Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Improvement in Cardiometabolic Risk Factors During Smoking Cessation Treatment in Patients with Type 2 Diabetes: A Retrospective Cohort Study
Authors Chen HJ , Huang WH, Chan HL, Hwang LC
Received 23 January 2021
Accepted for publication 27 March 2021
Published 16 April 2021 Volume 2021:14 Pages 1695—1702
DOI https://doi.org/10.2147/DMSO.S303446
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Hsin-Ju Chen,1 Wei-Hsin Huang,1 Hsin-Lung Chan,1 Lee-Ching Hwang1,2
1Department of Family Medicine, Mackay Memorial Hospital, Taipei, Taiwan; 2Department of Medicine, Mackay Medical College, New Taipei City, Taiwan
Correspondence: Lee-Ching Hwang
Department of Family Medicine, Mackay Memorial Hospital, No. 92, Sec. 2, Zhongshan North Road, Taipei City, 10449, Taiwan
Tel +886 2 2543 3535 #2136
Fax +886 2 2521 3847
Email [email protected]
Purpose: Smoking cessation reduces morbidity and mortality of cardiovascular diseases. The purpose of this study was to evaluate the effect during smoking cessation treatment on glycemic control and cardiometabolic risk factors, including blood pressure and lipid levels, in patients with type 2 diabetes.
Patients and Methods: This retrospective cohort study included patients with type 2 diabetes who participated in a smoking cessation program, which comprised health education and medication prescription at an outpatient clinic in combination with a 3-month follow-up by phone. Data on patient background characteristics, cardiometabolic factors, smoking status, body weight, and body mass index before and after the program were collected for analysis.
Results: The analysis included 241 participants with an average age of 58.6 ± 10.6 years. The rate of successful cessation at three months was 34.0%. Compared with the baseline levels, there were significant decreases in the levels of fasting plasma glucose (10.0 ± 46.8 mg/dL, P = 0.001), HbA1c (0.3 ± 1.4%, P = 0.004), systolic blood pressure (4.6 ± 17.5 mmHg, P < 0.001), diastolic blood pressure (2.9 ± 11.3 mmHg, P < 0.001), and total cholesterol (7.9 ± 42.8 mg/dL, P = 0.020) after participation in the smoking cessation program while there was no significant difference in body weight (0.1 ± 1.2 kg, P = 0.444). After adjustment for covariates, the decreases in HbA1c and total cholesterol levels were significantly better in younger participants and higher baseline nicotine dependence scores were associated with decreases in the levels of blood pressure, fasting plasma glucose, and triglycerides. However, the decrease in smoking amount was not associated with the changes in cardiometabolic factors.
Conclusion: Participation in a smoking cessation program was associated with improvements in glycemic control and cardiometabolic risk factors in patients with type 2 diabetes. The observed improvements were associated with participation in the program but not with the decrease in smoking amount.
Keywords: cardiometabolic risk factor, body weight, glycemic control, smoking cessation, type 2 diabetes
Introduction
The prevalence of type 2 diabetes is approximately 8.8% globally and 10.1% in Taiwan,1,2 and both numbers continue to increase.3,4 Cardiovascular diseases (CVDs) are the leading cause of mortality in patients with type 2 diabetes, with an estimated rate of nearly 52%.5 Importantly, cigarette smoking is associated with metabolic syndrome, intrahepatic fat,6 and CVDs.7 The prevalence of tobacco smoking, which is around 24.9% in the general population,8 is even higher in patients with type 2 diabetes, reaching up to 32.1% to 33.9%.9,10 Studies had established that smoking is associated with higher rates of overall mortality as well as higher rates of CVD risk and mortality among patients with diabetes.11,12 Several studies have also demonstrated that smoking patients with type 2 diabetes patients have worse disease control and more severe complications such as diabetic foot ulcers.13–16
On the other hand, smoking cessation has been shown to reduce cardiometabolic risk and improve glycemic control.17,18 One study showed that the hazard ratio of cardiovascular mortality compared to never smokers was 2.07 (95% CI 1.82 to 2.36) for current smokers and 1.37 (1.25 to 1.49) for former smokers, respectively.17 The other study revealed that HbA1c decreased linearly with an increase in years after smoking cessation in former smokers (P <0.001).18 However, there were few studies analyzing the factors influencing these effect of the smoking cessation. Besides, few studies analyzed the short-term effects on blood pressure, lipid profiles, and glycemic control during smoking cessation program.
