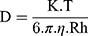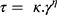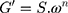Back to Journals » International Journal of Nanomedicine » Volume 15
Improved in vitro and in vivo Anti-Candida albicans Activity of Cymbopogon nardus Essential Oil by Its Incorporation into a Microemulsion System
Authors Gaspar de Toledo L , dos Santos Ramos MA, Bento da Silva P, Rodero CF, de Sá Gomes V, Noronha da Silva A, Pavan FR, da Silva IC , Bombarda Oda F , Flumignan DL , Gonzaga dos Santos A, Chorilli M, Gottardo de Almeida MT, Bauab TM
Received 4 August 2020
Accepted for publication 1 October 2020
Published 29 December 2020 Volume 2020:15 Pages 10481—10497
DOI https://doi.org/10.2147/IJN.S275258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Luciani Gaspar de Toledo,1 Matheus Aparecido dos Santos Ramos,1 Patrícia Bento da Silva,2 Camila Fernanda Rodero,3 Veridiana de Sá Gomes,1 Anderson Noronha da Silva,1 Fernando Rogério Pavan,1 Isabel Cristiane da Silva,1 Fernando Bombarda Oda,4 Danilo Luis Flumignan,5 André Gonzaga dos Santos,4 Marlus Chorilli,3 Margarete Teresa Gottardo de Almeida,6 Taís Maria Bauab1
1School of Pharmaceutical Sciences, Department of Biological Sciences, São Paulo State University (UNESP), São Paulo, Brazil; 2Department of Genetics and Morphology, University of Brasília (UnB), Institute of Biological Sciences, Brasília, Distrito Federal, Brazil; 3School of Pharmaceutical Sciences, Department of Drug and Medicines, São Paulo State University (UNESP), São Paulo, Brazil; 4School of Pharmaceutical Sciences, Department of Natural Active Principles and Toxicology, São Paulo State University (UNESP), São Paulo, Brazil; 5São Paulo Federal Institute of Education, Science and Technology (IFSP), São Paulo, Brazil; 6Faculty of Medicine of São José do Rio Preto (FAMERP), Department of Infectious Diseases, São José do Rio Preto, São Paulo, Brazil
Correspondence: Taís Maria Bauab
School of Pharmaceutical Sciences, Department of Biological Sciences, São Paulo State University (UNESP), Campus Araraquara, São Paulo 14800-903, Brazil
Tel +55-16-3301-6955
Fax +55-16-3322-0073
Email [email protected]
Purpose: Vulvovaginal candidiasis (VVC) is an opportunistic fungal infection that adversely affects a woman’s health, due to unpleasant symptoms, therapeutic challenges, and the emergence of resistant strains. The association of natural products and nanotechnology is important to improve the antifungal potential of medicinal plants. We aimed to evaluate the in vitro and in vivo anti-Candida albicans activity of unloaded (EO) and loaded (ME+EO) essential oil of Cymbopogon nardus in the microemulsion (ME).
Methods: The chemical analysis of the EO was performed by gas chromatography-mass spectrometry. The ME and ME+EO were characterized by scattering, zeta potential, polarized light microscopy, rheological assays, mucoadhesiveness and transmission electronic microscopy. The in vitro antifungal activity of the EO and ME+EO were evaluated by microdilution technique. The toxicity of EO and ME+EO was analyzed on human cell line HaCat and using alternative model assay with Artemia salina. The experimental in vivo VVC was performed in female mice (C57BL/6).
Results: The main compounds of the EO were found to be citronellal, geranial, geraniol, citronellol, and neral. The formulations exhibited suitable size, homogeneity, negative charge, isotropic behavior, highly organized structure, and pseudoplastic behavior, for vaginal application. TEM photomicrographs showed possible EO droplets inside the spherical structures. The EO, when loaded into the ME, exhibited an improvement in its antifungal action against C. albicans. The EO was not toxic against brine shrimp nauplii. An in vivo VVC assay showed that the use of the ME significantly improved the action of the EO, since only the ME+EO promoted the eradication of the fungal vaginal infection on the third day of treatment.
Conclusion: The EO and ME+EO are promising alternatives for the control of fungal infections caused by C. albicans, once the use of nanotechnology significantly improved the antifungal action of the EO, especially in an in vivo model of VVC.
Keywords: nanostructured lipid system, citronella, vulvovaginal candidiasis, therapeutic treatment
Introduction
Vulvovaginal candidiasis (VVC) is the second most common cause of vaginitis after bacterial vaginosis, which highlights it as an important women’s health issue.1 According to Denning et al, 2018, 75% of women have had VVC at least once during their lives and recurrent episodes may occur, leading to recurrent vulvovaginal candidiasis (RVVC). Although VVC is not a lethal disease, quality of life is drastically affected, both socially and economically, especially in cases of RVVC, which is considered the chronic phase of the disease.3
Candida albicans is the species responsible for over 90% of VVC cases, followed by C. tropicalis, C. glabrata, C. krusei, C. parapsilosis, and C. lusitaniae.1 VVC is common in women following antibiotic treatment, in pregnant women, and in women undergoing hormone replacement.2 The symptoms of VVC include pain, vulvar erythema, abrasions, pruritus, and a “curd-like” or watery vaginal discharge.4
The limitations of antifungal therapy, such as high cost, toxicity, drug interactions, and low drug bioavailability, are factors that contribute to the inefficiency of conventional antifungals, as represented by azole, polyenic, and echinocandin compounds.5 In addition, the emergence of drug-resistant strains has stimulated the search for new antifungal agents with different mechanisms of action.6
Plants are an important therapeutic possibility for treating fungal infections. The essential oil (EO) of the leaves of Cymbopogon nardus (L.) Rendle (citronella) is applied in cosmetic and perfumery industries and is used as an insect repellent. Geraniol, citral, citronellal and citronellol, the main chemical compounds from this EO, have various biological activities.7–9 Toledo et al (2016) reported the antifungal activity of the EO of C. nardus against standard and clinical strains of C. albicans, C. krusei, C. glabrata, C. tropicalis, and C. parapsilosis. Moreover, the EO was able to inhibit the main virulence factors, such as hyphal formation, of C. albicans and the mature biofilm of Candida species. The advantages presented by plant extracts have stimulated research on the application of alternative methods to improve the antifungal activity of plant derivatives, such as the use of nanotechnology, which allows the enhancement of biological activity, the control of drug release, and a reduction of side effects.11,12
Microemulsions (MEs) are potential nanostructured drug delivery systems. MEs present characteristics such as transparent emulsions, in which an oil is dispersed in an aqueous medium (or vice versa), containing a surfactant associated with an appropriate co-surfactant. The system is a thermodynamically stable system and shows internal phase with droplets of nanometer order, thus improving drug solubilization.12,13
Studies involving the use of natural products and nanotechnology in in vivo VVC models have been employed due to economic reasons, accessibility, and the anatomical similarity of the animal to humans. These studies have shown promising antifungal potential of the tested products, with remarkable results.14,15
The antifungal potential of C. nardus EO shown in previous study10 and the advantages of using nanostructured drug delivery systems, such as MEs, has stimulated the development of alternative antifungal agents. Furthermore, citronella EO production occurs on a large scale, which facilitates its use as a raw material for the production of herbal medicines. Furthermore, EO extraction by hydrodistillation shows advantages over the use of organic solvents for the production of plant extracts, as it takes into consideration green chemistry principles,16,17 reducing the environmental impact and the cost of the process.18,19
In this context, the aim of this study was to evaluate the possible improvement of the anti-C. albicans activity of the EO of C. nardus when loaded in a microemulsion (ME+EO). The mode of action was assessed by analyzing sorbitol and ergosterol interaction. The human cell line, HaCat, and an alternative model of Artemia salina were used to evaluate toxicity. Furthermore, in vivo VVC treatment was assessed in mice, in order to investigate the efficacy of vaginal administration of EO and ME+EO for the control of vaginal fungal infection.
