Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with recombinant human bone morphogenetic protein-2: a pilot in vivo study
Authors Lee J , Choi D , Jang I, Choi W
Received 26 November 2014
Accepted for publication 10 January 2015
Published 5 February 2015 Volume 2015:10(1) Pages 1145—1154
DOI https://doi.org/10.2147/IJN.S78138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Thomas Webster
Jae-Kwan Lee,1,4 Dong-Soon Choi,2,4 Insan Jang,2,4 Won-Youl Choi3,4
1Department of Periodontology, 2Department of Orthodontics, College of Dentistry, 3Department of Metal and Materials Engineering, 4Research Institute for Dental Engineering, Gangneung-Wonju National University, Gangneung, South Korea
Abstract: TiO2 nanotube arrays on the surface of dental implants were fabricated by two-step anodic oxidation. Their effects on bone-implant contact were researched by a pilot in vivo study. The implants were classified into four groups. An implant group with TiO2 nanotube arrays and recombinant human bone morphogenetic protein-2 (rhBMP-2) was compared with various surface implants, including machined surface, sandblasted large-grit and acid-etched surface, and TiO2 nanotube array surface groups. The diameter of the TiO2 nanotube window and TiO2 nanotube were ~70 nm and ~110 nm, respectively. The rhBMP-2 was loaded into TiO2 nanotube arrays and elution was detected by an interferometric biosensing method. A change in optical thickness of ~75 nm was measured by flow cell testing for 9 days, indicating elution of rhBMP-2 from the TiO2 nanotube arrays. For the in vivo study, the four groups of implants were placed into the proximal tibia of New Zealand white rabbits. In the implant group with TiO2 nanotube arrays and rhBMP-2, the bone-to-implant contact ratio was 29.5% and the bone volume ratio was 77.3%. Bone remodeling was observed not only in the periosteum but also in the interface between the bone and implant threads. These values were higher than in the machined surface, sandblasted large-grit and acid-etched surface, and TiO2 nanotube array surface groups. Our results suggest that TiO2 nanotube arrays could potentially be used as a reservoir for rhBMP-2 to reinforce osseointegration on the surface of dental implants.
Keywords: TiO2 nanotube arrays, drug reservoir, dental implant, bone morphogenetic protein
Introduction
Titanium and its alloys are currently used extensively in the field of dental implants because of its good mechanical properties and excellent biocompatibility. However, the native oxide layer of titanium cannot directly bond with bone to promote new bone formation in the early stage of osseointegration. Numerous studies have sought to optimize dental and orthopedic implants by modifying the implant surface chemistry and/or surface topography by methods such as blasting, plasma spraying of hydroxyapatite, sandblasting and etching, and anodic oxidation.1–7
TiO2 nanotube arrays can be formed as “columnar porous” titania layers by anodic oxidation on pure titanium or titanium alloy surfaces, and are of great interest due to their highly ordered nanostructure.8–12 The results of recent studies suggest that formation of a titanium implant surface with a nanostructure could reinforce osseointegration because the surface area is markedly increased and the surface topography can be nanomodified to resemble native bone tissue.12–14 Recent studies have demonstrated that the fabrication of ordered TiO2 nanotube arrays with controlled empty space diameters can be adjusted by controlling processing factors, such as voltage, current density, and electrolytes.8,15,16 TiO2 nanotube surfaces with the optimal length scale for cell adhesion and differentiation can induce the migration of osteoblasts and mesenchymal stem cells, and hence reinforce interactions between implant surfaces and cells.9,10,13,14
Recent advances in the fabrication, properties, and applications of TiO2 nanotube arrays have provided new opportunities for research in relation to their use in clinical practice. The more recent studies attempted localized drug delivery via TiO2 nanotubes.11,17 The empty space of TiO2 nanotubes could be used as a drug reservoir. Antibiotics, anti-inflammatory drugs, and growth factors can be prescribed to be taken orally, intravenously, or intramuscularly. Several drugs, however, are not effective when delivered via these routes. Moreover, systemic delivery of these drugs can produce adverse effects and organ toxicity associated with high concentrations. Therefore, local drug therapy has become an accepted type of treatment.11
Recombinant human bone morphogenetic protein-2 (rhBMP-2) has been shown to improve osteoblast differentiation and bone formation and remodeling.18,19 Several studies showed that application of rhBMP-2 at appropriate doses induced bone formation, whereas higher doses were associated with undesirable effects.19,20 Moreover, systemic delivery of rhBMP-2 can have adverse effects such as unwanted ectopic bone formation. Therefore, it is believed that rhBMP-2 should be immobilized on the implant surface to allow sufficient time to promote osseointegration.11
In this study, TiO2 nanotube arrays were formed on the surface of dental titanium implants by anodic oxidation. TiO2 nanotube arrays provided empty spaces for drug loading and demonstrated better biocompatibility than a machined surface. We designed a dental implant with TiO2 nanotube arrays as a suitable structure for inserting drugs such as antibiotics, anti-inflammatory agents, and growth factors. To improve bone-implant contact, the rhBMP-2 was selected and loaded in a storage room of TiO2 nanotube arrays. The effects of TiO2 nanotube arrays and rhBMP-2 on bone-implant contact in the dental implants were investigated in vitro and in an in vivo study in rabbits.
