Back to Journals » Journal of Inflammation Research » Volume 8
Implications of compromised zinc status on bone loss associated with chronic inflammation in C57BL/6 mice
Authors Chongwatpol P, Rendina-Ruedy E, Stoecker B, Clarke S, Lucas E, Smith B
Received 5 February 2015
Accepted for publication 17 April 2015
Published 13 July 2015 Volume 2015:8 Pages 117—128
DOI https://doi.org/10.2147/JIR.S82261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Pitipa Chongwatpol, Elizabeth Rendina-Ruedy, Barbara J Stoecker, Stephen L Clarke, Edralin A Lucas, Brenda J Smith
Department of Nutritional Sciences, Oklahoma State University, Stillwater, OK, USA
Abstract: Compromised zinc status and chronic inflammation are independent factors that can contribute to bone loss. However, zinc's role in regulating lymphoid and myeloid cell populations, combined with the interplay between the immune and skeletal systems raises the question as to the extent to which a low-grade inflammatory challenge in the context of marginal zinc deficiency would exacerbate bone loss. To address this question, young adult C57BL/6 male mice (n=32) were used in a 2×2 factorial design with dietary zinc (adequate or 35 ppm vs inadequate or −Zn =5 ppm) and lipopolysaccharide (LPS, 0 or 0.1 mg/kg body weight). Mice were fed their respective diets for 10 weeks. On the 6th week, mice had a slow release pellet implanted to induce a low-grade inflammation for the final 4 weeks of the study. −Zn induced a decrease in total white cell counts and peripheral lymphocytes, whereas LPS increased blood monocytes. LPS significantly reduced spine bone mineral density and trabecular bone volume and number of the vertebral body compared with both zinc adequate and inadequate without LPS groups. Likewise, the most pronounced effects on bone strength occurred with LPS, however, −Zn also had negative effects on the bone von Mises stresses. LPS induced an increase in TNF-α and this response was further increased with −Zn. Although the marginal zinc deficiency altered immune function, bone loss was not exacerbated with low-grade chronic inflammation in marginally zinc-deficient young adult mice. These findings demonstrate that in young adult animals an immune challenge modestly increases the inflammatory response and worsens bone biomechanics in the context of a marginal zinc deficiency, but not to the extent that more severe adverse outcomes are observed on bone structural parameters.
Keywords: chronic inflammation, zinc deficiency, osteoporosis
Introduction
According to recent National Health and Nutrition Examination Survey data, approximately 54 million Americans have osteoporosis or osteopenia.1 An important element of reducing lifetime risk for osteoporosis is achieving an optimal peak bone mass early in life. Major factors that influence peak bone mass include genetics and lifestyle factors such as weight-bearing activity and nutritional status.2 The emphasis on nutrition in promoting optimal bone mass has focused primarily on calcium and phosphorus, which form the main mineral constituents of bone’s hydroxyapatite, and vitamin D due to its calciotropic effects. However, a number of trace elements such as zinc, copper, and iron are required for the catabolic and anabolic phases of bone metabolism by osteoclasts and osteoblasts, respectively. In particular, failure to obtain adequate dietary zinc has been shown to increase the risk of compromised bone mineral density (BMD), impaired immune function, and increased risk of infection.3,4 Other than inadequate intake, zinc status can be compromised due to increased requirements for zinc, impaired intestinal absorption, and increased losses of zinc.5–7 There are certain groups who are at risk of developing zinc deficiency such as pregnant6 and lactating women,7 vegetarians,8 alcoholics, individuals with diseases (eg, gastrointestinal diseases, hemolytic anemias, renal diseases, and malabsorption syndromes),5 and young growing children.9
Functionally, zinc has catalytic and regulatory roles that are important for bone growth and mineralization, collagen synthesis, and bone resorption.10 The most well-known function of zinc in skeletal tissue is as a cofactor for alkaline phosphatase (ALP), a zinc metalloenzyme essential for bone mineralization. The relative abundance of bone ALP messenger (m)RNA and ALP activity have been reported to decrease in zinc-deficient rodents.11 Furthermore, in vitro human osteoblast-like cells supplemented with zinc exhibit increased activity and function as indicated by increased ALP activity and calcified nodule formation.12 Zinc also stimulates collagen synthesis and insulin-like growth factor-I expression in bone, which provides further insight into the reduction in bone formation (ie, reduced osteoid) that occurs with zinc deficiency.11,13 In addition to its effects on osteoblast’s bone forming activity, zinc is also involved in osteoclast activity. The activities of matrix metalloproteinases 2 and 9 and carbonic anhydrase-II decreased with increased zinc intake in young male rats, resulting in decreased osteoclastic resorption.14