Materials and Methods
Plant Material
The leaves of C. nardus (L.) Rendle were collected from April to June 2016, in the morning, in the Garden of Toxic and Medicinal Plants: “Profa. Dra. Célia Cebrian de Araújo Reis” (São Paulo State University [UNESP], Araraquara, São Paulo, Brazil). A voucher specimen (HRCB-60752) was deposited at Herbarium Rioclarense of the Institute of Biosciences (UNESP, Rio Claro, São Paulo, Brazil). This work was approved by the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) under license numbers A2B917A and AF35617/CNPJ 48.031.918/0001-24.
Extraction of Essential Oil from C. nardus
EO was extracted from fresh leaves of C. nardus (150 g) by hydrodistillation, using a Clevenger-type apparatus attached to a round bottom flask (3 L) with 1500 mL of deionized water (4 h). Residual water in the EO was separated from the sample by freezing. The yield of the EO was 0.7% (w/w). EO samples were stored at −20ºC until used for chemical analysis and biological assays.
Gas Chromatographic Analysis of Essential Oil from C. nardus
Gas Chromatography-Mass Spectrometry
Chemical analysis of EO from C. nardus was performed by gas chromatography-mass spectrometry (GC-MS), using a Shimadzu® GCMS-QP2010S gas chromatograph (mass detector: electron impact ionization; mass quadrupole analyzer), fitted with an Rtx-5ms capillary column (30 m x 0.25 mm, film thickness 0.25 µm; Shimadzu®, Kyoto, Japan). Helium was used as the carrier gas at a flow rate of 1.00 mL/min (58.8 kPa) and a linear velocity of 36.8 cm/s. The oven temperature program was 60–246ºC at 3ºC/min for 62 min, the injector temperature was 240ºC, the injection volume was 1 µL, the splitting ratio was 1:20, the ionization energy was 50 eV (280ºC), and the transfer line temperature was 250ºC. The EO was solubilized in hexane (chromatographic grade) at a ratio of 1:100 (v/v) and standard solutions were injected at concentrations of 5.0, 4.0, 3.0, 2.0, 1.0, 0.5, and 0.25 mg/mL. EO compounds were identified by comparing acquired mass spectra (from chromatogram peaks) with reference spectra of the National Institute of Standards and Technology mass-spectral library version 2.0 (2012) and data from the literature. Arithmetic retention indices20 were calculated as previously described (Adams, 2007), by linear interpolation relative to the retention times (tR) of a series of n-alkanes (C8–C20). These values were compared with a published retention indices.21 Relative amounts of EO components were calculated using the chromatogram peak area normalization method. The quantification of major EO compounds was performed using an analytical curve (external standard method), considering the commercial purity of each of the following standards (Sigma-Aldrich, St Louis, MO, USA): citronellal (95.0%), citronellol (95.0%), and geraniol (95.0%).
Microemulsion: Phase Diagram Development
MEs were prepared according to the method of Bonifácio et al22 (2015). MEs consisted of grape seed oil as the oil phase (OP), polyoxyethylene (23) lauryl ether (Brij35®) + soy phosphatidylcholine (2:1) as the surfactant (S), and phosphate-buffered saline (PBS) as the aqueous phase (AP).
The phase diagram development generated 36 formulations, mixing different concentrations of the constituents, but maintaining constant oil phase and surfactant proportions. The mixtures were sonicated (Q500; QSonica, Newtown, CT, USA) with a potency of 500 watts and a 20% amplitude, in discontinuous mode for 7 min, with an interval of 30 s in an ice bath every 59 s. The EO (2000 µg/mL) was loaded to the ME (ME+EO) by sonication for 30 s.
Microemulsion Characterization
Mean Diameter and Polydispersity Index
The mean diameter of the ME was performed by photon correlation using a dynamic laser scattering instrument (Brookhaven Instruments, Holtsville, NY, USA). The hydrodynamic radius of the colloidal particles of the Einstein-Stokes equation (Eq. [1]) was calculated as follows:
where D is the diffusion coefficient of the particles, K is the Boltzmann’s constant (1.3807 × 10−23 NmK−1), T is the absolute temperature (293.15 K), π = 3.141592, η is the viscosity (1.002 × 10−3 NM−2s), and Rh is the hydrodynamic radius.
The ME (without EO) and ME+EO were analyzed in the analysis chamber with a laser beam crossing throughout the dispersion. The system was maintained at 25°C, with a laser wavelength of 532 nm and the refractive index for each sample was analyzed according to the index observed. Ten determinations of the mean diameter and the polydispersity index (PDI) of droplets of each sample were performed (n = 3). This assay was performed after 24 h (T0) and 3 months (T3) of samples preparation in order to verify stability of formulations and possible alterations in particle diameter.
Zeta Potential
Zeta potential (ZP) was determined by assessing electrophoretic mobility using a Zetasizer Nano NS instrument (Malvern Instruments, Malvern, UK). Analyses were performed 24 h after ME preparation. The samples were diluted 1:10 in ultra-purified water, placed in the electrophoretic cell, and 3 determinations of surface potential were performed for each sample (ME and ME+EO). The mean and standard deviation were calculated. Zeta potential analyses were carried out after 24 h (T0) and 3 months (T3) of samples preparation in order to verify stability of formulations.
Polarized Light Microscopy
ME and ME+EO were placed on a glass slide, covered with a cover slip, and analyzed by polarized light microscopy (PLM; BX41 coupled with QColor3 Section; Olympus, Tokyo, Japan) at room temperature (25 ± 0.5°C).
Continuous Shear Rheology
An AR2000 pressure-controlled rheometer (TA Instruments, New Castle, DE, USA) was used for rheometry assays. Analyses were performed in triplicate, using cone/plate (40 mm diameter at a 52 μm gap) and plate/plate (40 mm diameter at a 200 μm gap) geometry at 37.0 ± 0.1°C. A controlled shear rate was used, ranging from 0 to 100 s−1, during 120 s (each step) with 10 s interval between curves. The shear was minimal and equilibrated 1 min prior to each analysis and the formulations were applied to the bottom plate. The consistency index and the flow index were determined using eq 2 for a quantitative analysis of flow behavior.
where τ is the shear stress, γ is the shear rate, κ is the consistency index, and η is the flow index.23
Oscillatory Rheology
Oscillatory rheometry was performed using the same parameters (rheometer, geometries, and temperature, 37.0 ± 0.1°C, to simulate the in vivo environment) as described for continuous shear rheometry. To establish the viscoelastic region of the formulations, a stress scan was performed, resulting in an ideal frequency condition ranging from 1 to 10 Hz. To perform a quantitative analysis of the dependence of G′ on the frequency, the exponent n and S were calculated using eq 3, which indicates the structure of the systems obtained.
where G′ is the storage modulus, ω is the oscillatory frequency, S is the formulation strength, and n is the viscoelastic exponent.23
Mucoadhesion Force
A TA.XTplus texture analyzer (Stable Micro Systems, Surrey, UK) was used, in the adhesion test mode, to evaluate the detachment force between porcine vaginal mucosa and the formulations tested (ME; ME+EO). Two mm thickness of porcine vaginal mucosa was fixed to the cylindrical probe (10 mm diameter) of the equipment. The temperature during the analyses was controlled at 37 ± 0.5°C. ME or ME+EO samples were added to small containers below the probe (same direction). The probe was lowered (constant rate, 1 mm/s) until the mucosa had direct contact (60 s) with the formulations (ME; ME+EO), without force employed during this period.