The aim of the present study was to evaluate the effects during a smoking cessation program on glycemic control and cardiometabolic risk factors including systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose, hemoglobin A1c (HbA1c), total cholesterol (TC), and triglycerides in patients with type 2 diabetes and to analyze the factors influencing the effect.
Patients and Methods
Design, Setting and Recruitment
This is a hospital-based, retrospective, cohort study. We included patients with type 2 diabetes who underwent Taiwan’s second-generation smoking cessation program between January 2017 and March 2019 at the outpatient clinics in Mackay Memorial Hospital, Taipei, Taiwan. All participants attended the program were over 18 years old either with a daily smoking amount more than 10 cigarettes and/or with an Fagerström test for nicotine dependence (FTND) score of at least 4 points (scores range from 0 to 10, with higher scores representing greater nicotine dependence). The diagnosis of type 2 diabetes was based on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code E11.0–11.9 in medical records or the laboratory data indicating a fasting glucose level of >126 mg/dL or a hemoglobin A1c (HbA1c) level >6.5% at first personal consultation for smoking cessation.
A total of 458 patients with results of blood tests in the smoking cessation program were included. 141 patients with missing data of fasting glucose or HbA1C before and after the program period were excluded in this study. We also excluded 42 patients lacking abstinence status and 34 patients with incorrectly recorded smoking years or daily amount. Eventually, 241 diabetes patients were enrolled in the study (Figure 1).
 |
Figure 1 Flow chart of the selection of study patients. |
Measurement and Methods
The smoking cessation program included health education and medical prescription by the physician at the outpatient clinic in combination with a 3-month follow-up by phone by the health promotion agency.
In the clinic, the patients received counseling from the physician. Smoking habits including daily consumption of cigarette, smoking year, and smoking pack-year were obtained and the FTND score was assessed at the first clinic visit. The possible side effects and contraindication of respective medication, withdrawal symptoms of smoking cessation, and the benefits from smoking cessation were informed and discussed. Varenicline and/or nicotine replacement therapy was prescribed based on shared decision-making during a maximum 8-week course divided into several clinic visits. The smoking status, adverse effects of medication, and exhaled carbon monoxide levels (ppm) were evaluated at each clinic visit.
The health promotion agency interviewed the patients in the clinic or by telephone. The assistance was provided including listening to the current experience of smoking cessation, understanding the obstacles, reinforcing the will and motivation, and the management of expected withdrawal symptoms and side effects of medication. The self-reported smoking status by telephone after the program was used to determine the success of smoking cessation at the 3rd month. If we could not contact the patient by phone, the individual would be categorized into failure in smoking cessation at the 3rd month.
We reviewed the patients’ medical records. The clinical characteristics of all participants, including sex, age, smoking years, smoking amount, FTND score, and baseline exhaled carbon monoxide, were recorded. The participants were defined to have previous diagnosis of hypertension and CVD if they had the diagnoses in their medical records. Additionally, data on the following parameters were obtained from the electronic medical record system: SBP, DBP, fasting plasma glucose, HbA1c, TC, triglycerides, smoking amount, body weight, body height, and body mass index (BMI) before and after the smoking cessation program. Using varenicline or not during the smoking cessation program was also recorded.
Ethics Statement
The present study was authorized by the Ethics Committee of Mackay Memorial Hospital (Institutional review board number: 17MMHIS049) based on the revised Helsinki Declaration. This study is a non-interventional, observational, and retrospective study with minimal risk for the participants, and the informed consent from the participants was not deemed necessary. The data were collected, analyzed, and used only for research purposes. The study was carried out in accordance with the local regulatory guidelines and international guidelines for good epidemiological practices.