Materials and methods
Fabrication of implants with TiO2 nanotube arrays and in vitro study
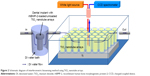
Dental implants with four different surface characteristics were used: a machined surface, a sandblasted large-grit and acid-etched SLA) implant (TS III™, Osstem Implants, Seoul, South Korea) as a positive control group, TiO2 nanotube arrays, and TiO2 nanotube arrays with rhBMP-2 loading were called I1 group, I2 group, I3 group, and I4 group, respectively. All implants were 3.5 mm in diameter and 8.5 mm in length. TiO2 nanotube arrays on the surface of the implants were fabricated by anodic oxidation in an electrolyte solution containing ethylene glycol with 0.5 wt% NH4F.21 Figure 1 shows a schematic diagram of the anodic oxidation procedure. To obtain a proper microstructure of TiO2 nanotube arrays, anodic oxidation voltages and the time provided by a DC power supply were computer-controlled by a LabVIEW program. Two-step anodic oxidation was conducted to obtain the clean surface and open windows of the TiO2 nanotube arrays. The implant was first anodized at a voltage of 60 V for 60 minutes. The TiO2 nanotube arrays fabricated by the first anodic oxidation were removed in ultrasonication. TiO2 nanotube arrays with clean and open windows were then fabricated by the second anodic oxidation. The voltage and duration of the second anodic oxidation were 60 V and 15 minutes, respectively. The thickness and open window size of the TiO2 nanotube arrays were determined by field emission scanning electron microscopy (Phillips/FEI XL 30SFEG, Eindhoven, the Netherlands) at the Korea Basic Science Institute. For the rhBMP-2-loaded TiO2 nanotube array implant, rhBMP-2 (Cowellmedi Co Ltd, Busan, South Korea) was loaded into the inner space of the TiO2 nanotube arrays on the surface of the dental implant by a dip-coating process in a vacuum chamber. The concentration of rhBMP-2 was 1.5 mg/mL, ie, the same concentration as that used by Lee et al19 and Wikesjo et al.20 Each implant was immersed three times in rhBMP-2 solution for 5 seconds and dried at a maximum temperature of 20°C.
  | Figure 1 Schematic diagram of anodic oxidation process. |
The elution of rhBMP-2 was observed by an interferometric biosensing method.22 Figure 2 shows a schematic diagram of the interferometric biosensing method using TiO2 nanotube arrays. TiO2 nanotube arrays fabricated on a flat titanium surface were also used as a sensing porous layer. White light from a tungsten lamp (Ocean Optics, Dunedin, FL, USA) was fed through one end of a bifurcated fiber optic cable and focused through a lens onto the surface of the TiO2 nanotube arrays at normal incidence. Reflected light was collected through the same optics, and the distal end of the bifurcated fiber optic cable was input to a S-4000 CCD spectrometer (Ocean Optics).23 Optical thickness (nL) was determined from the Fabry-Perot relationship (mλ =2 nL), where m is the fringe order, λ is the wavelength of maximum constructive interference for a spectral fringe of order m, n is the refractive index of the porous layer, and L is the thickness of the porous layer. The change in optical thickness over time was monitored.