At a constant velocity (0.5 mm/s), the probe was lifted until the mucosa detached from the formulations. The force required to detach the formulation from the mucosa was calculated from the force versus time curve. Five replicates were performed.24
Transmission Electron Microscopy
The morphology and structure of the ME and ME+EO were visualized by transmission electron microscopy (TEM), using a JEM-100CX2 transmission electron microscope (JEOL, Tokyo, Japan), with an acceleration of 100 kV. The samples were diluted in water (1:10 v/v), coated on a copper grid (200 mesh), and dried at room temperature (25 ± 0.5°C). Images were acquired at 50,000× magnification.25
Antifungal Activity
Fungal Strains
Candida albicans ATCC 10231 (CA-ATCC) and one clinical isolate (CAV – human vaginal origin) were used in this study. The clinical strain was donated from the Microbiology Laboratory of the Medicine School in Sao Jose do Rio Preto (FAMERP) for the purpose of scientific research after written consent from the donors. The Human Research Ethics Committee of FAMERP approved the use of this strain (project identification code 152/20066, December 2006).
Determination of Minimal Inhibitory Concentration
The minimal inhibitory concentrations (MICs) of EO and ME+EO were determined using a microplate dilution technique, according to protocol M27-A3,26 with some modifications. Roswell Park Memorial Institute (RPMI) 1640 medium (0.1 mL) was added to a 96-well microplate and was inoculated with 0.1 mL of a fungal suspension containing 2.5 × 103 colony-forming units (CFU)/mL. EO and ME+EO were tested at concentrations ranging from 7.8 to 500 µg/mL. Amphotericin B (AmB, Sigma-Aldrich) and fluconazole (Sigma-Aldrich) were used as positive controls. Culture medium, yeast growth, EO, ME+EO, ME, and solvent were used as additional (sterility) controls. Microplates were incubated at 37°C for 48 h. After incubation, 20 µL of an aqueous solution of 2% 2,3,5-triphenyltetrazolium chloride was added and the plates were incubated at 37°C for 2 h.27 All tests were performed in triplicate.
Determination of Minimal Fungicidal Concentration
To determine the minimum fungicidal concentration (MFC), an aliquot from each well that showed antifungal activity was plated on a Petri dish containing Sabouraud dextrose agar (DIFCO; BD Biosciences, Franklin Lakes, NJ, USA). The tests were carried out in triplicate. MFC was defined as the lowest concentration of EO and ME+EO that prevented visible growth on the solid medium.14
Ergosterol Assay
The binding of EO and ME+EO to membrane ergosterol in C. albicans was evaluated according to the method described by Peixoto et al28 (2017). Microplates were prepared according to protocol M27-A3,26 with some modifications. EO and ME+EO were evaluated at concentrations ranging from 5000 μg/mL to 2.44 μg/mL. C. albicans (ATCC 102331 and CAV) suspensions (103 cells/mL) were prepared (100 µL) in RPMI 1640 medium supplemented with 200 μg/mL exogenous ergosterol. Medium without exogenous ergosterol was used as a control. Microplates were incubated at 35°C and readings were performed after 2 d.
Sorbitol Assay
To evaluate the interactions of EO and ME+EO with the fungal cell wall, microplates were prepared as described in the MIC determination experiments, according to protocol M27-A3,26 with some modifications. EO and ME-EO were added to microplates at concentrations ranging from 1,000 μg/mL to 0.48 μg/mL. One hundred microliters of C. albicans (ATCC 102331 and CAV) suspensions (103 cells/mL), prepared in RPMI 1640 medium supplemented with 0.8 M sorbitol, were added. Medium without sorbitol was used as a control. Microplates were incubated at 35°C and readings were performed after 2 and 7 d.28
Toxicity Assays
HaCat Cell Line
Cytotoxicity assays were performed in the HaCat human keratinocyte cell line obtained from Banco de células do Rio de Janeiro (BCRJ: 0341), according to a previously described protocol29 using the resazurin reduction method. Cells (2.5 × 104 cells/mL) were added to a 96-well microplate (Costar, Cambridge, MA, USA) for 24 h. After this process, cells were treated with EO (from 7.8 to 1000 μg/mL) or ME+EO (from 0.1 to 30 μg/mL). After 24 h of incubation, the medium was removed and 50 mL of resazurin, diluted to 0.01% in DMEM, was added. The microplates were then incubated at 37°C for 3 h. Fluorescence was measured using a Synergy H1 microplate reader (BioTek, Winooski, VT, USA) using an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Untreated cells (viable cells) were used as negative controls, cells treated with 5 µg/mL doxorubicin (Sigma-Aldrich) were used as positive controls (dead cells), and 1% DMSO was used as the vehicle control. Assays were performed in triplicate. IC50 values, which represent the concentrations required to inhibit 50% of cells, were calculated by regression analysis from a calibration curve.
Artemia Salina Model
Brine shrimp cysts of Artemia salina, for use in the conventional Artemia test, were acquired from Artemia salina of RN, Natal, Brazil, produced by Flagner Sores de Souza. In vitro toxicity was assessed using an Artemia salina (brine shrimp) model, according to a previously described method (Parra et al30, 2001), with some modifications. Briefly, 25 mg of A. salina cysts was incubated for 24 h in artificial saline water consisting of 23 g of NaCl, 11 g of MgCl2.6H2O, 4 g of Na2SO4, 1.3 g of CaCl2.H2O, and 0.7 g of KCl dissolved in 1000 mL of distilled water. An oxygen pump was placed in the saline water and a light was coupled on top of the container to keep the temperature at 25°C during the experiment. After 24 h of incubation, the nauplii were fed with 0.6 g of Saccharomyces cerevisiae and incubated for another 24 h.
The next day, a suspension of nauplii (10–15 organisms/well) in a volume of 100 μL of saline water was added to a 96-well microplate. In a separate microplate, a serial dilution of the compounds was prepared with concentrations of EO and ME+EO ranging from 0.2 to 1000 µg/mL. The controls consisted of untreated nauplii (negative control), 2% DMSO (vehicle control), and potassium dichromate (positive control). After 24 h of treatment, the plate was examined under a binocular stereoscopic microscope and the number of dead nauplii in each well was counted to calculate the median lethal concentration (LC50). Larvae were considered dead if they did not exhibit any internal or external movement during several seconds of observation.
In vivo VVC Study in a Mouse Model
In vivo VVC assays were performed in a mouse model following the recommended guidelines of the Ethics Principles in Animal Experimentation of the Ethics Committee on the Use of Animals in Research, CEUA School of Pharmaceutical Sciences of Araraquara, UNESP, São Paulo State, Brazil, after approval protocol number 56/2016. Animals were purchased from Centro Multidisciplinar para Investigação Biológica na Área da Ciência em Animais de Laboratório, CEMIB, Unicamp, Campinas, São Paulo State, Brazil.
Female C57BL/6 mice (n = 56) at 8–10 weeks of age, weighing 20–25 g, were used for these experiments. For environmental adaptation, the animals remained in the experimental room for 4 weeks before starting the assay. During the entire experiment, the animals were kept in polypropylene boxes (50 × 30 × 20 cm), maintained with adequate temperature and ventilation and an alternating 12/12 h light/dark cycle.
The different experimental groups are listed in Table 1. C. albicans ATCC 10231 was used in this study. Initially, the pseudoestrus hormonal phase was induced in mice by subcutaneous administration (0.1 mL) of estradiol (Sigma-Aldrich) at a concentration of 2 mg/mL, at 6 and 3 days prior to infection (initial day). On the initial day, 20 μL of a C. albicans suspension containing 2.5 × 108 cells/mL was inoculated into the vaginal environment. At day 1, treatments (20 μL administered/animal) were initialized (once/day) and additional treatments were administered on days 3, 5, and 7. On days 2, 4, and 6, estradiol administration was continued to maintain the pseudoestrous phase. On days 2, 4, 6, and 8, vaginal lavages were performed with PBS.15,31 The infection was monitored by individual vaginal lavage of the mice (70 μL of sterile PBS), with repeated aspiration, shaking, and subsequent plating on Sabouraud agar + chloramphenicol (Acumedia, Lansing, MI, USA) to determine the number CFUs. After 48 h of incubation of the agar plates at 37°C, the colonies were counted. Colony counts are expressed as log CFU/mL.15,31 The animals were euthanized in a CO2 chamber.
