Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Implications of Seizure-Cluster Treatment on Healthcare Utilization: Use of Approved Rescue Medications
Authors Rabinowicz AL, Faught E , Cook DF, Carrazana E
Received 14 June 2022
Accepted for publication 29 September 2022
Published 25 October 2022 Volume 2022:18 Pages 2431—2441
DOI https://doi.org/10.2147/NDT.S376104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Adrian L Rabinowicz,1 Edward Faught,2 David F Cook,1 Enrique Carrazana1,3
1Clinical Development & Medical Affairs, Neurelis, Inc., San Diego, CA, USA; 2Emory Epilepsy Program, Emory University School of Medicine, Atlanta, GA, USA; 3John A. Burns School of Medicine, University of Hawaii, Honolulu, HI, USA
Correspondence: Edward Faught, Department of Neurology, Emory University School of Medicine, 12 Executive Park Drive, NE, Atlanta, GA, 30329, USA, Tel +1 404 778-3444, Email [email protected]
Purpose: People with epilepsy may experience seizure clusters despite a stable regimen of antiseizure medications. Such clusters have the potential to last ≥ 24 hours, typically occur in the community setting, and may progress to medical emergencies, such as status epilepticus, if untreated. Thus, long-acting rescue therapy for seizure clusters is needed that can be administered by nonmedical individuals outside a hospital. Benzodiazepines are the foundation of rescue therapy for seizure clusters. The approved outpatient treatments (ie, diazepam, midazolam) have differing profiles that may affect multiple aspects of health-care utilization. The current labeling of these medications allows for a second dose if needed to control the cluster. Although no head-to-head studies directly comparing rescue treatments have been conducted, differences between studies with generally similar designs may provide context for the potential importance of second doses of rescue therapy on health-care utilization.
Methods: For this analysis, large, long-term, open-label studies of approved seizure-cluster treatments designed for use by nonmedical caregivers were reviewed, and the percentage of seizure clusters for which a second dose was used or that were not controlled at 6, 12, and 24 hours was examined. Available data on hospitalizations were also collected.
Results: The 3 identified studies meeting the inclusion criteria were for use of diazepam rectal gel, intranasal midazolam, and diazepam nasal spray. Across these studies, the use of a second dose ranged from < 40% at 6 hours to < 13% at 24 hours. Hospitalizations and serious treatment-emergent adverse events were reported variably across these studies.
Conclusion: These results demonstrate the importance of second doses of rescue therapy for seizure clusters for optimizing health-care utilization. Need for second doses should be included as one component. In turn, when second doses are needed, they have the potential to curtail emergency department use and hospitalization and to prevent further seizure clusters.
Keywords: diazepam, midazolam, nasal, rectal, acute repetitive seizures, twenty-four hours
Plain Language Summary
Some people with epilepsy who take daily medicine may still have seizures. Groups of these seizures might occur in clusters, and they are an emergency. These clusters are more likely to happen at home than in a hospital. If not treated, these clusters can last 24 hours or more and lead to status epilepticus. The 3 approved treatments are diazepam rectal gel, diazepam nasal spray, and intranasal midazolam. If a cluster does not stop, care partners can give a second dose of these drugs. Seizure clusters can be a burden to people with epilepsy and their families, and clusters may need an extra dose and even lead to a hospital visit. The 3 types of rescue therapy may have different needs for second doses and hospital visits. Until now, though, no studies have compared rescue therapies for these differences. We searched for large, published studies on the treatment for seizure clusters. There were 3 similar studies, with one for each drug. For these studies, we looked at the number of times second doses were used or needed at 6, 12, and 24 hours after the first dose. We found that the need for second doses was different in each study. The diazepam nasal spray study had the lowest use of second doses. The different studies described hospital visits in ways that were not directly comparable. Differences between treatments may affect epilepsy burden. Results of our comparison may help with choosing a rescue therapy.