Animal models can potentially provide insights into the physiological effects of compromised zinc status on bone, but one of the challenges has been the significant decrease in food intake that occurs in rodents consuming a zinc-deficient diet.15–17 In previous studies,16,17 severe zinc deficiency (<2 ppm in the diet) significantly decreased food intake. Despite pair feeding, this decrease in food intake can have negative consequences on bone growth and metabolism due to deficits in other nutrients (eg, calcium, magnesium, and potassium) and can confound the interpretation of the findings in relation to zinc. To address this issue, Scrimgeour et al set out to develop a model of marginal zinc deficiency. The investigators fed young rats a zinc inadequate diet (ie, 5 ppm) for 45 days and induced a marginal zinc deficiency without negatively affecting food intake.15 They reported that rats experiencing marginal zinc deficiency had compromised bone biomechanical properties (ie, lower load to failure) but no differences were detected in whole body BMD in the marginally zinc-deficient animals compared with control animals.15
In addition to the direct effects of zinc on bone metabolism, this trace element also plays a key role in both adaptive and innate immunity.18 Suboptimal zinc in young animals produces thymic atrophy (70%–80%) along with a reduction in the CD4+ and CD8+ T cells by 38%.19 Likewise, a significant decline in pre- and immature B cells has been observed in adult mice with zinc deficiency.20 Monocyte populations in bone marrow have been reported to be increased by 70% in marginal zinc-deficient mice compared with the cohort on the zinc adequate diet.19 Zinc deficiency also resulted in the deterioration in macrophage function in 6-week-old mice.21 Individuals with zinc deficiency have been reported to experience impaired immunity and increased susceptibility to infections.18
Alterations in immune cell function induced with zinc deficiency have been characterized by an increase in T-cell and monocyte activation and cytokine production.18,22,23 T-cell activation in zinc-deficient individuals is reported to be normalized with zinc supplementation.22 In vitro, monocyte-macrophages exposed to low levels of zinc upregulate interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α expression.23 Chronic elevation of these pro-inflammatory cytokines can disrupt bone remodeling and ultimately lead to bone loss by increasing osteoclast activity and/or decreasing osteoblast activity.24 In particular, TNF-α directly induces the differentiation of monocytes into pre-osteoclasts.25 Furthermore, TNF-α, receptor activator of NF-κB ligand, IL-1β, and lipopolysaccharide (LPS) can activate NF-κB in pre-osteoclasts and upregulate nuclear factor of activated T cells c1, a key transcription factor in osteoclast differentiation.26,27 In addition to promoting an increase in bone resorption, pro-inflammatory cytokines also alter osteoblast differentiation and function. For example, TNF-α inhibits osteoblast gene expression of RUNX2, a transcriptional factor associated with osteoblast differentiation,27,28 and activates the NF-κB pathway, which decreases osteoblast function.29 Given the role that zinc plays in immune function and bone metabolism, it raises the question of whether or not marginal zinc deficiency would exacerbate the effects of an immune challenge on bone health. Such a scenario would be anticipated clinically in both adults and young children who have inadequate zinc intake and then experience a secondary immune challenge (eg, infection or a chronic inflammatory state).
Our laboratory has previously developed a model of very low-grade chronic inflammation using a slow release pellet impregnated with LPS to examine the effects of low-grade chronic inflammation on bone.30–32 We showed that the chronic inflammatory state characterized by an increase in TNF-α in the bone decreased bone mineral content (BMC), BMD, and the trabecular bone compartment was primarily affected.31 Importantly, this chronic inflammatory state does not alter food intake and the animals show no alterations in grooming behavior or clinical signs of infection. Previous studies examined the effects of zinc deficiency or an inflammatory state alone on bone in young growing animals, but to date no studies have examined whether marginal zinc deficiency primes the immune system so that an exacerbated response occurs. Therefore, this study was designed to test the hypothesis that low-grade chronic inflammation in the context of marginal zinc deficiency exarcerbates bone loss in young growing C57BL/6 mice.
