(3):327–334. doi:10.1016/j.ejps.2003.07.002
7. Mandel L, Carunchio MJ. Rampant caries from oral transmucosal fentanyl citrate lozenge abuse. J Am Dent Assoc. 2011;142(4):406–409. doi:10.14219/jada.archive.2011.0195
8. Lambropoulos J, Spanos GA, Lazaridis NV, et al. Development and validation of an HPLC assay for fentanyl and related substances in fentanyl citrate injection, USP. J Pharm Biomed Anal. 1999;20(4):705–716. doi:10.1016/S0731-7085(99)00077-1
9. Payne R, Coluzzi P, Hart L, et al. Long-term safety of oral transmucosal fentanyl citrate for breakthrough cancer pain. J Pain Symptom Manage. 2001;22(1):575–583. doi:10.1016/S0885-3924(01)00306-2
10. Abuse, N.I.o.D. Overdose Death Rates. Bethesda, MD: NIDA; 2017.
11. Chandler S. Symptoms of an Opiate Overdose. Live Strong; May 17, 2012.
12. Bernards CM, Luger T, Malmberg AB, et al. Liposome encapsulation prolongs alfentanil spinal analgesia and alters systemic redistribution in the rate. Anesthesiology. 1992;77(3):529–535. doi:10.1097/00000542-199209000-00019
13. Wallace MS, Yanez AM, Ho RJ, Shen DD, Yaksh TL. Antinociception and side effects of liposome-encapsulated alfentanil after spinal delivery in rats. Anesth Analg. 1994;79(4):778–786. doi:10.1213/00000539-199410000-00028
14. Isackson J, Mllace MS, Ho RJY, Shed DD, Yaksh TL. Antinociception and side effects of L-and D-dipalmitoylphosphatidyl choline liposome-encapsulated alfentanil after spinal delivery in rats. Pharmacol Toxicol. 1995;77:333–340. doi:10.1111/j.1600-0773.1995.tb01037.x
15. Souto E, Almeida A, Müller R. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: structure, protection and skin effects. J Biomed Nanotechnol. 2007;3(4):317–331. doi:10.1166/jbn.2007.049
16. Hatefi L, Farhadian N. A safe and efficient method for encapsulation of ferrous sulfate in solid lipid nanoparticle for non-oxidation and sustained iron delivery. Colloid Interface Sci Commun. 2020;34:100227. doi:10.1016/j.colcom.2019.100227
17. Zhuang C-Y, Li N, Wang M, et al. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int J Pharm. 2010;394(1–2):179–185. doi:10.1016/j.ijpharm.2010.05.005
18. Ebrahimi S, Farhadian N, Karimi M, Ebrahimi M. Enhanced bactericidal effect of ceftriaxone drug encapsulated in nanostructured lipid carrier against gram-negative Escherichia coli bacteria: drug formulation, optimization, and cell culture study. Antimicrob Resist Infect Control. 2020;9(1). doi:10.1186/s13756-020-0690-4
19. Pezeshki A, Ghanbarzadeh B, Mohammadi M, et al. Encapsulation of vitamin A palmitate in nanostructured lipid carrier (NLC)-effect of surfactant concentration on the formulation properties. Adv Pharm Bull. 2014;4(Suppl 2):563. doi:10.5681/apb.2014.047
20. Saporito F, Sandri G, Bonferoni MC, et al. Essential oil-loaded lipid nanoparticles for wound healing. Int J Nanomedicine. 2018;13:175. doi:10.2147/IJN.S152529
21. Lacatusu I, Arsenie LV, Badea G, et al. New cosmetic formulations with broad photoprotective and antioxidative activities designed by amaranth and pumpkin seed oils nanocarriers. Ind Crops Prod. 2018;123:424–433. doi:10.1016/j.indcrop.2018.06.083
22. Hu F-Q, Jiang S-P, Du Y-Z, et al. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B Biointerfaces. 2005;45(3–4):167–173. doi:10.1016/j.colsurfb.2005.08.005
23. Pardeike J, Weber S, Haber T, et al. Development of an Itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int J Pharm. 2011;419(1–2):329–338. doi:10.1016/j.ijpharm.2011.07.040
24. Anderson C, MacKay M. Stability of fentanyl citrate, hydromorphone hydrochloride, ketamine hydrochloride, midazolam, morphine sulfate, and pentobarbital sodium in polypropylene syringes. Pharmacy. 2015;3(4):379–385. doi:10.3390/pharmacy3040379
25. Pongpeerapat A, Itoh K, Tozuka Y, et al. Formation and stability of drug nanoparticles obtained from drug/PVP/SDS ternary ground mixture. J Drug Deliv Sci Technol. 2004;14(6):441–447. doi:10.1016/S1773-2247(04)50082-5
26. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi:10.1016/0304-3959(77)90130-0
27. Myers RH, Montgomery DC, Vining GG, et al. Response surface methodology: a retrospective and literature survey. J Qual Technol. 2004;36(1):53–77. doi:10.1080/00224065.2004.11980252
28. Haaland PD. Experimental Design in Biotechnology. United State of America: Marcel Dekker. Inc; 1989.
29. Chauhan B, Gupta R. Application of statistical experimental design for optimization of alkaline protease production from Bacillus sp. RGR-14. Process Biochem. 2004;39(12):2115–2122. doi:10.1016/j.procbio.2003.11.002
30. Li H, Qiao H, Lu H, et al. Evaluation of the peripheral analgesic effect of sufentanil lipid nanoparticles. J Anesth. 2014;28(5):702–707. doi:10.1007/s00540-014-1795-9
31. Zeashan H, Amresh G, Rao CV, et al. Antinociceptive activity of Amaranthus spinosus in experimental animals. J Ethnopharmacol. 2009;122(3):492–496. doi:10.1016/j.jep.2009.01.031
32. Bahrami MA, Farhadian N. Experimental study and mathematical modeling for encapsulation of fentanyl citrate drug in nanostructured lipid carrier. Biomol Struct Dyn. 2020;38:1263–1271. doi:10.1080/07391102.2019.1599732
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