Introduction
About 3.4 million persons in the United States live with epilepsy,1 and associated health-care spending for seizure or epilepsy was recently estimated to represent an incremental cost of $24.5 billion annually, a figure that has increased with time.2 Refractory epilepsy increases the risk of seizure emergencies and health-care utilization.3 Seizure clusters, also referred to as acute repetitive seizures, often occur outside the hospital4 and may persist for 24 hours or more.5 An empirical definition for a seizure cluster of ≥2 seizures across a period of time different from the person’s normal seizure pattern has been used in the literature.5,6 Weekly, monthly, and annual seizure cycles have also been described.7 Prompt, effective treatment of seizure clusters is critical to prevent prolonged seizures or status epilepticus.8 Untreated, seizure emergencies often require paramedic or emergency room (ER) treatment, creating a burden for the person with epilepsy, their family, and the healthcare system. Thus, seizure clusters may lead to a higher overall burden of epilepsy due to hospitalizations, reduced quality of life, and possibly increased risk of mortality.3
Benzodiazepines are the cornerstone of rescue therapy for people with epilepsy who experience seizure clusters.6 Rectal diazepam was approved by the US Food and Drug Administration (FDA) for the treatment of seizure clusters more than 20 years ago.8 However, this route of administration may raise concerns for many people with epilepsy and caregivers (eg, social acceptability) and may have substantial interperson variability in bioavailability.6,9
Intranasal administration of rescue therapy offers advantages over other routes of administration, including being more socially acceptable than rectal administration, ease of administration, and rapid onset of action.10 Nasal spray formulations of midazolam and diazepam are now FDA approved for treatment of seizure clusters.11,12
Epilepsy may be associated with a high economic burden, with estimated annual per-person direct costs due to epilepsy of nearly $8000 to more than $11,000 per person (2013).13 In particular, substantial burden is associated with lack of seizure control. For people with uncontrolled epilepsy, annual epilepsy-related overall health-care costs were $6658 and $9713 higher (2007–2009) for adults and children, respectively, when compared with adults and children whose epilepsy was controlled.14,15 The odds ratios for epilepsy-related ER usage and hospitalization were about twice as high in uncontrolled epilepsy, and were associated with 33.2% (adults) and 47.1% (children) of the additional cost.14,15 Before enrollment in a study of diazepam rectal gel, 24% of people with epilepsy who had seizure-cluster episodes required treatment by paramedics or in the ER.16 An analysis of efficacy results from that study suggested that rescue with diazepam rectal gel may reduce the need for ER treatment by ≥50% vs placebo.16
Indications and approved rescue treatments reflect regulatory differences across regions. In the United States, labeling of approved rescue medications for seizure clusters allows for a second dose, if needed to control the cluster in order to potentially reduce additional morbidity and more extensive health-care utilization. Although no head-to-head studies directly comparing rescue treatments have been conducted, the side-by-side examination of studies and use of second doses suggestive of real-world experience described here may help to form context for understanding how the use of second doses to control clusters may impact overall epilepsy cost burden.
Materials and Methods
A literature search was conducted to identify long-term open-label studies of seizure-cluster drug treatments for use in people with epilepsy by nonmedical caregivers in the community setting. Only studies of ≥100 people with epilepsy and ≥1000 treated seizure clusters with a duration of ≥12 months were to be included, in order to capture a sufficient number of clusters that were not controlled by the first dose, given the sporadic nature of the clusters.
PubMed was searched using the search string “seizure clusters AND open label AND treatment”, which returned 31 records (Figure 1). Article titles were then screened for appropriateness; 19 records were excluded for not reporting the following: treatment for epilepsy, people with epilepsy, original research, seizure clusters, drug treatment, and nonintravenous treatment. Full-text article contents were reviewed for the remaining 12 records; 9 of these articles were excluded: 6 that were secondary reports from studies with published primary results, 2 that included <100 people with epilepsy, and 1 because the treatment was subsequently approved for an indication other than seizure clusters.
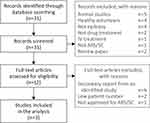 |
Figure 1 PRISMA diagram for literature search via PubMed. Adapted from Page MJ, McKenzie, JE, Bossuyt, PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;n71.17 Abbreviations: ARS, acute repetitive seizures; IV, intravenous; SC, seizure cluster. |
The remaining 3 studies were reviewed to gather details, including initial rescue dose as well as requirement for a second rescue dose. Other data collected included the following:
- Number of doses given (if second dose not available per protocol, lack of control after first dose was used as a proxy for need for a second dose; this is explained in further detail as relevant to the results from one of the studies).
- Characteristics of participant populations.
- Available information on serious treatment-emergent adverse events (TEAEs) and overall relationship to study drug.
Brief details on the history of the rescue therapies, study design, and general study results were summarized to provide context for the results of the analysis.
Analysis
This analysis summarizes the proportion of treated seizure clusters that require a second dose. The percentage of seizure clusters was calculated based on number of initial doses of treatment compared with clusters requiring a second dose before 6, 12, and 24 hours, and relative number needed to treat across the different formulations was calculated. No adjustment was made for baseline hospitalization rates or severity of epilepsy.