Materials and methods
Animal care
After a 7-day acclimation period, 8-week-old C57BL/6 male mice (n=32; Charles River Laboratories, Wilmington, MA, USA) were randomly assigned to either zinc adequate (35 ppm zinc = control diet or Con) or zinc inadequate diet (5 ppm zinc = −Zn) for a 10-week study. All diets were adjusted for macronutrient and micronutrient content with the exception of zinc. Beginning the final 4 weeks of the 10-week period, mice were exposed to LPS either 0 (−LPS) or 0.1 mg LPS/kg body weight/day (+LPS) by subcutaneously implanted slow release pellets (Innovative Research of America, Saratosa, FL, USA). All procedures were approved by and strictly adhered to the guidelines set forth by the Institutional Animal Care and Use Committee at Oklahoma State University.
Throughout the study, food intake was monitored and body weights were recorded weekly. At the end of the 10-week experiment, the mice were anesthetized using a ketamine/xylazine cocktail (60 mg ketamine and 6 mg xylazine/kg body weight), administered by an intraperitoneal injection. Whole body dual-energy X-ray absorptiometry (DXA) scans (Lunar PIXImus; GE Medical System, Madison, WI, USA) were performed for determination of bone density and body composition. A sample of whole blood (25 μL) was taken from the carotid artery for total white cell counts. Whole blood smears were prepared, fixed in methanol, and stained with CAMCO Stain Pak (Cambridge Diagnostic Products, Fort Lauderdale, FL, USA) for differential counts (ie, lymphocytes, neutrophils, monocytes, basophils, and eosinophils). The remaining blood was collected in ethylenediaminetetraacetic acid-coated tubes, centrifuged at 730× g, 4°C for 20 minutes, and plasma aliquots were stored at −80°C. Spleen and thymus specimens were harvested and their weights were recorded. Livers were snap frozen in liquid nitrogen, and then stored at −80°C. Tibias were cleaned of soft tissues and were fixed in 10% neutral buffered formalin and the spines were trimmed of soft tissues and stored at −20°C.
Bone densitometry assessment
Whole body BMD, BMC, and bone mineral area (BMA) were determined using PIXImus Series Software (version 1.4x) from whole body DXA scans that were performed at the end of the study. Excised spine specimens were also scanned to assess the lumbar spine (ie, fourth and fifth lumbar vertebra) BMD, BMC, and BMA. Additionally, whole body DXA scans were used to determine the body composition (ie, fat mass and fat free mass) of the animals.
X-ray micro-computed tomography analyses
Micro-computed tomography (MicroCT) analyses (μCT40; SCANCO Medical, Brüttisellen, Switzerland) were performed on the lumbar vertebral body (L4), proximal tibial metaphysis, and the tibial mid-diaphysis to determine alterations in trabecular and cortical bone microarchitecture. Tibial specimens were scanned at a high resolution of 2,048×2,048 pixels. Analysis of the proximal tibia was performed on a volume of interest (VOI) that included a region of secondary spongiosa that was 30 μm from the growth plate and extended in the distal direction 600 μm (100 images). A 300 μm VOI (50 images) at the mid-point of the tibia was used for cortical bone analysis. Scanning of the vertebra was performed at medium resolution or 1,024×1,024 pixels. The VOI included a region of secondary spongiosa that was approximately 2.56 mm (160 images ×16 μm each) and approximately 80 μm from the dorsal and caudal growth plates. Analysis of all specimens was performed at the threshold of 340, a sigma of 1.2, and a support setting of 2. The trabecular analysis for the proximal tibial metaphysis and vertebral body included the following: relative bone volume (BV/TV), trabecular number (TbN), trabecular thickness (TbTh), trabecular separation (TbSp), connectivity density (ConnDens), structural model index (SMI), apparent density, material density, and degree of anisotropy. Cortical analyses of the tibial mid-diaphysis included cortical thickness, cortical area, medullary area, and porosity.