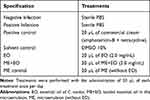 |
Table 1 Specification and Treatments Used in the in vivo VVC Study |
Statistical Analysis
Results are reported as mean ± standard deviation (SD). Statistical analyses were performed with Prism 7.00 software (GraphPad, San Diego, CA, USA) using a two-way ANOVA, with a post-hoc Tukey’s test for multiple comparisons. A Student’s t test was used to compare means between only two groups. It was considered to be statistically significant if p < 0.05.
Results and Discussion
Chemical Composition of Cymbopogon nardus Essential Oil
The chemical profile (qualitative and quantitative composition) of the EO from C. nardus, as determined by GC-MS, is shown in Table 2. The following oxygen-containing monoterpenes (89.93%) were the major constituents: citronellal (27.34%), geraniol (23.21%), geranial (13.37%), citronellol (12.49%), and neral (10.31%). These monoterpenes are biosynthetically related and are derived from geranyl diphosphate.32
 |
Table 2 Chemical Composition of C. nardus Essential Oil |
The citronellal concentration calculated from the analytical curve (area = 5,575,407, R2 = 0.997) was 2.653 mg/mL, which corresponds to a citronellal concentration in the EO of 26.5% (w/w). The citronellol concentration (area = 2,196,889, R2 = 0.996) was 1.116 mg/mL, which can be expressed as 11.1%. The geraniol concentration (area = 4,318,673, R2 = 0.997) was 2.458 mg/mL, which corresponded to 24.6%. These values are consistent with the quantification from the chromatogram peak area normalization method by GC-MS.
EOs from the genus Cymbopogon have monoterpenes as their main chemical constituents. Variations in the chemical composition of EOs may occur due to several factors, such as climate, genetics, environment, seasonal variation, geographical conditions, and extraction methods.33–36
This EO chemical profile corroborated the results of a previous study that showed a citronellal concentration of 27.87% in the EO of C. nardus (Toledo et al, 2016), which is similar to the concentration found in this study (27.34%) and in a study by Wei and Wee (2013),37 which reported a citronellal concentration of 29.6%. Studies performed by Kandimalla et al38 (2016) in India and Nakahara et al39 (2003) in Japan showed low concentrations of citronellal in the OE of C. nardus (6.06% and 5.8%, respectively). Kandimalla et al38 (2016) found that the main constituents were citral (38.75%) and 2,6-octadienal,3,7-dimethyl (31.02%) and Nakahara et al39 (2003) reported geraniol as the main chemical component, with a concentration of 35.7%.
Identification of the chemical compounds in the EO responsible for antimicrobial activity is difficult, since the essential oils are complex mixtures with different chemical compounds that can act synergistically.40,41 A previous study has shown that the EO of C. nardus has greater antifungal activity against planktonic cells of Candida species than citronellal, which is its main chemical compound. This indicates a possible synergic action among the chemical compounds present in the EO.10
Microemulsion Formation
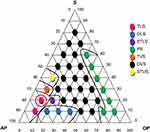
A phase diagram (Figure 1) was constructed to demonstrate the 36 points of formulations obtained from the mixtures of different constituents and a visual classification was evaluated for each formulation. The formulations were visually classified as a transparent liquid system (TLS), an opaque liquid system (OLS), a semi-transparent liquid system (STLS), a phase separation (PS), a transparent viscous system (TVS), an opaque viscous system (OVS), or a semi-transparent viscous system (STVS). Therefore, different regions of the phase diagram were delineated and the best formulation for loading the essential oil in the ME was selected. The ME with the TLS characteristic (circled red point – Point 9) was chosen for use in in vitro and in vivo assays. It was composed of 70% PBS, 20% Brij35 + soy phosphatidylcholine (2:1), and 10% grape seed oil. The EO (2000 µg/mL) was loaded in the ME (point 9, ME+EO).
Microemulsion Characterization
Mean Diameter, Polydispersity Index, and Zeta Potential
The mean values and standard deviation of particle size and PDI for the ME and ME+EO are demonstrated in Table 3. According to Formariz et al42 (2010) the particle size of MEs range from 10 to 250 nm. The results obtained in this study corroborate these data, since the particles sizes were 55.23 and 70.14 nm for the ME and ME+EO, respectively. Small differences in particle diameter size were observed between the ME and ME+EO, which suggested the incorporation of the EO in the ME. PDI is an index that evaluates the relative homogeneity of the particle sizes distributed in the sample.43,44 Therefore, these results demonstrated that the ME and ME+EO were homogeneous. These data confirmed the results of a previous study of an ME containing orange oil, with varying oil concentrations of 20%, 25%, and 30%, which exhibited a particle size of 70 nm and an IPD of 0.22 nm.45
 |
Table 3 Mean Diameter of the Particle Size, Polydispersity (PDI) and Zeta Potential for ME and ME+EO After 24 h (T0) and 3 Months (T3) of Samples Preparation |
An analysis of the ZP of the ME and ME+EO showed a negative charge, which is likely due to the presence of soy phosphatidylcholine in the system that may favor the negative potential because of free esters present in the lipid structure.46 However, phosphatidylcholine provides a phosphate group (negative charge) and a choline group (positive charge), which can cancel out each other, generating a zero charge at neutral pH. Thus, the hydration layers, lipid headgroups, and water polarization are factors that can be considered as the cause of the negative ZP.47,48 No significant changes were observed between time 0 and time 3 months, in relation to the particle diameter, IPD and zeta potential, which indicates stability of ME and ME+EO.
PLM
The ME and ME+EO were analyzed by PLM to evaluate the structure of the formulations. Figure 2 shows the PLM photomicrographs of the ME (A) and ME+EO (B). Photomicrography A (10% oil phase) showed isotropic behavior (dark field), which, in the plane of polarized light, did not deviate light, suggesting the formation of a microemulsified system. Photomicrography B (10% oil phase), after the incorporation of the EO in the ME, also showed a dark field and therefore, the lipid system containing the active ingredient maintains the characteristics of a microemulsified system.