Results
Three studies met the inclusion criteria.16,18,19 These studies evaluated diazepam rectal gel, intranasal midazolam, and diazepam nasal spray. Although these studies looked at different time points for use of second doses, the designs of the 3 studies were generally similar, enrolling populations with highly intractable epilepsy and seizure clusters, and their indications in the United States are identical,20–22 allowing for side-by-side evaluation. However, there were some differences such as age range, inclusion of concomitant benzodiazepines, and exclusion owing to a history of status epilepticus. The diazepam rectal gel study included 149 people with epilepsy who were treated for 1578 total seizure clusters; duration of treatment ranged from 2 months to 2.5 years, with 48% on the study for more than 2 years.16 In the intranasal midazolam study, 161 people were treated for 1998 total seizure clusters, and the median duration on the study was 16.8 months.19 In the diazepam nasal spray study, 163 people were treated for 3853 total seizure clusters, and the median duration on the study was 15.0 months.18 None of these studies addressed individual responsiveness to treatment, precluding this level of analysis.
Diazepam Rectal Gel
Diazepam rectal gel was approved by the FDA in 1997 and is intended for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from the person’s usual seizure pattern in people with epilepsy 2 years of age and older.20 It became the mainstay of rescue therapy for seizure clusters outside the hospital setting in the United States.8,20 Owing to social concerns about the route of administration, this formulation is more likely to be used for younger children rather than adults or older children.23
People with epilepsy in the diazepam rectal gel study were aged 2 to 76 years; 48.3% were 11 years or younger (median age not reported).16 Included participants had been enrolled in 1 of 3 previous studies of diazepam rectal gel and had not been terminated from that study owing to an adverse event (AE). Participants were excluded if they had habitual progression to status epilepticus despite intervention or significant psychiatric disorder. Caregivers of people with epilepsy enrolled in the study were instructed on the administration of diazepam rectal gel, which was provided in a prefilled single-use syringe. The participant’s dose of study drug was prescribed by a physician based on age and weight: 2 to 5 years, 0.5 mg/kg; 6 to 11 years, 0.3 mg/kg; adults (12–76), 0.2 mg/kg. Caregivers were provided with a booklet in which to record the episode treated, respiratory rates for 4 hours, and seizure control and AEs for 12 hours.
Because only 1 of the previous source studies had required multiple doses of diazepam rectal gel (2 for children, 3 for adults) per episode (N = 125, diazepam rectal gel vs placebo),24 there was limited provision for a second dose of diazepam rectal gel in this study.16 If the investigator determined it was necessary for treating the episode, participants continuing from that earlier study were allowed 2 doses, 4 hours apart. Other participants received a single dose for each episode.16 Current labeling permits a second dose.20
Intranasal Midazolam
Intranasal midazolam was approved by the FDA in 2019 for acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from a person’s usual seizure pattern.11,21 It is approved for use in people with epilepsy aged 12 years and older.21
People with epilepsy in the intranasal midazolam study were aged 12 to 62 years, with 5.0% younger than 18 years (mean age, 32.9 years).19 Included participants had completed a prior study evaluating the safety and efficacy of intranasal midazolam for outpatient treatment of seizure clusters (N = 292, intranasal midazolam vs placebo), and >75% of participants had focal seizures.25 Participants were on stable antiseizure-drug regimens but were excluded if they had status epilepticus due to cluster progression or a major depressive episode, psychosis, or active suicidality.19 Caregivers of people with epilepsy enrolled in this study received a treatment kit with 2 doses (ie, first dose and, if needed, a second dose) of 5 mg intranasal midazolam at their first study visit. The 5-mg dose was used for all participants regardless of age or weight. A trial workbook was provided to caregivers in which to record seizure events. Caregivers were to use a single dose for treating a seizure-cluster episode; a second dose could be used if the seizure cluster lasted more than 10 minutes or another seizure happened 10 minutes to 6 hours after the initial dose, provided the person with epilepsy did not require emergency treatment. Success was assessed as seizure termination within 10 minutes and no seizure recurrence 10 minutes to 6 hours after drug administration. In cases in which seizure activity persisted beyond use of a second dose, caregivers were instructed to begin rescue protocol per the person’s individualized management plan.19
Diazepam Nasal Spray
Diazepam nasal spray was approved by the FDA in 2020 for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from a person’s usual seizure pattern in people with epilepsy.12,22 It is approved for use in people with epilepsy aged 6 years and older.22 Compared with diazepam rectal gel, the FDA states that the route of administration for diazepam nasal spray makes it less invasive, more comfortable, and easier to administer.26