In vivo study
This study was approved by the ethical committee on animal research at the Institute of Gangneung-Wonju National University (GWNU-2013-3). Three New Zealand white rabbits (weighing 3,060±167 g and aged 13–14 weeks at the start of the experiment) were used in the study. The surgical procedures were performed aseptically under intramuscular anesthesia with a combination of 0.7 mL/kg of ketamine (Yuhan Corporation, Seoul, South Korea) and 0.2 mL/kg of xylazine (Bayer, Leverkusen, Germany). Prior to surgery, the operative sites were shaved and carefully washed with iodine solution and injected with 2% lidocaine containing 1:100,000 epinephrine (Huons, Seoul, South Korea). A skin incision was made along the proximal one third of the tibia using sterile surgical technique. After full-thickness flap reflection, two holes were drilled about 7 mm apart with copious irrigation. The drilling procedures followed the manufacturer’s instructions. All 12 implants were surgically placed. Each rabbit received the four different types of implant. Two different types of implant were placed at each tibia in random circulating order into the left and right sides of the tibia to ensure unbiased comparisons. The middle third of each implant was engaged by upper cortical bone only. After surgery, all rabbits received antibiotic cover, ie, 0.15 mL/kg gentamicin sulfate (Dongwha Pharm Co Ltd, Seoul, South Korea) and analgesia, ie, 0.20 mL/kg Sulpyrine (Green Cross Veterinary Products Co Ltd, Yongin, South Korea) by intramuscular injection. The animals were kept in separate cages and allowed full weight bearing after surgery.
For fluorochrome labeling, subcutaneous injections with alizarin red (30 mg/kg; Sigma-Aldrich, St Louis, MO, USA) and calcein green (10 mg/kg; Sigma-Aldrich) were performed 3 and 6 weeks, respectively, after the operation. The rabbits were euthanized in a CO2 chamber 8 weeks after inserting the implants, and 1 cm ×1 cm bone fragments with dental implants were collected.
After euthanasia, the implant areas were scanned using a 1173 micro-computed tomography scanner (Skyscan, Kontich, Belgium). The voltage and current of the X-ray tube were 130 kV and 60 μA, respectively, with an exposure time of 500 msec. X-ray projections were obtained at 0.3 degree intervals with a scanning angular rotation of 360 degrees. The image pixel size was set at 6.04 μm.
The samples were fixed by immersion in a 10% neutral-buffered formalin solution (Accustain™, Sigma-Aldrich, Steinheim, Germany) for one day, then dehydrated in a graded series of ethanol solutions and embedded in methylmethacrylate resin (Technovit 7200 VLC, Kulzer Co GmbH, Friedrichsdorf, Germany). After dehydration, the specimens were polymerized in a light-based polymerization unit (Exakt Apparatebau, Norderstedt, Germany). The implants were cut mid-axially in a mediolateral plane into 200 μm thick sections using a band saw with a diamond blade (Exakt cutting-grinding system, Exakt Apparatebau). The final section was ground to no thicker than about 20 μm using an Exakt microgrinder and polished to an optical finish utilizing the cutting-grinding technique described by Donath and Breuner.24
All the sections were first examined by immunofluorescence microscopy (Leica Microsystems, Wetzlar, Germany). The specimens were then stained by the Masson-Goldner trichrome method. Images of the specimens were obtained by optical microscopy (BX-50, Olympus America, Melville, NY, USA). One examiner performed the histomorphometric analysis in a blinded manner. The bone-to-implant contact ratio (%) and bone volume ratio (%) was measured in the cortical bone area using Image Pro Plus analysis software (Media Cybernetics Inc, Rockville, MD, USA). The bone-to-implant contact ratio was defined as the percentage of bone contact measured along the entire surface length of two implant threads. The bone volume ratio was the percentage of total bone tissue within the area of two threads.
Results and discussion
Fabrication of implant with TiO2 nanotube arrays and in vitro study
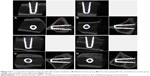
Figure 3 shows field emission scanning electron microscopic images of the implants with various surface treatments. Figure 3A and B shows typical images and microstructures of a machined titanium implant. It has mainly unidirectional machined grooves from the manufacturing instrument. The smooth surface by machining with a CNC lathe is also shown in Figure 3B. The surfaces of the SLA implant have very rough surfaces (Figure 3C and D). The rough and irregular sharp surface results from a grit-blasting procedure. The rough morphology enhances osseointegration. The rough surface was additionally obtained by anodic oxidation. Figure 3E and F show the rough surface of TiO2 nanotube arrays fabricated by anodic oxidation. The surface with the TiO2 nanotube arrays is less rough than the SLA surface. However, the TiO2 nanotube surface has nanosized holes for loading drugs such as BMP-2, PEP7, and ibuprofen.