 |
Figure 2 PLM photomicrographs of ME (A) and ME+EO (B) at magnification 20×. |
Oscillatory Rheology
The viscoelastic behavior of the formulations (ME and ME+EO) was evaluated by oscillatory measurements. Rheological analyses are relevant, as they provide parameters for the characterization of the formulations, including structure and viscosity, which are important for the development of formulations for topical use.49 G′ represents the storage modulus of the energy of stress, which can be restored when the stress is released and G″ represents the flow resistance of the sample.50
Figure 3 shows the elastic modulus (G′) and viscous modulus (G″) of the formulations. The ME and ME+EO showed elastic behavior (G′ > G″), indicating a highly organized structure. These results demonstrated that the incorporation of EO in the system did not alter the viscoelastic behavior of the formulations. Viscoelasticity is important for vaginal application, due to the ease of administration and suitable scattering in the vaginal environment.51
 |
Figure 3 Rheological behavior in oscillatory flow conditions of ME and ME+EO. |
Continuous Shear Rheology
Continuous shear rheology data for the ME and ME+EO are shown in Figure 4. The ascending (filled symbol) and descending curves (empty symbol) indicate the flow behavior of the formulations. Table 4 shows the properties related to flow behavior (n) and consistency of the formulations (k). The ME and ME+EO demonstrated non-Newtonian characteristics, with pseudoplastic behavior (n < 1). This result is relevant for the appropriate application of pseudoplastic formulations in the vaginal environment, since an increase in viscosity occurs under the application of shear stress; however, when the force is removed, the system structure returns to its initial properties.52
 |
Table 4 Flow Behavior (n) and Consistency Index (k) of ME and ME+EO by Continuous Flow Rheology |
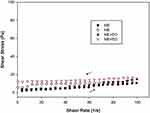 |
Figure 4 Continuous shear rheogram of ME and ME+EO. Filled symbol represents the ascending curve and empty symbol indicates the descending curve; up arrow: ascending; down arrow: descending. |
These results are in agreement with the work of Ramos et al24 (2019), who showed that a methanolic extract of Syngonanthus nitens loaded in a nanoemulsion showed pseudoplastic behavior and allowed effective application for vulvovaginal candidiasis treatment. Another study demonstrated the pseudoplastic behavior of multiple emulsions loaded with clotrimazole as an adequate topical (vaginal) delivery system, showing effective antifungal activity when compared to clotrimazole, a commercial antimycotic drug.53
Mucoadhesion Properties
Suitable mucoadhesion characteristics are relevant for the attachment of a formulation to vaginal mucosal membranes, in order to promote extended contact between the formulation and the vaginal cavity, thus allowing an increased residence time and bioavailability of the drug. Moreover, adequate mucoadhesion avoids drainage of the formulation outside the vagina, which is an unfortunate condition for users.54
The peaks of mucoadhesion of the ME and ME+EO are shown in Figure 5. There was no significant difference (p > 0.05) in mucoadhesion between the ME and ME+EO, indicating that, after incorporation of the EO in the ME, mucoadhesion remained stable. The formulations showed adequate mucoadhesion for use in an in vivo VVC assay, which was in agreement with the results of studies by Bonifácio et al22 (2015) and Ramos et al24 (2019), in which the mucoadhesivity of loaded vegetal extracts in nanoemulsioned formulations were suitable for application in in vivo vulvovaginal candidiasis studies.
 |
Figure 5 Peak of mucoadhesion (N.s) of ME and ME+EO. Each value represents the mean (± standard deviation) of at least five replicates. |
Transmission Electron Microscopy
Figure 6 shows TEM images of the ME and ME+EO at a magnification of 50,000×. The photomicrographs of the ME and ME+EO showed spherical structures; however, ME+EO images showed possible EO droplets inside the spherical structures, which suggested incorporation of the EO.55 The particle sizes of the formulations obtained from DLS measurements were confirmed by TEM photomicrographs, which showed particles ranging from 50 to 200 nm.
 |
Figure 6 TEM photomicrographs of the ME (A) and ME+EO (B) at magnification of 50,000×. |
Antifungal Activity
MIC and MFC of EO and ME+EO
The MIC and MFC results for EO and ME+EO are shown in Table 5. The EO showed antifungal activity with MIC values of 500 µg/mL and >500 µg/mL against C. albicans ATCC and CAV, respectively. ME+EO showed the lowest MIC (31.2 µg/mL) against CAATCC and CAV (62.5 µg/mL). Thus, the use of an ME considerably improved the action of the EO. The growth control showed satisfactory results, whereas the solvent and ME controls did not show any antifungal activity.
 |
Table 5 MIC and MFC of EO and ME+EO Against C. albicans |
Several studies have demonstrated the anti-Candida activity of EOs.56–59 The genus Cymbopogon has been shown to have effective antifungal activity against pathogenic fungi. A previous study demonstrated the antifungal activity of the EO of Cymbopogon citratus against different Candida species, showing MIC values ranging from 125 to 175 µg/mL.60 Another study demonstrated the anti-Candida action of the EO from C. nardus against C. albicans, with MIC values ranging from 32 to 64 µg/mL. Furthermore, the EO of C. nardus inhibits the adherence of C. albicans to dental implants and cover screws.61
The use of nanotechnology as an alternative to improving the antifungal activity of natural products has been used with promising results. In an in vivo study of the prophylactic action of a methanolic extract of Syngonanthus nitens (Bong.) Rhulland loaded into a drug delivery system, the prevention of VVC development caused by C. krusei was demonstrated.62 Bonifácio et al22 (2015) studied the antifungal activity of an ethanolic extract from Astronium urundeuva (Engl.), unloaded or loaded in an ME, against C. albicans and showed an improvement in the inhibitory capacity of the extract after incorporation into the nanostructured system.
The incorporation of oils63 and chemical compounds from EOs64 in nanostructured delivery systems has been used to combat microorganisms. A previous study showed greater antifungal activity against Candida species using clotrimazole loaded into lipid nanoparticles containing EOs compared to prolonged in vitro delivery of clotrimazole. Moreover, increased anti-Candida action was observed for loaded clotrimazole nanoparticles prepared with EOs of Lavandula or Rosmarinus.65
The use of MEs is important to enhance the solubilization of the EO, which improves the safety and stability of the system and consequently, may enhance the antifungal activity of the EO.66
Ergosterol and Sorbitol
Table 6 shows MIC values for EO, ME+EO, and AmB, in the absence and presence of exogenous ergosterol, against C. albicans ATCC 10231 and CAV. The MIC values of EO and ME+EO increased 5-fold and 20-fold, respectively, in the presence of exogenous ergosterol. These results suggested that EO and ME+EO bound to ergosterol in the fungal membrane, which required higher concentrations of EO and ME+EO to promote fungal cell death, demonstrating action on the ionic permeability of the fungal cell membrane. The positive control (AmB) showed the same mode of action. The antifungal activities of EO and ME+EO are not related to the biosynthetic pathways of the cell wall, since the MIC values were maintained in the presence of sorbitol.
 |
Table 6 MIC Values of EO, ME+EO and Amphotericin-B (AMB) in Absence (A) and Presence (P) of Exogenous Ergosterol Against C. albicans ATCC 10231 and CAV |
A previous study investigated the mode of action of Thyme vulgaris EO, against Rhizopus oryzae, via interaction with ergosterol, and verified that this EO can interact with the fungal membrane, as evidenced by increasing MIC values in the presence of ergosterol.67 Another study demonstrated that Laurus nobilis EO may interfere have two mechanisms of action against C. albicans, (cell wall biosynthesis and ionic permeability of the fungal cell membrane), considering that increased MIC values for this EO are observed in the presence of ergosterol and sorbitol.28
Toxicity Studies
HaCat Cell Line
Table 7 shows the cytotoxic activity (IC50) of the EO, ME+EO, and ME against HaCat cells. The EO showed lower cytotoxicity than ME+EO and ME. Koba et al68(2009) compared the cytotoxicity of EOs from the genus Cymbopogon (C. nardus and C. citratus) against HaCat cells and showed that the C. nardus EO had lower cytotoxicity than the C. citratus EO, with IC50 values of 450 µL/mL and 150 µL/mL, respectively.
 |
Table 7 Cytotoxic Activity (IC50) of EO, ME+EO and ME Against HaCat Cell Line |
The IC50 values of the ME+EO and ME were similar to conventional drugs used in the clinical practice, such as AmB (IC50 = 4.0 μg/mL).24 Topical administration is proposed for the formulations in this study. The route of administration is important for toxicity parameters, since topical administration exhibits lower toxicity than oral administration.69
Brine Shrimp Lethality
Table 8 shows the lethal concentration (LD50) of the EO, ME+EO, and ME against brine shrimp (A. salina) nauplii. Several studies have described the toxicity of EOs against brine shrimp nauplii.70–72 However, the results of this study showed that EO was not toxic against brine shrimp nauplii, with LD50 values higher than 1000 μg/mL. Moreover, brine shrimp nauplii treated with ME+EO and ME were found to be viable, with LD50 values >30 μg/mL, which was the highest tested concentration of these formulations in this assay.