People with epilepsy in the diazepam nasal spray study were aged 6 to 65 years, with 27.6% younger than 12 years (mean age, 23.1 years).18 Included participants were on a stable antiseizure-drug regimen and had an anticipated need for benzodiazepine intervention for seizure control on average 6 times per year according to investigator opinion, and a history of status epilepticus or concomitant benzodiazepine use was permitted. Participants could not have active major depression, past suicide attempt, or history of suicidal ideation.18 Participation in a prior study of diazepam nasal spray was not required, and people with epilepsy and caregivers were trained in the proper administration of the diazepam nasal spray device at screening and as needed. Dose was based on the person’s age (6–11, 0.3 mg/kg; ≥12 years, 0.2 mg/kg) and weight, as 5, 10, 15, or 20 mg, with the 5- and 10-mg doses administered as 1 spray, and the 15- and 20-mg doses as 1 spray in each nostril. A diary was to be used to record seizures and administration of diazepam nasal spray. People with epilepsy and caregivers were instructed that a second dose could be administered, if needed, 4 to 12 hours after the initial dose for a seizure cluster.18 Use of second doses was assessed at 6, 12, and 24 hours.28,29
Data from these 3 studies are outlined in Table 1. Across the studies, median treatment durations ranged from ~1 to 2 years. Cohort age ranges differed, particularly with regard to the inclusion of pediatric participants. Calculated frequency of seizure episodes per person per year varied, with approximately twice as many in the diazepam nasal spray study than in the other studies.
 |
Table 1 Summary of Data from the Included Seizure-Cluster Studies |
Observation of Second-Dose Administration
Use of a second dose appeared to vary substantially across benzodiazepines and formulations. The proportions of seizure clusters for which no second dose was needed at 6, 12, and 24 hours is illustrated for the 3 studies in Figure 2. Because of the differences in studies regarding the timing of recording second doses, all studies are not represented for all time periods. At 6 and 12 hours, diazepam nasal spray had a higher percentage of seizure clusters controlled with a single dose compared with the other study represented in the time period, and only the diazepam nasal spray study reported results for 24 hours.18
 |
Figure 2 Seizure episodes treated with only one dose for control at 6, 12, and 24 hours. aIn the midazolam study, an additional 28 doses were administered more than 6 hours after the first dose. |
Seizure episode data at 6, 12, and 24 hours are reported as these were available for the 3 studies for first doses, second doses, and mean number of doses per episode (Table 2). For 6 and 12 hours, the mean number of doses was lower in both time periods for diazepam nasal spray compared with the other study represented in the time period. Based on the proportion of seizure clusters treated with a second dose at 6 hours, participants receiving midazolam nasal spray needed an average of 1.38 doses per cluster, while participants receiving diazepam nasal spray received and average of 1.06 doses per cluster, and, relative to midazolam nasal spray, the number needed to treat with diazepam nasal spray to prevent need for 1 second dose was 3.1. Likewise, at 12 hours, relative to diazepam rectal gel, the number needed to treat with diazepam nasal spray to prevent need for 1 second dose was 6.7.
 |
Table 2 Number of Doses Used per Seizure Episode at 6, 12, and 24 Hours |
Hospitalizations and Serious TEAEs
Hospitalizations and serious TEAEs may sometimes occur when a seizure cluster is not controlled by the second dose, and these measures were reported variably across the studies. In the rectal diazepam study,16 16 of 363 seizure clusters were subsequently treated in the ER. As previously noted, there was no provision for a second dose in this study, and not all hospitalizations were due to seizures. Serious TEAEs were not specifically reported; 2 deaths were not considered related to diazepam rectal gel.16
In the intranasal midazolam study,19 hospitalizations were not reported. Four participants each had 1 serious TEAE categorized as possibly (although unlikely) treatment related (convulsion, status epilepticus, dysesthesia, and upper gastrointestinal hemorrhage). Eighteen (11.2%) participants had a total of 45 serious TEAEs; most common were seizure cluster and convulsion in 4 people (2.5%) each, and epilepsy and status epilepticus in 2 people (1.2%) each.19
In the diazepam nasal spray study, hospitalizations were not summarized in the published paper.18 No serious TEAEs were considered treatment related. One participant died (not treatment related). Serious TEAEs occurred in 50 participants (30.7%), and those occurring in ≥2 participants were seizure in 24 people (14.7%); status epilepticus and pneumonia in 7 people (4.3%) each; epilepsy in 5 people (3.1%); and seizure cluster, pancreatitis, vomiting, pyrexia, aspiration pneumonia, anxiety, and scoliosis in 2 people (1.2%) each. Overall, 44 people (27.0%) had serious TEAEs that required or prolonged hospitalization.18
Discussion
This is a review of large, long-term studies of FDA-approved seizure-cluster treatments designed for use by nonmedical caregivers outside the hospital setting. These were all open-label studies, and participants could withdraw at any time. The duration of exposure in all studies is suggestive that the medications were viewed as effective, so people with epilepsy and their care partners would have had an incentive to adhere to treatment recommendations. Percentages of clusters controlled by the initial dose were compared at 6, 12, or 24 hours, with duration of activity and need for a second dose varying substantially among the studies. In the diazepam rectal gel study, 77% of administrations prevented further seizures in 12 hours. In the midazolam study, a second dose was not administered within 6 hours in 61.5% of administrations. In the diazepam nasal spray study, second doses were not administered for 94.2% in 6 hours, 91.7% in 12 hours, and 87.4% in 24 hours.