Finite element analysis
Finite element analysis (FEA) software (SCANCO Medical) was used to evaluate the mechanical properties of trabecular bone by simulating a compression test on reconstructed 3-dimensional images of the lumbar vertebral body and the proximal tibial metaphysis. For the purpose of these analyses, voxels within the VOI were converted into equally eight-node linear brick elements and were incorporated into a micromechanical finite element (FE) model. The tests were performed on the trabecular bone compartment. The following FEA-derived parameters were determined: total force, stiffness, size-independent stiffness, and von Mises stresses.
Zinc content of bone
Inductively coupled plasma mass spectrometry was used to determine the zinc content of bone (ie, humerus) according to the procedure described previously33 with minor modifications. Briefly, the humerus was placed in the acid-washed borosilicate glass tube and weighed, and then air-dried for 24 hours at 105°C. The dry weight was recorded prior to exposing the samples to wet ashing with the addition of deionized water, concentrated nitric acid, and concentrated hydrogen peroxide with the tubes maintained in a heating block at 100°C. After the samples were completely dry, the dry-ash cycle was initiated by placing the tube with sample into the muffle furnace at 375°C for 48 hours. The wet ashing and dry ashing were repeated until all black carbon particles were digested. Ash weight was recorded in mg and then zinc content was determined using inductively coupled plasma mass spectrometry (ELAN9000; PerkinElmer, Waltham, MA, USA). The percent of the bone that was ash was derived from the ash weight being divided by the initial bone weight and then multiplied by 100. Zinc expressed as a percentage of dry weight was calculated by dividing the total zinc content of the bone by the ash weight and multiplying by 100.
ELISA quantification of liver cytokine
Liver tissues were weighed (~100 mg each) and then homogenized in 1 mL lysis buffer (300 mM NaCl, 30 mM Tris, 2 mM MgCl2, 2 mM CaCl2, 1% Triton X-100, 1× Protease inhibitor 100× [Catalog #5872; Cell Signaling, Danvers, MA, USA]; pH 7.4). Homogenates were incubated at 4°C for 30 minutes, and then centrifuged at 1,500× g at 4°C for 15 minutes. Supernatants were stored at −80°C until analyses. Liver homogenates were used to measure TNF-α and IL-1β protein levels using commercially available ELISA kits (TNF-α: Catalog #MTA00B, IL-1β: Catalog #MLB00C; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocols.
Hepatic gene expression
Quantitative real-time PCR (qPCR) analyses were used to determine hepatic expression of genes encoding for proteins that respond to changes in zinc status or are involved in the inflammatory response. Total RNA from mouse liver (n=6/group) was extracted using Trizol Reagent (Life Technology, Rockville, MD, USA). The quantity and the quality of RNA (A260/A280 ratio) were obtained using a NanoDrop spectrophotometer (Rockland, DE, USA). Total RNA (2 μg) was used to prepare cDNA by first treating with DNase I followed by reverse transcription using Superscript II (Invitrogen, Carlsbad, CA, USA). cDNA (50 ng) was used in a final volume of 10 μL for each qPCR. All reactions were performed in duplicate using SYBR green chemistry (SABiosciences, Valencia, CA, USA) and the samples were read on the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The comparative cycle number at threshold (CT) method (User Manual #2; Applied Biosystems) was used to calculate qPCR results using cyclophilin b (Ppib) as a reference gene. The following primer sequences were used: MT2, 5′-catcacgctcctagaactcttc-3′ and 5′-tgcaggaagtacatttgcattg-3′; Slc30a1 (ZnT1), 5′-ccaacaccagcaattccaac-3′ and 5′-gcag aaacactcctcgcata-3′; Nrf2, 5′-cccggttgcccacattc-3′ and 5′-tgtctctgccaaaagctgcat-3′; CD14, 5′-gccgccaccgcttct-3′ and 5′-acacgttgcggaggttca-3′; TLR4, 5′-actgttcttctcctgc ctgaca-3′ and 5′-tgatccatgcattggtaggtaata-3′; TNF-α, 5′-ctgaggtcaatctgcccaagtac-3′ and 5′-cttcacagagcaatgactc caaag-3′; Slc39A14 (Zip14), 5′-gagtgggccgggataatgtt-3′ and 5′-agctaaagcacgtggagaggtt-3′; Ppib, 5′-tggagagcaccaagaca gaca-3′ and 5′-tgccggagtcgacaatgat-3′.