Statistical Analysis
All statistical analyses were performed using SPSS software (version 22.0, SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as means ± standard deviation, and categorical variables were expressed as numbers with percentages. Paired t test was used to compare daily smoking amount (stick/day), glycemic control, cardiometabolic risk factors, body weight, and BMI before and after the smoking cessation program. Logistic regression was used to determine factors associated with improvements in cardiometabolic factors. Multiple logistic regression analysis with covariates of age, sex, daily smoking amount, duration of smoking, and FTND score was performed to compare patients categorized into those with and without decreases in the levels for SBP, DBP, fasting glucose, HbA1c, TC, and triglycerides after the cessation program.
Results
The retrospective study cohort finally enrolled a total of 241 patients. The characteristics of the enrolled patients are summarized in Table 1. The cohort comprised 34 (14.1%) female patients, and the average age was 58.6 ± 10.6 years. The average body height was 165.2 ± 6.8 cm, the average body weight was 73.7 ± 13.8 kg, and the average BMI was 26.9 ± 4.7 kg/m2. There were 165 (68.5%) and 83 (34.4%) patients with previous diagnoses of hypertension and CVD, respectively. The average baseline FTND score, smoking duration, baseline carbon monoxide level, and baseline smoking amount were 6.3 ± 2.4 points, 35.4 ± 11.8 years, 18.0 ± 3.5 ppm, and 21.4 ± 12.9 sticks/day, respectively. After the smoking cessation program, the average smoking amount was 12.8 ± 12.7 sticks/day. There were 186 (77.2%) patients who ever used varenicline in the cessation program. The successful cessation number was 82 (34.0%) at the 3-month follow-up by phone.
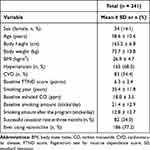 |
Table 1 Characteristics of the Study Participants |
The comparisons of cardiometabolic factors before and after participation in the smoking cessation program are summarized in Table 2. The smoking amount was decreased by 8.7 ± 12.3 sticks/day after the smoking cessation program (P < 0.001). The decreases in the levels of SBP, DBP, fasting glucose, HbA1c, TC, and triglycerides were found in 91 (37.8%), 92 (38.2%), 135 (56.0%), 107 (44.4%), 86 (52.4%), and 90 (71.8%) patients, respectively. Compared with the baseline values, the decreases after participation in the smoking cessation program were significant for the levels of fasting glucose (10.0 ± 46.8 mg/dL, P = 0.001), HbA1c (0.27% ± 1.4%, P = 0.004), SBP (4.6 ± 17.5 mmHg, P < 0.001), DBP (2.9 ± 11.3 mmHg, P < 0.001), and TC (7.9 ± 42.8 mg/dL, P = 0.02). However, the triglyceride levels did not show a significant change after participation in the smoking cessation program (26.7 ± 198.1 mg/dL, P = 0.078). Additionally, there was no significant difference in body weight (0.1 ± 1.2 kg, P = 0.444) or BMI (0.01 ± 0.47 kg/m2, P = 0.737) with participation in the smoking cessation program.
 |
Table 2 Comparison of Changes in Cardiometabolic Factors After Participation in the Smoking Cessation Program |
The analysis of factors associated with improvements in cardiometabolic factors is summarized in Table 3. In multiple logistic regression models, patient age was associated with decreases in HbA1c (odds ratio [OR] 0.95, 95% confidence interval [CI] 0.92–0.99) and TC (OR 0.95, 95% CI 0.92–0.99) levels, baseline smoking amount was associated with a decrease in DBP (OR 0.96, 95% CI 0.93–0.98), and baseline FTND score was associated with decreases in fasting glucose (OR 1.15, 95% CI 1.01–1.31), SBP (OR 1.22, 95% CI 1.06–1.40), DBP (OR 1.15, 95% CI 1.00–1.31), and triglyceride (OR 1.16, 95% CI 1.00–1.315) levels. However, baseline smoking duration and the decrease in smoking amount did not show significant associations with improvement in any of the cardiometabolic factors included in the analyses.