Figure 4 shows field emission scanning electron microscopic images of the TiO2 nanotube arrays and rhBMP-2-loaded TiO2 nanotube arrays on the surface of the implants. The TiO2 nanotube arrays were fabricated by two-step anodic oxidation.25 There were traces of the TiO2 nanotube arrays by first anodic oxidation on the surface of the newly grown TiO2 nanotube arrays. The diameter of the TiO2 nanotube window and TiO2 nanotube were ~70 nm and ~110 nm, respectively. The windows of the TiO2 nanotubes were clean and open, and these structures were appropriate for loading a drug. The thickness of the TiO2 nanotube arrays was ~17 μm, as shown in the inset in Figure 4A. The window of the TiO2 nanotube, as shown in Figure 4D, was slightly clogged with rhBMP-2 loading. The diameter of the TiO2 nanotube windows was decreased to ~50 nm. The surface-loaded rhBMP-2 was expected to improve the osseointegration.
To observe the elution of rhBMP-2 from the TiO2 nanotube arrays on the surface of the dental implant, an interferometric biosensing method with a flow cell was used.22,26 During this process, deionized water flowed through the dental implant with rhBMP-2-loaded TiO2 nanotube arrays. The change in optical thickness with rhBMP-2 was monitored in real time. Figure 5 shows the change in optical thickness of the dental implant with rhBMP-2-unloaded TiO2 nanotube arrays (I3 group) and rhBMP-2-loaded TiO2 nanotube arrays (I4 group) for 10 days. A baseline was established using the optical thickness of deionized water for ~20 hours. The solution was subsequently introduced by deionized water passed through the dental implant with TiO2 nanotube arrays. The optical thickness remained constant for 10 days in I3 group. However, in the I4 group, optical thickness was dramatically increased by elution of rhBMP-2 from the TiO2 nanotube arrays and slowly increased for 9 days. A change in optical thickness of ~75 nm confirms the elution of rhBMP-2 from TiO2 nanotube arrays.
In vivo study
This study was designed to evaluate the possibility of using TiO2 nanotube arrays as a drug reservoir, and rhBMP-2 was selected as a drug that might improve the induction of new bone formation and osseointegration around an implant surface. Dental implants were classified into four groups: machined surface implants, SLA surface implants, TiO2 nanotube array surface implants, and TiO2 nanotube array surface implant with rhBMP-2 (groups I1, I2, I3, and I4, respectively). Four group implants were placed into the proximal tibia of rabbits for 8 weeks. Clinical healing was uneventful, with no cases of implant exposure or loss in any of the rabbits. After 8 weeks, all the implants were histologically in direct contact with the surrounding bones along the threads.
Figure 6 and Table 1 show the results of the histomorphometric analysis. The highest mean bone-to-implant contact ratio was found in the I4 group (29.5%), followed by the I3 group, I2 group, and I1 group (16.3%, 14.7%, and 11.1%, respectively). The bone volume ratios were measured around the implant threads. The highest bone volume ratio (77.3%) was also found in the I4 group, with values of 67.2%, 53.7%, and 66.9% in the I3 group, I2 group, and I1 group, respectively. These results suggest that the TiO2 nanotube array surface had more of an osseointegration effect than the machined surface or the SLA surface implant, and that rhBMP-2 loaded into TiO2 nanotube arrays had the biochemical effect of bone induction.
Our findings concerning the effect of TiO2 nanotube arrays are similar to those of previous studies reporting an enhanced bone formation and cell adhesion effect with the TiO2 nanotube array implant.9,12,14 Bjursten et al12 reported that TiO2 nanotubes significantly improved bone bonding strength, bone-implant contact, and new bone formation when compared with titanium grit-blasted surfaces. Because TiO2 nanotubes have a good oxide microstructure for growing new bone, TiO2 nanotube arrays are able to influence protein interactions and components of bone for rapid and permanent bone bonding.
A number of previous studies have reported the osteoinduction effect of the rhBMP-2-coated implant.18–20,27 However, conventional rhBMP-2 coating methods cannot provide a long-lasting osteoinduction effect by rhBMP-2. As suggested by Çalişkan et al28 TiO2 nanotube arrays enhance water wettability, and can therefore act as a drug reservoir. Recently, a TiO2 nanotube implant combined with rhBMP-2 was reported to improve bone formation.29 In this study, the I4 group in which rhBMP-2 was loaded into TiO2 nanotube arrays showed the greatest bone-to-implant contact ratio and bone volume ratio 8 weeks after implantation. This can be taken as evidence of the long-lasting effect of rhBMP-2, because bone remodeling, bone formation, and bone reduction occurs very slowly. The slow elution of rhBMP-2 as shown in Figure 5 indicates that rhBMP-2 has a long-lasting effect.