Available data on hospitalizations were also collected. In the diazepam rectal gel study, 16 seizure clusters were subsequently treated in the ER. In the intranasal midazolam study, 4 participants each had 1 serious AE categorized as possibly (unlikely) treatment related; possible association with second dose was not reported. In the diazepam nasal spray study, no serious AEs were treatment related; overall, 27.0% of participants had serious TEAEs that required or prolonged hospitalization.
All 3 study populations had highly intractable epilepsy and seizure clusters, but specific inclusion and exclusion criteria varied. Enrollment requirements for the 3 studies selected participants with seizure clusters; however, differences in inclusion/exclusion criteria (eg, history of status epilepticus) likely contributed to between-study differences in seizure cluster prevalence. The rectal diazepam and diazepam nasal spray studies included young pediatric participants (ie, ≥2 and ≥6 years, respectively), while the intranasal midazolam study included participants aged 12 years and older. In the midazolam study, people were ineligible if they used benzodiazepine antiepileptic medication maintenance therapy or status epilepticus due to cluster progression within 2 years,25 potentially due to concerns about respiratory depression, while these criteria did not result in exclusion from the other 2 trials. In addition, participants in the diazepam nasal spray appear to have had roughly twice as many seizure clusters per year, suggestive of a high degree of intractability in that population. Notably, the diazepam nasal spray study included children with developmental and epileptic encephalopathies (30 of 78 patients 6–17 years of age received concomitant clobazam30), while a substantial majority of the intranasal midazolam population were adults with a focal form of seizures,19 suggesting that the diazepam nasal spray population may have had a higher level of seizure intractability. Although people with allergic rhinitis were not excluded from the midazolam study, the impact of this condition was not formally evaluated.19 In the diazepam nasal spray study, exploratory analyses of the groups taking concomitant benzodiazepines, with higher and lower frequencies of usage, and having a history of seasonal allergies/rhinitis found that use of second doses was consistent with the overall study.18,27,31,32
Seizure clusters may persist 24 hours or longer, as was shown in a study of the seizure diaries of 1177 people with epilepsy and seizure clusters, in which 58.5% documented a seizure occurring 6 to 24 hours after the initial seizure.5 Thus, rescue therapies for seizure clusters that provide long-lasting activity (eg, 6, 12, 24 hours) are necessary, and formulation-specific characteristics including bioavailability (midazolam nasal spray, 44%; diazepam rectal gel, 90%; diazepam nasal spray, 97%)20–22 should be considered. However, few data are available in the literature for rescue therapies across these time periods after the start of the cluster. Thus, this review, which examines rescue therapy dosing of seizure clusters for the FDA-approved medications at 6, 12, and 24 hours, provides data to address that gap.