Statistical analysis
Statistical analysis was performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Significant differences between treatment groups were analyzed as 2×2 factorial design with dietary zinc (Con vs −Zn) and LPS treatment (−LPS vs +LPS) as factors. Two-way ANOVA, post hoc analysis, and Fisher’s least significant difference were calculated using the general linear model procedure. The α was set at 0.05 and results expressed as mean ± SE.
Results
Body weights, body composition, and tissue weights
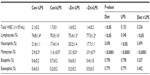
Over the course of the 10-week study, body weight and food intake were not affected by dietary zinc or LPS treatment (Table 1). The evaluation of body composition revealed that the LPS-treated animals exhibited a reduction in fat mass (P<0.05), which coincided with a significant decrease in percent body fat (Table 1). Although zinc is recognized as an important micronutrient for skeletal growth, no significant differences were observed in the tibial length (Table 1) with marginal zinc deficiency or LPS in this study.
Thymic involution has been associated with prolonged zinc deficiency,21,34 but in this study no decrease in thymus weight was reported in response to moderate zinc deficiency (Table 1). Thymus hypertrophy did occur in conjunction with LPS treatment (P<0.05), and the effect tended (P=0.054) to be more pronounced in the animals receiving the zinc adequate diet as opposed to those receiving the zinc-deficient diet. The same response to zinc and LPS was observed when thymus weight was expressed relative to body weight (data not shown). There tended to be an interaction between zinc adequate diet and LPS in spleen weight (P=0.072), but this response did not reach the level of statistical significance (Table 1).
Zinc content of bone
Zinc content of bone is one of the indicators of total body zinc status due to the large proportion of total body zinc deposited in the skeleton.35 Although there was no change in total mineral content (ash weight), mineral content of the humerus expressed as a percent of dry weight of the bone was reduced in the zinc inadequate groups (Table 1). The −Zn diet did result in decrease in the total zinc content of the bone compared with the mice on the control (35 ppm) diet (Figure 1A). The percent of zinc expressed per unit of humerus dry weight (Zn [% dry weight]) confirmed that the difference in humeral zinc was not due to difference in bone size but from the relative abundance of zinc in the bone (Figure 1B). Similar results were observed in the LPS-treated cohort in that the zinc content and zinc expressed per unit of bone dry weight were also reduced with LPS treatment (Figure 1A and B).
Complete blood counts
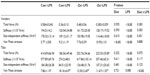
Leukopenia, a reduction in total white blood cells, is characteristic of early zinc deficiency and has been shown to contribute to compromised immunity.36,37 In this study, mice fed the −Zn diet experienced decreased total white blood cell counts in peripheral blood (Table 2). The percentage of lymphocytes was significantly increased in the zinc inadequate cohort, while the combination of control diet and LPS treatment decreased the percentage of lymphocytes in these animals. Neutrophils were not affected by the −Zn diet (Table 2), but the percentage of neutrophils was decreased by the LPS treatment as anticipated. The percentage of peripheral blood monocytes increased in response to LPS treatment, but was in turn suppressed with the −Zn diet. Additionally, the animals fed control diet together with LPS treatment had significantly elevated monocytes compared with other groups. These findings indicate that marginal zinc deficiency and LPS treatment alone impaired the immune system but the combination of LPS treatment in the context of mild zinc deficiency did not exacerbate this response.
Bone density analysis
In order to determine the effects of moderate zinc deficiency followed by a subsequent chronic inflammatory challenge on BMD, whole body and spine BMD were assessed. There were no alterations in whole body BMD with LPS administration (Table 1), but LPS resulted in a significant reduction in BMD of the lumbar vertebra (Figure 1C). Marginal zinc deficiency reflected by a decrease in bone zinc content had no effect on whole body or lumbar vertebra BMD, BMC, or BMA (data not shown).