 |
Table 3 Factors Associated with Improvements in Cardiometabolic Factors During Smoking Cessation Treatment in Patients with Type 2 Diabetes |
Discussion
The present study was designed to evaluate the effect during a smoking cessation program on glycemic control and cardiometabolic risk factors in patients with type 2 diabetes and to determine factors associated with the observed effect. Our analyses revealed that improvements in glycemic control and cardiometabolic risk factors were noted by three months after participation in the smoking cessation program. The effect was more predominant in patients who were younger and in those with lower baseline smoking amount and higher baseline FTND scores. Our analyses also demonstrated that the decrease in smoking amount was not associated with the observed improvements in glycemic control and cardiometabolic factors. These findings are consistent with those of a previous study showing that smoking cessation intervention was associated with lower mortality compared with usual care, although successful smoking cessation was achieved in a minority of participants.19
The positive cardiometabolic effects of smoking cessation observed in our study was reported by several other studies, indicating that smoking cessation improves glycemic control and is associated with cardiovascular benefit.18,20 Additionally, one study showed that smoking cessation improved both mental and physical health-related quality of life among patients with type 2 diabetes.21 However, several studies reported that smoking cessation might deteriorate glycemic control and cardiometabolic factors and that these changes were primarily secondary to weight gain.22–25 One study also demonstrated that smoking cessation without instruction might contribute to the development of metabolic syndrome.26 In the present study, participants did not experience a significant body weight change after the smoking cessation program and the effects of participation in the program on glycemic control and cardiometabolic factors were in contrast to the findings of the abovementioned studies. These results suggest that adequate weight control during smoking cessation might contribute to improvements in glycemic control and cardiometabolic factors.
Studies revealed that behavioral modification improved glycemic control in patients with type 2 diabetes.27,28 Motivational interviewing is especially favorable for weight management in type 2 diabetes.27 The smoking cessation program in our study provided health education to help patients control weight. One study showed that behavior change techniques achieved significant reductions in HbA1c at three and six months,28 which was compatible with the duration of the smoking cessation program in our study. Thus, we hypothesize that observed improvements in glycemic control and cardiometabolic factors in patients with type 2 diabetes after their participation in the smoking cessation program might be due to the modification of views and behaviors in association with the health education and enhanced adherence during the program and not due to a decrease in smoking amount.
As above-mentioned, studies had established the long-term effects of successful smoking cessation on cardiometabolic factors. Some studies also revealed that the reduction of vascular inflammation was noted during smoking cessation in less than 6 months.29,30 However, few studies focused on the short-term effects on the blood pressure, lipid profiles, and glycemic control during smoking cessation. The present study revealed that the immediate improvement of cardiometabolic factors among diabetes patients during the smoking cessation program can be seen in three months.
Since participation in a smoking cessation program is associated with improvements in glycemic control and cardiometabolic factors and as the effect could be observed in three months, we strongly suggest that clinicians in general practice should recommend patients with type 2 diabetes to participate in a smoking cessation program.
The present study has several limitations. First, since information was retrieved from medical records, we could not include information on waist circumference, changes in diet, and changes in physical activities, which are significant factors contributing to improvements in cardiometabolic factors. Second, we enrolled patients with newly diagnosed type 2 diabetes as well as those stable patients under diabetes treatment for longer time periods. It remains possible that patients with a new diagnosis might have affected the average blood glucose levels of the study cohort. However, our statistical analyses after the exclusion of 12 patients with newly diagnosed type 2 diabetes yielded comparable results. Third, the study sample size was relatively small and limited to one medical hospital. The study participants were also followed for approximately three months during the smoking cessation program, and the long-term effects require further validation. However, despite these limitations, the study findings reflect the real-world situation and highlight the need for further investigation into the impact of smoking cessation programs on cardiometabolic factors in patients with type 2 diabetes.
Conclusion
Participation in a smoking cessation program was associated with improvements in glycemic control and cardiometabolic risk factors in patients with type 2 diabetes in 3 months. Moreover, younger patients exhibited better improvement in HbA1c and TC than older patients, whereas patients with a lower baseline smoking amount experienced better improvement in DBP. The Improvements in fasting glucose, SBP, DBP, and triglycerides were better in patients with higher baseline FTND scores as well. However, these observed improvements in cardiometabolic factors were associated with participation in the program but not with the decrease in smoking amount.