Route of administration and formulation of rescue therapies may have roles in effectiveness and use of a second dose. Although diazepam rectal gel has well-established safety and efficacy,33 diazepam nasal spray is less intrusive and offers advantages for caregivers and people with epilepsy with comparable safety and pharmacokinetics.9,34–37 The pharmacokinetic profiles of midazolam and diazepam differ, and there are also differences in their intranasal formulations that potentially may contribute to differing levels of effectiveness in individuals over time. The midazolam intranasal formulation includes several organic solvents including ethanol, PEG-6 methyl ether, polyethylene glycol 400, and propylene glycol.21 The diazepam nasal spray formulation includes dodecyl maltoside (Intravail® A3; 0.25% w/v), an alkylsaccharide, and vitamin E.10,22,38
Limitations
This review is limited by the number of studies fulfilling the inclusion criteria at the time of the search. There is no clear definition of seizure clusters,39 and it is not defined by the International League Against Epilepsy as a specific syndrome.40 The studies included in this review were all based in the United States, where regulatory authorities recognize seizure clusters as an entity. Also, comparing the included studies may be difficult because of unrecognized differences in populations of people with epilepsy, including severity of epilepsy and historic duration of seizure clusters, availability of second doses, study designs, and how seizure clusters were defined in each study. Two of the studies included people with epilepsy who had completed a prior study for the rescue therapy (ie, rectal diazepam and intranasal midazolam); these populations may have been more tolerant to the study medication than those in the general epilepsy population. All studies required subjects have seizure clusters; however, the likelihood that a treated seizure would have resulted in a seizure cluster might have varied between studies. Additionally, the number of treated seizure clusters differed (~5 seizure clusters per person per year in the diazepam rectal gel study, ~9 in the midazolam nasal spray study, and ~19 in the diazepam nasal spray study).
In addition, data relied on diary entries by participants and caregivers, reporting only seizure clusters treated with the study drug. Furthermore, the reports of these studies did not provide details on any changes to the daily antiseizure medications, seizure triggers if the cause could be determined, or type of seizures in the clusters. However, this review does address parallel outcomes between the 3 approved rescue therapies for seizure clusters that are available for use by nonmedical individuals outside the hospital setting, and this preliminary study provides the foundation for further research with existing databases and real-world data.
Conclusions
Beyond the initial dose of rescue therapy treatment for seizure clusters, health-care utilization analysis should include a second dose and even hospitalization. Because seizure clusters may last 24 hours or longer, long-acting rescue therapies are needed to maximize control over an extended period of time with single doses. When needed for additional control, second doses may contribute to prevention of further episodes—and need for related health-care utilization—in a seizure cluster.
Across the noncomparative open-label studies reviewed here, the need for a second dose with the approved agents ranged from <40% at 6 hours to <10% at 12 hours. Differences among approved therapies and routes of administration appear to have the potential to affect healthcare burden and should be considered when selecting rescue therapy for treatment of seizure clusters.
Abbreviations
AE, adverse event; ARS, acute repetitive seizure; ER, emergency room; FDA, US Food and Drug Administration; IV, intravenous; SC, seizure cluster; TEAE, treatment-emergent adverse event.
Acknowledgments
Medical writing support was provided at the direction of the authors by Laura J. Herold, MA, of The Curry Rockefeller Group, LLC (Tarrytown, NY), which also provided additional editorial assistance including formatting and proofreading. This support was funded by Neurelis, Inc. (San Diego, CA).
Portions of this paper were presented at the Academy of Managed Care Pharmacy 2020 Annual Meeting, the Academy of Managed Care Pharmacy Nexus 2020, and the American Epilepsy Society 2020 Annual Meeting as poster presentations with interim findings. Final results were presented at the American Academy of Neurology 2021 Annual Meeting and the 2021 International Epilepsy Congress as poster presentations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Neurelis, Inc.
Disclosure
Drs Rabinowicz and Cook are employees of and have received stock options from Neurelis, Inc. Dr Faught has been a member of scientific advisory boards for Eisai Ltd., Neurelis, Inc., SK Life Science, Sage Pharmaceuticals, and Biogen, and has received research support from UCB Pharma. Dr Carrazana is an employee of and has received stock and stock options from Neurelis, Inc. The authors report no other conflicts of interest in this work.