Trabecular and cortical bone microarchitecture
In the vertebra, LPS administration significantly decreased trabecular BV/TV (~23%) compared with the animals receiving the LPS placebo pellet (Figure 1D). This loss of trabecular bone in response to LPS coincided with a decrease in TbN, an increase in TbSp, but no change in TbTh (Table 3). These alterations in trabecular bone morphometric parameters of the vertebra occurred irrespective of zinc status. LPS also induced a decrease in ConnDens of the trabecular struts of the vertebra, but no change in material density of the bone. Evaluation of the arrangement of the trabecular struts based on SMI indicated that the trabecular bone was arranged in a weaker more rod-like pattern in response to LPS (Table 3).
In this present study, the observations in the proximal tibial metaphysis were somewhat different compared with the vertebra. LPS produced an approximately 20% reduction in BV/TV of the tibia, but this response failed to reach the level of statistical significance (Figure 1E). No alterations in the TbN, TbTh, TbSp, ConnDens, and degree of anisotropy of the tibia were observed with LPS. The only alterations in trabecular bone to LPS in the tibial metaphysis were an increase in the SMI (Table 3). Marginal zinc deficiency did lead to a significant reduction in TbN of the tibia.
Evaluation of cortical bone at the mid-diaphysis of the tibia revealed that neither the LPS administration nor the −Zn diet altered the cortical thickness or area, medullary area, and porosity of the tibia (Table 3).
Biomechanical properties of trabecular bone
Alterations in trabecular microarchitecture resulted in lower total force, stiffness, and size-independent stiffness and increased von Mises stresses of the trabecular bone of the vertebra in the LPS-treated cohort (Table 4). The only negative effect of the −Zn diet on the biomechanical properties at this site was an increase in the von Mises stresses. Similar observations were made in regard to alterations in biomechanical properties of the trabecular bone in the proximal tibial metaphysis; that is total force, stiffness, and size-independent stiffness were decreased with LPS administration (Table 4). However, there were no significant effects of −Zn at this site.
Hepatic gene expression
In order to evaluate the systemic response to −Zn and chronic LPS, qRT-PCR was performed to determine the alterations in gene expression associated with zinc status in liver tissue. Metallothionein, which is highly correlated with liver zinc status, and the zinc transporter were assessed.38,39 The relative abundance of MT2 and ZnT1 mRNA was not significantly altered by −Zn or LPS (Figure 2A and B). However, the −Zn diet suppressed the relative abundance of Nrf2 (Figure 2C), which plays an important role against oxidative stress by acting to induce detoxifying genes that subsequently leads to antioxidant responses. Zinc has been shown to be involved in the regulation of Nrf2 antioxidant function,40 which coincides with the finding of this study that Nrf2 gene expression is affected by diet.
In terms of inflammatory responses, CD14 mRNA expression was altered by diet, LPS treatment, and also the interaction between these two factors in this study (Figure 2D). Marginal zinc deficiency and LPS treatment alone significantly decreased the relative abundance of CD14 in liver and the CD14 gene expression was suppressed most in zinc inadequate groups regardless of LPS treatment. Upon recognition of LPS, CD14 presents LPS to Toll-like receptor (TLR4) activating a downstream signaling cascade upregulating inflammatory cytokines such as TNF-α and IL-1, which in turn stimulate immune responses.41 Interestingly, the relative abundance of CD14 gene was lower in Con/+LPS group than in Con/−LPS group. However, no alteration in gene expression of either TLR4 or TNF-α was observed (Figure 2E and F).
Administration of low-dose LPS upregulated the relative abundance of Zip14 in these animals (Figure 2G), which is comparable to other studies that showed under inflammatory conditions, pro-inflammatory cytokines escalated zinc influx by stimulating Zip14.42,43 Increased hepatic zinc accumulation is one of the characteristic features during inflammatory responses (eg, zinc’s involvement in hepatic acute-phase protein production).43 However, Zip14, a zinc transporter, was not altered at the transcriptional level by zinc inadequate diet, which coincides with our findings relative to MT2 and ZnT1.