 |
Table 8 Lethal Concentration (LD50) of EO, ME+EO and ME Against Brine Shrimp Nauplii |
Parra et al30 (2001) used the A. salina model to determine the toxicity of 20 plant extracts. The authors found a good correlation between in vivo and in vitro tests, concluding that brine shrimp lethality studies are a useful alternative to predict the acute oral toxicity of plant extracts. Moreover, the A. salina model is a useful tool for toxicity studies, due to the benefits of the method, which include quickness, cost-effectiveness, and reproducibility.73
In vivo VVC Assay
Table 9 shows the results of fungal burden (CFU) from vaginal lavages collected at days 2, 4, 6, and 8. The EO of C. nardus was found to have significant therapeutic activity against VVC caused by C. albicans in mice. All animals treated with ME+EO were cured on the third day of treatment (experimental day 5). Moreover, incorporation of the EO into an ME enhanced the antifungal action of the EO (free), which was able to eradicate fungal infection in 50% of the animals. In the positive infection, solvent, and ME control experimental groups, the infection remained constant on all days of treatment, with a high fungal load present in 100% of the animals.
 |
Table 9 Fungal Burden (CFU) from the Vaginal Lavages Collected at Days 2, 4, 6 and 8, During Treatment Period |
These data are significant, since the ME+EO demonstrated superior antifungal action compared with both free EO and a commercial cream used in clinical therapy (AmB + tetracycline), thus demonstrating the effectiveness of the nanotechnology application as an alternative to improve the antifungal activity of natural products.
Studies using in vivo experimental models of mucosal infections caused by Candida are typically performed with mammals (rats, mice) because of economic, ethical, and anatomical reasons, and because these animals are immunologically similar to humans.74–76
Previous studies have shown that plant extracts incorporated into nanostructured lipid systems are effective in the treatment rat models of VVC caused by C. albicans. Plant extracts loaded into nanostructured drug delivery systems were shown to be significantly more effective than unloaded plant extracts or the clinical practice drug, AmB, at the complete eradication of the vaginal fungal infection.14,22
Rodero et al15 (2018) described the use of liquid crystalline systems to improve the action of curcumin in a mouse model of VVC. The authors showed effective antifungal activity of curcumin, loaded in the liquid crystalline system, against C. albicans. In addition, the loaded curcumin was effective at modulating the inflammatory response to the infection.
Pietrella et al77 (2011) evaluated Mentha suaveolens EO in a mouse model of VVC (C. albicans). The authors reported that the EO was able to decrease the fungal load of the vaginal infection compared to the saline control, thus accelerating the removal of the infection.
Another study investigated the antifungal activity of clove EO in an emulsion and in a liposomal form in mice with VVC. After 8 d of treatment, a reduction in vaginal fungal load was observed for both forms tested. However, the liposomal form was found to be more effective than the emulsion form of the EO. In addition, the free EO was only able to eliminate the infection after 14 d.
These data corroborate the results of the present study, in which EOs were shown to have improved action when incorporated into a drug delivery system. In addition to improved antimicrobial activity, these drug delivery systems increase the stability and bioavailability of the plant extracts.12
Conclusion
The main compounds of the EO of C. nardus were found to be the oxygen-containing monoterpenes, citronellal, geranial, geraniol, citronellol, and neral. The ME+EO showed adequate technological properties, providing stability of the system and improvement of solubilization of the EO for incorporation in the ME. The EO and ME+EO exhibited effective in vitro and in vivo antifungal activity against C. albicans and demonstrated possible actions on the ionic permeability of the fungal cell membrane. Moreover, the use of nanotechnology significantly improved the antifungal action of the EO, especially in an in vivo model of VVC, in which the ME+EO promoted the eradication of the fungal vaginal infection on the third day of treatment. These encouraging results suggest that the incorporation of the essential oil of C. nardus in an ME can considerably enhance the antifungal action of the EO against C. albicans. Additionally, EO extraction complies with the principles of green chemistry, being a viable process that facilitates the development and industrial-scale production of antifungal herbal medicines, based on citronella EO.
Abbreviations
AmB, amphotericin-B; AP, aqueous phase; GC/MS, gas chromatography-mass spectrometry; DMEM, Dulbecco’s Modified Eagle’s Medium; DMSO, dimethylsulfoxide; EO, essential oil; FLU, fluconazole; ME, microemulsion; ME+EO, loaded essential oil in the microemulsion; OP, oil phase; PBS, phosphate-buffered saline; RPMI, Roswell Park Memorial Institute; S, surfactant SDA, Sabouraud Dextrose Agar; SDB, Sabouraud Dextrose broth; SisGen, National System for the Management of Genetic Heritage and Associated Traditional Knowledge; VVC, vulvovaginal candidiasis.
Acknowledgments
We thank São Paulo Research Foundation-FAPESP (grant #2015/23959-7, grant #2017-21356-9, grant #2016/08559-5, grant #2014/50926-0 and grant #2020/13057-4). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior-Brasil (CAPES) – Finance code-001.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lopez JEM. Candidiasis (vulvovaginal). Clin Evid (Online). 2015;3:1–23.
2. Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis. 2018;18(11):e339–e347. (). doi:10.1016/S1473-3099(18)30103-8
3. Cassone A, Sobel JD. Experimental models of vaginal candidiasis and their relevance to human candidiasis. Infect Immun. 2016;84(5):1255–1261. doi:10.1128/IAI.01544-15
4. Blostein F, Mph EL, Wagner J, Foxman B. Annals of epidemiology recurrent vulvovaginal candidiasis. Ann Epidemiol. 2017;27(9):575–582.e3. doi:10.1016/j.annepidem.2017.08.010
5. Ferreira D, Grenouillet F, Blasco G, et al. Outcomes associated with routine systemic antifungal therapy in critically ill patients with Candida colonization. Intensive Care Med. 2015;41(6):1077–1088. doi:10.1007/s00134-015-3791-4
6. Perfect JR. Expert opinion on emerging drugs “ Is there an emerging need for new antifungals ?”. Expert Opin Emerg Drugs. 2016;21(2):129–131. doi:10.1517/14728214.2016.1155554
7. Zore GB, Thakre AD, Rathod V, Karuppayil SM. Evaluation of anti-Candida potential of geranium oil constituents against clinical isolates of Candida albicans differentially sensitive to fluconazole: inhibition of growth, dimorphism and sensitization. Mycoses. 2011;54(4):99–109. doi:10.1111/j.1439-0507.2009.01852.x
8. Aini MNN, Said MI, Nazlina I, Hanina MN, Ahmad IB. Screening for antiviral activity of sweet lemon grass (Cymbopogon nardus (L.) Rendle) fractions. J Biol Sci. 2006;6(3):507–510. doi:10.3923/jbs.2006.507.510
9. Feyaerts AF, Mathé L, Luyten W, De Graeve S, Van Dyck K. Essential oils and their components are a class of antifungals with potent vapour-phase-mediated anti -Candida activity. Sci Rep. 2018;8:1–10. doi:10.1038/s41598-018-22395-6
10. Toledo LG, Ramos MAS, Spósito L, et al. Essential Oil of Cymbopogon nardus (L.) Rendle: a strategy to combat fungal infections caused by Candida species. Int J Mol Sci. 2016;17(8):1–16. doi:10.3390/ijms17081252
11. Ghosh V, Saranya S, Mukherjee A, Chandrasekaran N. Antibacterial microemulsion prevents sepsis and triggers healing of wound in wistar rats. Colloids Surfaces B Biointerfaces. 2013;105:152–157. doi:10.1016/j.colsurfb.2013.01.009
12. Ramos MAS, Silva PB, Spósito L, et al. Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. Int J Nanomedicine. 2018;13:1179–1213. doi:10.2147/IJN.S146195
13. Muzaffar F, Singh UK, Chauhan L. Review on microemulsion as futuristic drug delivery. Int J Pharm Pharm Sci. 2013;5(3):39–53.