References
1. Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821–825. doi:10.15585/mmwr.mm6631a1
2. Moura L, Karakis I, Zack MM, Tian N, Kobau R, Howard D. Drivers of US health care spending for persons with seizures and/or epilepsies, 2010–2018. Epilepsia. 2022;63 (8) :2144–2154. doi:10.1111/epi.17305
3. Jafarpour S, Hirsch LJ, Gainza-Lein M, Kellinghaus C, Detyniecki K. Seizure cluster: definition, prevalence, consequences, and management. Seizure. 2019;68:9–15. doi:10.1016/j.seizure.2018.05.013
4. Maglalang PD, Rautiola D, Siegel RA, et al. Rescue therapies for seizure emergencies: new modes of administration. Epilepsia. 2018;59(suppl 2):207–215. doi:10.1111/epi.14479
5. Fisher RS, Bartfeld E, Cramer JA. Use of an online epilepsy diary to characterize repetitive seizures. Epilepsy Behav. 2015;47:66–71. doi:10.1016/j.yebeh.2015.04.022
6. Penovich PE, Buelow J, Steinberg K, Sirven J, Wheless J. Burden of seizure clusters on patients with epilepsy and caregivers: survey of patient, caregiver, and clinician perspectives. Neurologist. 2017;22(6):207–214. doi:10.1097/NRL.0000000000000140
7. Leguia MG, Andrzejak RG, Rummel C, et al. Seizure cycles in focal epilepsy. JAMA Neurol. 2021;78(4):454–463. doi:10.1001/jamaneurol.2020.5370
8. Haut SR. Seizure clusters: characteristics and treatment. Curr Opin Neurol. 2015;28(2):143–150. doi:10.1097/WCO.0000000000000177
9. Hogan RE, Gidal BE, Koplowitz B, Koplowitz LP, Lowenthal RE, Carrazana E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61(3):455–464. doi:10.1111/epi.16449
10. Cloyd J, Haut S, Carrazana E, Rabinowicz AL. Overcoming the challenges of developing an intranasal diazepam rescue therapy for the treatment of seizure clusters. Epilepsia. 2021;62(4):846–856. doi:10.1111/epi.16847
11. Epilepsy Foundation. FDA news: Nayzilam (midazolam) nasal spray approved for seizure clusters. Available from: https://www.epilepsy.com/article/2019/5/fda-news-nayzilam-midazolam-nasal-spray-approved-seizure-clusters.
12. Epilepsy Foundation. New drug: Valtoco (diazepam nasal spray) approved as rescue therapy for seizures. Available from: https://www.epilepsy.com/article/2020/1/new-drug-valtoco-diazepam-nasal-spray-approved-rescue-therapy-seizures.
13. Begley CE, Durgin TL. The direct cost of epilepsy in the United States: a systematic review of estimates. Epilepsia. 2015;56(9):1376–1387. doi:10.1111/epi.13084
14. Cramer JA, Wang ZJ, Chang E, et al. Healthcare utilization and costs in adults with stable and uncontrolled epilepsy. Epilepsy Behav. 2014;31:356–362. doi:10.1016/j.yebeh.2013.09.046
15. Cramer JA, Wang ZJ, Chang E, et al. Healthcare utilization and costs in children with stable and uncontrolled epilepsy. Epilepsy Behav. 2014;32:135–141. doi:10.1016/j.yebeh.2014.01.016
16. Mitchell WG, Conry JA, Crumrine PK, et al. An open-label study of repeated use of diazepam rectal gel (Diastat) for episodes of acute breakthrough seizures and clusters: safety, efficacy, and tolerance. North American Diastat Group. Epilepsia. 1999;40(11):1610–1617. doi:10.1111/j.1528-1157.1999.tb02047.x
17. Page MJ, McKenzie, JE, Bossuyt, PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;n71. doi:10.1136/bmj.n71
18. Wheless JW, Miller I, Hogan RE, et al. Final results from a Phase 3, long-term, open-label, repeat-dose safety study of diazepam nasal spray for seizure clusters in patients with epilepsy. Epilepsia. 2021;62(10):2485–2495. doi:10.1111/epi.17041
19. Wheless JW, Meng TC, Van Ess PJ, Detyniecki K, Sequeira DJ, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters: an open-label extension trial. Epilepsia. 2019;60(9):1809–1819. doi:10.1111/epi.16300
20. Diastat® C-IV (diazepam rectal gel) [prescribing information]. Bridgewater, NJ: Bausch Health US, LLC; 2021.
21. NAYZILAM® (midazolam nasal spray) [prescribing information]. Smyrna, GA: UCB, Inc; 2021.
22. VALTOCO® (diazepam nasal spray) [prescribing information]. San Diego, CA: Neurelis, Inc; 2022.
23. Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105(3):362–367. doi:10.1016/j.eplepsyres.2013.02.018
24. Dreifuss FE, Rosman NP, Cloyd JC, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med. 1998;338(26):1869–1875. doi:10.1056/NEJM199806253382602
25. Detyniecki K, Van Ess PJ, Sequeira DJ, Wheless JW, Meng TC, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters-a randomized, double-blind, placebo-controlled trial. Epilepsia. 2019;60(9):1797–1808. doi:10.1111/epi.15159
26. US Food and Drug Administration. Clinical superiority findings. Available from: https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/clinical-superiority-findings.