Hepatic inflammatory mediators
As anticipated, hepatic TNF-α protein was elevated in response to LPS. Although zinc deficiency has been shown to increase pro-inflammatory cytokine production, the marginal zinc deficiency alone that was achieved in this study did not alter TNF-α protein in the liver. However, TNF-α in the −Zn/+LPS group was significantly higher than the Con/−LPS (~25%) and Con/+LPS groups (~19%) and tended to be elevated (P=0.072) in the −Zn/−LPS group (Figure 3).
Discussion
Zinc is a trace mineral with a wide array of functions, including regulation of the immune system and bone metabolism. This study was designed to investigate whether a low-grade chronic inflammatory challenge would exacerbate the effects of a marginal zinc deficiency on bone structural and biomechanical parameters. The animals were fed a zinc inadequate diet (ie, 5 ppm) in order to induce marginal zinc deficiency. Signs and symptoms of severe zinc deficiency, including decreased food intake, thymic atrophy, and stunted growth, were not observed in animals fed −Zn diet over a 10-week period in this study. However, a decrease in bone zinc in the −Zn groups was observed, which suggests a compromise in zinc status was achieved. In contrast to the skeletal response, no alteration in the gene expression of hepatic zinc transporters was observed in response to mild zinc deficiency. These findings are similar to Blalock et al who observed that metallothionein mRNA levels in the liver were only altered by severe (<2 ppm Zn/day) dietary zinc deficiency.44 However, previous studies using rodent models have shown that zinc-deficient animals, fed <2 ppm zinc/day, significantly decreased hepatic expression of MT1 and -239,45 with no significant change in ZnT1.39 Taken together, these findings demonstrate that a model of zinc deficiency was produced that would allow for the study of physiological changes associated with a marginal compromise in zinc status.
Physiologically, zinc is known to play a key role in the development and function of innate and adaptive aspects of immunity. Cells associated with the innate immune function such as neutrophils, natural killer cells, and macrophages require zinc for normal cellular development and function.46 Likewise, the effect of zinc on T-cell and B-cell function is well established.18–20 The influence of zinc status on T and B cells is of particular interest in relation to bone metabolism due to their role in regulating osteoclastogenesis by serving as a major source of receptor activator of NF-κB ligand and osteoprotegerin in the bone marrow.47,48 The chronic LPS model utilized in this study has been previously shown to induce bone loss coincident with a low-level pro-inflammatory state characterized by upregulated TNF-α, IL-1β, and cyclooxygenase-2 in bone.30,31 Because both zinc deficiency and chronic inflammation are known to contribute independently to bone loss, this study was to determine the extent to which bone mass and biomechanical properties were altered in mice with marginal zinc deficiency that are immunologically challenged by a low-grade inflammation.
The findings of this study revealed that bone loss, as indicated by whole body BMD, was not observed with marginal zinc deficiency or chronic LPS in young growing mice. Notably, whole body BMD reflects changes primarily in cortical bone as opposed to trabecular bone due to cortical bone comprising approximately 80% of the skeleton. Evaluation of the spine BMD, a site with a greater proportion of trabecular bone, revealed that LPS reduced bone density, but no alterations occurred in response to −Zn. Similar results were observed with marginal zinc deficiency by Erben et al49 and Scrimgeour et al15 in that zinc status did not significantly alter BMA, BMC, and BMD in a rat model. While BMD provides important information relative to the overall density of the bone, it was important to further assess the alterations occurring within the trabecular and cortical bone compartments. MicroCT analyses revealed that marginal zinc status resulted in a trend (P<0.1) toward reduced numbers of trabecular struts in both the tibial proximal metaphysis and the vertebral body. Whether or not these changes in trabecular bone would have reached the level of statistical significance with an extended study duration remains in question. In agreement with our previous reports,30,31 mice receiving the chronic LPS treatment had induced deterioration of trabecular bone within the lumbar vertebra (ie, decreased BV/TV, TbN, and ConnDens) compared with control animals. It is important to note that this model utilizes doses of LPS that are 50–100× lower than the doses used in traditional sepsis studies. The results in this current study indicate alterations in trabecular bone at the vertebra site, primarily were affected by LPS administration, but no changes in cortical bone microarchitectural parameters were observed over the 10-week study period. LPS treatment in a context of marginal zinc deficiency did not exacerbate deterioration of bone microarchitecture in these young growing animals.