Abbreviations
BMI, Body mass index; DBP, Diastolic blood pressure; FTND, Fagerström test for nicotine dependence; HbA1c, hemoglobin A1c; ICD-10-CM, The International Classification of Diseases, 10th Revision, Clinical Modification; SBP, Systolic blood pressure; TC, Total cholesterol.
Data Sharing Statement
Data are available from the Outpatient Smoking Cessation Treatment Database established by Taiwan Health Promotion Administration. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the Taiwan Health Promotion Administration (http://www.hpa.gov.tw).
Ethics Approval
The Institutional Review Board of Mackay Memorial Hospital approved the study protocols with application number, 17MMHISO049.
Acknowledgments
The authors thank smoking cessation education managers in Mackay Memorial Hospital for help rendered for this study. We also thank the Taiwan Health Promotion Administration which established the Outpatient Smoking Cessation Treatment Database and provided general support.
Author Contributions
Lee-Ching Hwang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Lee-Ching Hwang. Acquisition of data: Hsin-Ju Chen, Wei-Hsin Huang, and Lee-Ching Hwang. Analysis and interpretation of data: Hsin-Ju Chen, Wei-Hsin Huang, Hsin-Lung Chan, and Lee-Ching Hwang
Drafting of the manuscript: Hsin-Ju Chen and Lee-Ching Hwang. Critical revision of the manuscript for important intellectual content: Hsin-Ju Chen, Wei-Hsin Huang, Hsin-Lung Chan, and Lee-Ching Hwang. Statistical analysis: Hsin-Ju Chen and Lee-Ching Hwang. Study supervision: Lee-Ching Hwang and Wei-Hsin Huang.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors declare no conflict of interest.
References
1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
2. Sheen YJ, Hsu CC, Jiang YD, Huang CN, Liu JS, Sheu WH. Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J Formos Med Assoc. 2019;118 Suppl 2:S66–S73. doi:10.1016/j.jfma.2019.06.016
3. Bennett JE, Stevens GA, Mathers CD. NCD countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet (London, England). 2018;392(10152):1072–1088. doi:10.1016/S0140-6736(18)31992-5
4. Wang CY, Wu YL, Sheu WH, Tu ST, Hsu CC, Tai TY. Accountability and utilization of diabetes care from 2005 to 2014 in Taiwan. J Formos Med Assoc. 2019;118(Suppl 2):S111–S121. doi:10.1016/j.jfma.2019.08.010
5. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44 Suppl 2:S14–S21. doi:10.1007/PL00002934
6. Kato A, Li Y, Ota A, et al. Smoking results in accumulation of ectopic fat in the liver. Diabetes Metab Syndr Obes. 2019;12:1075–1080. doi:10.2147/DMSO.S212495
7. Lee YA, Kang SG, Song SW, Rho JS, Kim EK. Association between metabolic syndrome, smoking status and coronary artery calcification. PLoS One. 2015;10(3):e0122430. doi:10.1371/journal.pone.0122430
8. WHO global report on trends in prevalence of tobacco use 2000–2025, third edition. Geneva: World Health Organization; 2019.
9. Schipf S, Schmidt CO, Alte D, et al. Smoking prevalence in type 2 diabetes: results of the Study of Health in Pomerania (SHIP) and the German National Health Interview and Examination Survey (GNHIES). Diabet Med. 2009;26(8):791–797. doi:10.1111/j.1464-5491.2009.02784.x
10. Awadalla H, Almobarak AO, Ahmed MH. Prevalence of smoking in Sudanese individuals with diabetes and associated complications: population-based study. Diabetes Metab Syndr Obes. 2018;12(5):749–751. doi:10.1016/j.dsx.2018.04.038
11. Pan A, Wang Y, Talaei M, Hu FB. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132(19):1795–1804. doi:10.1161/CIRCULATIONAHA.115.017926
12. Banks E, Joshy G, Korda RJ, et al. Tobacco smoking and risk of 36 cardiovascular disease subtypes: fatal and non-fatal outcomes in a large prospective Australian study. BMC Med. 2019;17(1):128. doi:10.1186/s12916-019-1351-4
13. National Center for Chronic Disease P, Health Promotion Office on S, Health. Reports of the surgeon general. In: The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014.