27. Segal EB, Tarquinio D, Miller I, et al. Evaluation of diazepam nasal spray in patients with epilepsy concomitantly using maintenance benzodiazepines: an interim subgroup analysis from a phase 3, long-term, open-label safety study. Epilepsia. 2021;62(6):1442–1450. doi:10.1111/epi.16901
28. Sperling MR, Wheless JW, Hogan RE, et al. Use of second doses of Valtoco® (diazepam nasal spray) across 24 hours after the initial dose for out-of-hospital seizure clusters: results from a phase 3, open-label, repeat-dose safety study. Epilepsia. 2022;63(4):836–843. doi:10.1111/epi.17177
29. Cascino GD, Tarquinio D, Wheless JW, et al. Lack of clinically relevant differences in safety and pharmacokinetics after second-dose administration of intranasal diazepam within 4 h for acute treatment of seizure clusters: a population analysis. Epilepsia. 2022;63(7):1714–1723. doi:10.1111/epi.17249
30. Tarquinio D, Dlugos D, Wheless JW, Desai J, Carrazana E, Rabinowicz AL. Safety of diazepam nasal spray in children and adolescents with epilepsy: results from a long-term Phase 3 safety study. Pediatr Neurol. 2022;132:50–55. doi:10.1016/j.pediatrneurol.2022.04.011
31. Miller I, Wheless JW, Hogan RE, et al. Consistent safety and tolerability of Valtoco® (diazepam nasal spray) in relationship to usage frequency in patients with seizure clusters: interim results from a phase 3, long-term, open-label, repeat-dose safety study. Epilepsia Open. 2021;6(3):504–512. doi:10.1002/epi4.12494
32. Vazquez B, Wheless J, Desai J, Rabinowicz AL, Carrazana E. Lack of observed impact of history or concomitant treatment of seasonal allergies or rhinitis on repeated doses of diazepam nasal spray administered per seizure episode in a day, safety, and tolerability: interim results from a phase 3, open-label, 12-month repeat-dose safety study. Epilepsy Behav. 2021;118:107898. doi:10.1016/j.yebeh.2021.107898
33. Garnett WR, Barr WH, Edinboro LE, Karnes HT, Mesa M, Wannarka GL. Diazepam autoinjector intramuscular delivery system versus diazepam rectal gel: a pharmacokinetic comparison. Epilepsy Res. 2011;93(1):11–16. doi:10.1016/j.eplepsyres.2010.10.001
34. Penovich P, Wheless JW, Hogan RE, et al. Examining the patient and caregiver experience with diazepam nasal spray for seizure clusters: results from an exit survey of a phase 3, open-label, repeat-dose safety study. Epilepsy Behav. 2021;121(Pt A):108013. doi:10.1016/j.yebeh.2021.108013
35. Higdon LM, Sperling MR. A review of a diazepam nasal spray for the treatment of acute seizure clusters and prolonged seizures. Expert Rev Neurother. 2021;21(11):1207–1212. doi:10.1080/14737175.2021.1965880
36. Hogan RE, Tarquinio D, Sperling MR, et al. Pharmacokinetics and safety of VALTOCO (NRL-1; diazepam nasal spray) in patients with epilepsy during seizure (ictal/peri-ictal) and nonseizure (interictal) conditions: a Phase 1, open-label study. Epilepsia. 2020;61(5):935–943. doi:10.1111/epi.16506
37. Tanimoto S, Simons FE, Lockey R, Lieberman P, Kaliner M, Lowenthal R. A phase 1, five-period, five-treatment, randomized crossover study of the pharmacokinetics (PK) and pharmacodynamics (PD) of epinephrine after administration of intranasal (IN) ARS-1 and intramuscular (IM) epinephrine to healthy volunteers. J Allergy Clin Immunol. 2020;145(2):AB77. doi:10.1016/j.jaci.2019.12.668
38. Rabinowicz AL, Carrazana E, Maggio ET. Improvement of intranasal drug delivery with Intravail® alkylsaccharide excipient as a mucosal absorption enhancer aiding in the treatment of conditions of the central nervous system. Drugs R D. 2021;21(4):361–369. doi:10.1007/s40268-021-00360-5
39. Buchhalter J, Shafer PO, Buelow JM, et al. Preferred practices for rescue treatment of seizure clusters: a consensus-driven, multi-stakeholder approach. Epilepsy Behav. 2021;117:107836. doi:10.1016/j.yebeh.2021.107836
40. Wirrell EC, Nabbout R, Scheffer IE, et al. Methodology for classification and definition of epilepsy syndromes with list of syndromes: report of the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1333–1348. doi:10.1111/epi.17237
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