Figure 7 shows the micro-computed tomographic images for the four implant groups. Vertical bone ingrowth in I4 group was increased, and the I3 and I4 groups showed more bone deposition around the implant threads than the I1 and I2 groups.
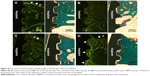
Figure 8 shows the fluorescence and histologic staining images for the four groups as observed by optical microscopy. The fluorescence and histologic staining images in Figure 8A–D are shown on the right and left sides, respectively. The fluorescence images were formed by fluorochrome labeling with alizarin red and calcein green. Different patterns of bone formation and bone remodeling were observed between the four groups. The red and green areas represent new bone formation at 3 weeks and 6 weeks after implant installation, respectively. Bone formation and bone remodeling, as indicated by white arrows, were mainly observed near the periosteum in the I1, I2, and I3 groups. However, the bone formation and remodeling in the I4 group were observed not only near the periosteum but also all around the implant threads.
The bone formation and bone remodeling in the I1, I2, and I3 groups can be explained by an osteoblast-rich periosteum. In the I4 group, the active bone formation and bone remodeling at the implant thread site are due to the osteogenic effect of rhBMP-2 eluted from the TiO2 nanotube arrays. The I4 group also showed stronger fluorochrome labeling. Fluorochrome labeling near the periosteum indicates bone formation, whereas fluorochrome labeling around the implant thread is thought to indicate bone remodeling and improving osseointegration. The results suggest that TiO2 nanotube arrays with rhBMP-2 can enhance bone formation and bone remodeling around an implant, and thus reinforce osseointegration on the surface of dental implants.
The aim of the present study was to fabricate thicker TiO2 nanotubes on the surface of dental implants for slower release of rhBMP-2 than that in a previous report.29 Slower release of rhBMP-2 from the nanotubes was observed in our in vitro study, and a higher bone-to-implant contact ratio was observed in the I4 group in our in vivo study. However, a weak point in our study was the small number of samples which precluded a statistical analysis. Further, the mechanical analysis like a removal torque and its evaluation on the different time points which could not be not performed due to same reason. A further study with a larger number of samples and various evaluation methods is necessary to clarify the effects of TiO2 nanotube arrays on the surface of dental implants with rhBMP-2.
Conclusion
TiO2 nanotube arrays on the surface of titanium dental implants were fabricated by anodic oxidation. To load rhBMP-2 into TiO2 nanotube arrays, clean and open windows of TiO2 nanotubes were obtained by two-step anodic oxidation. Elution of rhBMP-2 from the TiO2 nanotube arrays was measured by an interferometric biosensing method with a flow cell. An optical thickness change of ~75 over 9 days indicated slow release of rhBMP-2. Implants with a TiO2 nanotube array surface containing rhBMP-2 had the highest bone-implant contact and enhanced bone remodeling in the in vivo study. TiO2 nanotube arrays on the surface of the implant demonstrated the feasibility of the drug reservoir and were helpful for bone formation and cell adhesion. This structure could be very useful as a drug reservoir for various treatments, such as bone growth factors, anti-inflammatory drugs, and antibacterial agents.
Acknowledgment
This research was financially supported by the Ministry of Education, Science, and Technology and by the National Research Foundation of Korea through the Human Resources Training Project for Regional Innovation (NRF-2012H1B8A2026009).
Disclosure
The authors report no conflicts of interest in this work.