14. Peng K, Chen G, Liu C, et al. Association between smoking and glycemic control in diabetic patients: results from the Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diabetes. 2018;10(5):408–418. doi:10.1111/1753-0407.12625
15. Su J, Qin Y, Shen C, et al. Association between smoking/smoking cessation and glycemic control in male patients with type 2 diabetes. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(11):1454–1459. doi:10.3760/cma.j.issn.0254-6450.2017.11.003
16. Jalilian M, Ahmadi Sarbarzeh P, Oubari S. Factors related to severity of diabetic foot ulcer: a systematic review. Diabetes Metab Syndr Obes. 2020;13:1835–1842. doi:10.2147/DMSO.S256243
17. Mons U, Müezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ (Clinical Research Ed). 2015;350:h1551. doi:10.1136/bmj.h1551
18. Ohkuma T, Iwase M, Fujii H, et al. Dose- and time-dependent association of smoking and its cessation with glycemic control and insulin resistance in male patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. PLoS One. 2015;10(3):e0122023. doi:10.1371/journal.pone.0122023
19. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. doi:10.7326/0003-4819-142-4-200502150-00005
20. Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2014;7:169–183. doi:10.2147/DMSO.S61438
21. Dehesh T, Dehesh P, Gozashti MH. Metabolic factors that affect health-related quality of life in type 2 diabetes patients: a multivariate regression analysis. Diabetes Metab Syndr Obes. 2019;12:1181–1188. doi:10.2147/DMSO.S208689
22. Iino K, Iwase M, Tsutsu N, Iida M. Smoking cessation and glycaemic control in type 2 diabetic patients. Diabetes Obes Metab. 2004;6(3):181–186. doi:10.1111/j.1462-8902.2004.00329.x
23. Lycett D, Nichols L, Ryan R, et al. The association between smoking cessation and glycaemic control in patients with type 2 diabetes: a THIN database cohort study. Lancet Diabetes Endocrinol. 2015;3(6):423–430. doi:10.1016/S2213-8587(15)00082-0
24. Athyros VG, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Effect of tobacco smoking and smoking cessation on plasma lipoproteins and associated major cardiovascular risk factors: a narrative review. Curr Med Res Opin. 2013;29(10):1263–1274. doi:10.1185/03007995.2013.827566
25. Kim K, Choi S, Lee JK, et al. Weight change after smoking cessation and incident metabolic syndrome in middle-aged Korean men: an observational study. Sci Rep. 2019;9(1):3103. doi:10.1038/s41598-019-39811-0
26. Takayama S, Takase H, Tanaka T, Sugiura T, Ohte N, Dohi Y. Smoking cessation without educational instruction could promote the development of metabolic syndrome. J Atheroscler Thromb. 2018;25(1):90–97. doi:10.5551/jat.40063
27. Ekong G, Kavookjian J. Motivational interviewing and outcomes in adults with type 2 diabetes: a systematic review. Patient Educ Couns. 2016;99(6):944–952. doi:10.1016/j.pec.2015.11.022
28. Cradock KA, Ólaighin G, Finucane FM, Gainforth HL, Quinlan LR, Ginis KA. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2017;14(1):18. doi:10.1186/s12966-016-0436-0
29. Komiyama M, Wada H, Ono K, et al. Smoking cessation reduces the lectin-like low-density lipoprotein receptor index, an independent cardiovascular risk marker of vascular inflammation. Heart Vessels. 2018;33(1):9–16. doi:10.1007/s00380-017-1026-z
30. Toffolo MCF, Gomes ADS, Van Keulen HV, et al. Alteration of inflammatory adipokines after four months of smoking abstinence in multidisciplinary intervention program. Nutr Hosp. 2018;35(2):434–441. doi:10.20960/nh.1548
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