14. Dos Santos Ramos MA, de Toledo LG, Fioramonti Calixto GM, et al. Syngonanthus nitens Bong. (Rhul.)-loaded nanostructured system for Vulvovaginal candidiasis treatment. Int J Mol Sci. 2016;17(8):1368. doi:10.3390/ijms17081368
15. Rodero CF, Fioramonti Calixto GM, Cristina Dos Santos K, et al. Curcumin-loaded liquid crystalline systems for controlled drug release and improved treatment of vulvovaginal candidiasis. Mol Pharm. 2018;15(10):4491–4504. doi:10.1021/acs.molpharmaceut.8b00507
16. Farias LA, Fávaro DI. Vinte Anos de química verde: conquistas e desafios. Quim Nova. 2011;34(6):1089–1093. doi:10.1590/S0100-40422011000600030
17. Prado AGS. Química verde, os desafios da química do novo milênio. Quim Nova. 2003;26(5):738–744. doi:10.1590/S0100-40422003000500018
18. Paroul N, Grzegozeski LP, Chiaradia V, Treichel H. Solvent-free production of bioflavors by enzymatic esterification of citronella (Cymbopogon winterianus) essential oil. Appl Biochem Biotechnol. 2012;166:13–21.
19. Chanthai S, Prachakoll S, Ruangviriyachai C, Luthria DL. Influence of extraction methodologies on the analysis of five major volatile aromatic compounds of citronella grass (Cymbopogon nardus) and lemongrass (Cymbopogon citratus) grown in thailand. J AOAC Int. 2012;95(3):763–772. doi:10.5740/jaoacint.11-335
20. Vandendool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr. 1963;11(3):463–471. doi:10.1016/S0021-9673(01)80947-X
21. Adams R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry.
22. Bonifácio BV, Dos Santos Ramos MA, Silva P. Nanostructured lipid system as a strategy to improve the anti-Candida albicans activity of Astronium sp. Int J Nanomedicine. 2015;10:5081–5092. doi:10.2147/IJN.S79684
23. Calixto G, Yoshii AC, Rocha H, Stringhetti B, Cury F, Chorilli M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: preparation and characterization. Pharm Dev Technol. 2014;7450:1–7.
24. Dos Ramos MAS, da Silva PB, de Toledo LG, et al. Intravaginal delivery of syngonanthus nitens (Bong.) Ruhland fraction based on a nanoemulsion system applied to vulvovaginal candidiasis treatment. J Biomed Nanotechnol. 2019;15(5):1072–1089. doi:10.1166/jbn.2019.2750
25. Eloy JO, Petrilli R, Ribeiro HM, et al. Co-loaded paclitaxel/rapamycin liposomes: development, characterization and in vitro and in vivo evaluation for breast cancer therapy. Colloids Surf B Biointerfaces. 2016;141:74–82. doi:10.1016/j.colsurfb.2016.01.032
26. Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts.
27. Duarte MCT, Figueira GM, Sartoratto A, Rehder VLG, Delarmelina C. Anti- Candida activity of Brazilian medicinal plants. J Ethnopharmacol. 2005;97:305–311. doi:10.1016/j.jep.2004.11.016
28. Peixoto LR, Rosalen PL, Ferreira GLS, et al. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch Oral Biol. 2017;73:179–185. doi:10.1016/j.archoralbio.2016.10.013
29. Page B, Page M, Noël C. A new fluorometric assay for cytotoxicity measurements in vitro. Int J Oncol. 1993;3:473–476.
30. Parra AL, Yhebra RS, Sardiñas IG, Buela LI. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8(5):395–400. doi:10.1078/0944-7113-00044
31. Miró MS, Rodríguez E, Vigezzi C, et al. Contribution of TLR2 pathway in the pathogenesis of vulvovaginal candidiasis. Pathog Dis. 2018;75(7):1–10.
32. Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. Vol. 53. 2009.
33. Ganjewala D, Luthra R. Essential oil biosynthesis and regulation in the genus cymbopogon. Nat Prod Commun. 2010;5(1):163–172.
34. Wei LS, Wee W. Chemical composition and antimicrobial activity of Cymbopogon nardus citronella essential oil against systemic bacteria of aquatic animals. Iran J Microbiol. 2013;5(2):147–152.
35. Zarshenas M, Samani S, Petramfar P, Moein M. Analysis of the essential oil components from different Carum copticumL. samples from Iran. Pharmacognosy Res. 2013;6(1):62. doi:10.4103/0974-8490.122920
36. Ghaffari Z, Rahimmalek M, Sabzalian MR. Variations in essential oil composition and antioxidant activity in Perovskia abrotanoides kar. collected from different regions in Iran. Chem Biodivers. 2018;15:6.
37. Kandimalla R, Kalita S, Choudhury B, Dash S, Kalita K, Kotoky J. Chemical composition and anti-candidiasis mediated wound healing property of Cymbopogon nardus essential oil on chronic diabetic wounds. Front Pharmacol. 2016;7:
38. Nakahara K, Alzoreky NS, Yoshihashi T, Nguyen HTT, Trakoontivakorn G. Chemical Composition and Antifungal Activity of Essential Oil from Cymbopogon nardus (Citronella Grass). Japan Agric Res Q. 2003;37(4):249–252. doi:10.6090/jarq.37.249
39. Espina L, Somolinos M, Lorán S, Conchello P, García D, Pagán R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control. 2011;22(6):896–902. doi:10.1016/j.foodcont.2010.11.021
40. Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3:1–24.
41. Formariz TP, Chiavacci LA, Scarpa MV, et al. Structure and viscoelastic behavior of pharmaceutical biocompatible anionic microemulsions containing the antitumoral drug compound doxorubicin. Colloids Surfaces B Biointerfaces. 2010;77(1):47–53. doi:10.1016/j.colsurfb.2010.01.004
42. Moghimipour E, Salimi A, Eftekhari S. Design and characterization of microemulsion systems for naproxen. Adv Pharm Bull. 2013;3(1):63–71.
43. Zalak BP, Kruti SP, Ankit SS, Naazneen IS. of rosuvastatin calcium. J Pharm Bioallied Sci. 2012;4:118–120. doi:10.4103/0975-7406.94164
44. Jantrawut P, Boonsermsukcharoen K, Thipnan K, Chaiwarit T, Hwang K-M, Park E-S. Enhancement of antibacterial activity of orange oil in pectin thin film by microemulsion. Nanomaterials. 2018;8(7):545. doi:10.3390/nano8070545
45. Silva PB, De Souza PC, Maria G, et al. In Vitro Activity of Copper (II) complexes, loaded or unloaded into a nanostructured lipid system, against mycobacterium tuberculosis. Int J Mol Sci Artic. 2016;17:745–7557. doi:10.3390/ijms17050745
46. Kotyńska J, Figaszewski ZA. Binding of trivalent metal ions (Al3+, In3+, La3+) with phosphatidylcholine liposomal membranes investigated by microelectrophoresis. Eur Phys J E. 2018;41:5. doi:10.1140/epje/i2018-11679-6
47. Klasczyk B, Knecht V, Lipowsky R, Dimova R. Interactions of alkali metal chlorides with phosphatidylcholine vesicles. Langmuir. 2010;26(24):18951–18958. doi:10.1021/la103631y
48. Pénzes T, Ildikó C, István E. Rheological analysis of the structural properties effecting the percutaneous absorption and stability in pharmaceutical organogels. Rheol Acta. 2004;43:457–463. doi:10.1007/s00397-004-0396-1
49. Carvalho C, Barbi MS, Hugo V, et al. Surfactant systems for nasal zidovudine delivery: structural, rheological and mucoadhesive properties. J Pharm Pharmacol. 2010;430–439.