References
Albrektsson T, Branemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scan. 1981;52:155–170. | ||
Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1 – review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–543. | ||
Das K, Balla VK, Bandyopadhyay A, et al. Surface modification of laser-processed porous titanium for load-bearing implants. Scr Mater. 2008;59:822–825. | ||
Fujibayashi S, Neo M, Kim HM, Kokubo T, Nakamura T. Osteoinduction of porous bioactive titanium metal. Biomaterials. 2004;25:443–450. | ||
Han Y, Xu K. Photoexcited formation of bone apatite-like coatings on micro-arc oxidized titanium. J Biomed Mater Res A. 2004;71:608–614. | ||
Sul YT, Johansson CB, Kang Y, Jeon DG, Albrektsson T. Bone reactions to oxidized titanium implants with electrochemical anion sulphuric acid and phosphoric acid incorporation. Clin Implant Dent Relat Res. 2002;4:78–87. | ||
Albrektsson T, Wennerberg A. Oral implant surfaces: Part 2 – review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004;17:544–564. | ||
Zwilling V, Aucouturier M, Darque-Ceretti E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim Acta. 1999;45:921–929. | ||
Brammer KS, Oh S, Cobb CJ, et al. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009;5:3215–3223. | ||
Balasundaram G, Yao C, Webster TJ. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesion. J Biomed Mater Res A. 2008;84:447–453. | ||
Popat KC, Eltgroth M, LaTempa TJ, Grimes CA, Desai TA. Titania nanotubes: a novel platform for drug-eluting coatings for medical implants. Small. 2007;3:1878–1881. | ||
Bjursten LM, Rasmusson L, Oh S, Smith GC, Brammer KS, Jin S. Titanium dioxide nanotubes enhance bone bonding in vivo. J Biomed Mater Res A. 2010;92:1218–1224. | ||
Popat KC, Leoni L, Grimes CA, Desai TA. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007;28:3188–3197. | ||
Park J, Bauer S, Schlegel KA, Neukam FW, von der Mark K, Schmuki P. TiO2 nanotube surfaces: 15 nm – an optimal length scale of surface topography for cell adhesion and differentiation. Small. 2009;5:666–671. | ||
Ishizawa H, Ogino M. Formation and characterization of anodic titanium oxide films containing Ca and P. J Biomed Mater Res. 1995;29:65–72. | ||
Macák JM, Tsuchiya H, Schmuki P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew Chem Int. Ed. 2005;44:2100–2102. | ||
Lee SJ, Oh TJ, Bae TS, et al. Effect of bisphosphonates on anodized and heat-treated titanium surfaces: an animal experimental study. J Periodontol. 2011;82:1035–1042. | ||
Wikesjo UM, Qahash M, Huang YH, Xiropaidis A, Polimeni G, Susin C. Bone morphogenetic proteins for periodontal and alveolar indications; biological observations – clinical implications. Orthod Craniofac Res. 2009;12:263–270. | ||
Lee JK, Cho LR, Um HS, Chang BS, Cho KS. Bone formation and remodeling of three different dental implant surfaces with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in a rabbit model. Int J Oral Maxillofac Implants. 2013;28:424–430. | ||
Wikesjö UM, Qahash M, Polimeni G, et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol. 2008;35:1001–1010. | ||
Park H, Kim W-R, Jeong H-T, et al. Fabrication of dye-sensitized solar cells by transplanting highly ordered TiO2 nanotube arrays. Solar Energy Materials and Solar Cells. 2011;95:184–189. | ||
Mun K-S, Alvarez SD, Choi W-Y, Sailor MJ. A stable, label-free optical interferometric biosensor based on TiO2 nanotube arrays. ACS Nano. 2010;4:2070–2076. | ||
Schwartz MP, Alvarez SD, Sailor MJ. Porous SiO2 interferometric biosensor for quantitative determination of protein interactions: binding of protein A to immunoglobulins derived from different species. Anal Chem. 2007;79:327–334. | ||
Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. J Oral Pathol Med. 1982;11:318–326. | ||
Wang Y, Wen C, Hodgson P, Li Y. Biocompatibility of TiO2 nanotubes with different topographies. J Biomed Mater Res A. 2014;102:743–751. | ||
Li J, Sailor MJ. Synthesis and characterization of a stable, label-free optical biosensor from TiO2-coated porous silicon. Biosens Bioelectron. 2014;55:372–378. | ||
Bessho K, Carnes DL, Cavin R, Chen HY, Ong JL. BMP stimulation of bone response adjacent to titanium implants in vivo. Clin Oral Implants Res. 1999;10:212–218. | ||
Çalişkan N, Bayram C, Erdal E, Karahaliloğlu Z, Denkbaş EB. Titania nanotubes with adjustable dimensions for drug reservoir sites and enhanced cell adhesion. Mater Sci Eng C Mater Biol Appl. 2014;35:100–105. | ||
Bae IH, Yun KD, Kim HS, et al. Anodic oxidized nanotubular titanium implants enhance bone morphogenetic protein-2 delivery. J Biomed Mater Res B Appl Biomater. 2010;93:484–491. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.