50. Sanz R, Clares B, Mallandrich M, Suñer-carbó J, Jesús M, Calpena AC. Development of a mucoadhesive delivery system for control release of doxepin with application in vaginal pain relief associated with gynecological surgery. Int J Pharm. 2018;535(1–2):393–401. doi:10.1016/j.ijpharm.2017.11.027
51. Salmazi R, Calixto G, Bernegossi J, et al. A curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int J Nanomedicine. 2015;10:4815–4824.
52. Soriano-ruiz JL, Suñer-carbó J, Calpena-campmany AC, et al. Clotrimazole multiple W/O/W emulsion as anticandidal agent: characterization and evaluation on skin and mucosae. Colloids Surfaces B Biointerfaces. 2019;175:166–174. doi:10.1016/j.colsurfb.2018.11.070
53. Rencber S, Ay Z, Katip I, Universitesi C, Limoncu MH. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: formulation, preparation, and in vitro/in vivo evaluation. Pharm Dev Technol. 2016;1–11.
54. Oliveira CA, Gouvêa MM, Antunes GR, de Freitas ZMF, Marques FFC, Ricci-Junior E. Nanoemulsion containing 8-methoxypsoralen for topical treatment of dermatoses: development, characterization and ex vivo permeation in porcine skin. Int J Pharm. 2018;547(1–2):1–9. doi:10.1016/j.ijpharm.2018.05.053
55. Malik S, Santos L, Mesquita S De, Silva CR. Chemical Profile and. Biological activities of essential oil from artemisia vulgaris L. cultivated in Brazil. Pharmaceuticals. 2019;12(49):1–10.
56. Bona E, Cantamessa S, Pavan M, et al. Sensitivity of Candida albicans to essential oils: are they an alternative to antifungal agents? J Appl Microbiol. 2016;121:1530–1545. doi:10.1111/jam.13282
57. Rivera-yañez CR, Terrazas LI, Jimenez-estrada M, et al. γ -Terpinene — an in vitro study. Molecules. 2017;22:2095–2108. doi:10.3390/molecules22122095
58. Helal IM, El-bessoumy A, Al-bataineh E, et al. Antimicrobial efficiency of essential oils from traditional medicinal plants of asir region, saudi arabia, over drug resistant isolates. Biomed Res Int. 2019;2019:1–9. doi:10.1155/2019/8928306
59. Khosravi AR, Sharifzadeh A, Nikaein D, Almaie Z, Nasrabadi HG. Chemical composition, antioxidant activity and antifungal effects of five Iranian essential oils against Candida strains isolated from urine samples. J Mycol Med. 2018;28(2):355–360. doi:10.1016/j.mycmed.2018.01.005
60. Trindade LA, de Araújo Oliveira J, de Castro RD. de Oliveira Lima E. Inhibition of adherence of C. albicans to dental implants and cover screws by Cymbopogon nardus essential oil and citronellal. Clin Oral Investig. 2015;19(9):2223–2231. doi:10.1007/s00784-015-1450-3
61. Ramos MAS, Calixto G, Toledo LG. et al. Liquid crystal precursor mucoadhesive system as a strategy to improve the prophylactic action of Syngonanthus nitens (Bong.) Ruhland against infection by Candida krusei. Int J Nanomedicine;2015. 7455. doi:10.2147/IJN.S92638
62. Joe MM, Bradeeba K, Parthasarathi R, et al. Development of surfactin based nanoemulsion formulation from selected cooking oils: evaluation for antimicrobial activity against selected food associated microorganisms. J Taiwan Inst Chem Eng. 2012;43(2):172–180. doi:10.1016/j.jtice.2011.08.008
63. Chang Y, Mclandsborough L, Mcclements DJ. Physicochemical properties and antimicrobial efficacy of carvacrol nanoemulsions formed by spontaneous emulsification. J Agric Food Chem. 2013;61:8906–8913. doi:10.1021/jf402147p
64. Carbone C, Musumeci T. Clotrimazole-loaded mediterranean essential oils NLC: a synergic treatment of Candida skin infections. Pharmaceutics. 2019;11:231–252. doi:10.3390/pharmaceutics11050231
65. Pang J, Dong W, Li Y, et al. Oil Using macroporous resin followed by microemulsion encapsulation to improve its safety and antiviral activity. Molecules. 2017;22:1–16. doi:10.3390/molecules22020293
66. de Mota KS, de Oliveira Pereira F, de Oliveira W, Lima I, de Oliveira Lima E. Antifungal activity of thymus vulgaris l. essential oil and its constituent phytochemicals against rhizopus oryzae: interaction with ergosterol. Molecules. 2012;17(12):14418–14433. doi:10.3390/molecules171214418
67. Koba K, Sanda K, Guyon C, Raynaud C, Chaumont JP, Nicod L. In vitro cytotoxic activity of Cymbopogon citratus L. and Cymbopogon nardus L. essential oils from Togo. Bangladesh J Pharmacol. 2009;4(1):29–34.
68. Mcpherson ML, Pharm D, Cimino NM, Pharm D. Topical NSAID formulations. Pain Med. 2013;14:35–39. doi:10.1111/pme.12288
69. Radulović NS, Mladenović MZ, Blagojević PD, et al. Toxic essential oils. Part III: identification and biological activity of new allylmethoxyphenyl esters from a Chamomile species (Anthemis segetalis Ten.). Food Chem Toxicol. 2013;62:554–565. doi:10.1016/j.fct.2013.09.017
70. Martins MDR, Arantes S, Candeias F, Tinoco MT, Cruz-Morais J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J Ethnopharmacol. 2014;151(1):485–492. doi:10.1016/j.jep.2013.10.063
71. Da Silva Ramos R, Rodrigues ABL, Farias ALF, et al. Chemical composition and in vitro antioxidant, cytotoxic, antimicrobial, and larvicidal activities of the essential oil of mentha piperita L. (Lamiaceae). Sci World J. 2017;2017.
72. Déciga-Campos M, Rivero-Cruz I, Arriaga-Alba M, Gabriela Castañeda-Corral GE, Angeles-López AN, Mata R. Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. J Ethnopharmacol. 2007;110:334–342. doi:10.1016/j.jep.2006.10.001
73. Chamilos G, Lionakis MS, Lewis RE, Kontoyiannis DP. Role of mini-host models in the study of medically important fungi. Lancet Infect Dis. 2007;7(1):42–55. doi:10.1016/S1473-3099(06)70686-7
74. Break TJ, Jaeger M, Solis NV, et al. CX 3 CR1 Is dispensable for control of mucosal Candida albicans infections in mice and humans. Infect Immun. 2015;83(3):958–965. doi:10.1128/IAI.02604-14
75. França CM, Ferreira LR, Prates RA, et al. Antimicrobial photodynamic therapy as a new approach for the treatment of vulvovaginal candidiasis: preliminary results. Lasers Med Sci. 2018;33(9):1925–1931. doi:10.1007/s10103-018-2557-y
76. Pietrella D, Angiolella L, Vavala E, et al. Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement Altern Med. 2011;11(1):18. doi:10.1186/1472-6882-11-18
77. Ahmad N, Alam MK, Shehbaz A, et al. Antimicrobial activity of clove oil and its potential in the treatment of vaginal candidiasis. J Drug Target. 2005;13(10):555–561. doi:10.1080/10611860500422958
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.