In addition to reporting the structural changes in bone, it is also important to understand how the biomechanical properties of bone are altered by zinc status and LPS which will ultimately determine fracture risk. Our data show that in the vertebra, the von Mises stresses were increased in mice treated with the −Zn diet. This is an expected response based on the fact that von Mises stresses represent the amount of stress the trabecular bone was undergoing at a given load in the FE model. These findings demonstrate greater stress in the −Zn animals compared with the controls and consequently greater risk of mechanical failure. Scrimgeour et al15 reported that moderate zinc deficiency in young adult Sprague Dawley rats compromised bone biomechanical properties of the tibia. These findings take into consideration with the findings of the current study indicate that marginal zinc deficiency leads to compromised bone biomechanical properties despite the lack of a change in bone density or trabecular microarchitecture. Aside from the effects of zinc on bone strength, the changes induced by LPS were also examined. A reduction in the trabecular bone biomechanical properties in the mice treated with LPS was observed in both the spine and the proximal tibial metaphysis, which is similar to our previous findings.30 Interestingly, the decrease in trabecular bone strength in the tibia occurred despite the lack of bone histomorphometric changes. This observation would suggest that the changes occurring in bone strength with LPS were not solely dependent on the amount of trabecular bone present or its spatial arrangement, but were a result of alterations in the protein matrix or some other factor that can affect bone strength. Collectively, the FEA data indicate that although −Zn and LPS negatively affected biomechanical properties on an independent basis, this response was not exacerbated when these two factors were combined.
The question of whether or not the alterations in the immune system occurring with zinc deficiency would augment the effects of LPS on the systemic immune response was a key aspect of this study. LPS is known to bind to CD14 on macrophages, especially the hepatic Kupffer cells, which makes the liver an appropriate target organ for the assessment of the systemic inflammatory response.50 We showed that the relative abundance of CD14 RNA was suppressed by the −Zn diet, but this response was not altered with LPS treatment. We are not aware of previous reports of decreased hepatic CD14 with a marginal dietary zinc deficiency, but this response may provide an explanation as to why the response to chronic LPS exposure was not as pronounced as expected in the context of zinc deficiency in this study. Because CD14 is a co-receptor with TLR4 and MD-2, involved in the recognition of LPS and the subsequent inflammatory signaling, downregulating CD14 is likely to increase the risk of infection. In this study, we did show that no alteration in the relative abundance of TLR4 and TNF-α RNA occurred, but Zip14 which is known to be upregulated in response to inflammation,42,43 was increased in the LPS-treated groups. Although TNF-α was not altered at the transcriptional level, hepatic TNF-α protein production was significantly increased in the LPS-treated mice. Importantly, this increase in hepatic TNF-α was even more pronounced in the −Zn/+LPS group. These findings demonstrate that the low-dose chronic inflammation challenge utilized in this study in conjunction with a marginal zinc deficiency produces a modest increase in the inflammatory response, but not to the extent that detrimental effects are observed on bone.
Conclusion
The results of this study suggest that marginal zinc deficiency resulting in a modest reduction of zinc content of bone did not significantly alter BMD or cortical bone, but did have detrimental effects on some trabecular bone parameters and biomechanical properties over the 10-week study period in young growing animals. A more robust effect on bone was observed in response to the low-grade chronic inflammatory state produced by the chronic LPS model, which resulted in loss of trabecular bone and impaired biomechanical properties from LPS administration. Despite the fact that an LPS challenge in the context of a marginal zinc deficiency further increased hepatic TNF-α production, this response did not translate to more detrimental effects on bone structural parameters. It remains to be determined whether the same skeletal protection from the combined effect of −Zn and LPS observed in the young growing mice in this study would be afforded in aging models in which immunosenescence may occur.
Acknowledgments
We would like to express our sincere gratitude to Kelsey Hembree and Yan Wang for their technical assistance with the project.
Disclosure
The authors report no conflicts of interest in this work.
References
Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. | |
Heaney R, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. | |
Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third National Health and Nutrition Examination Survey. J Nutr. 2002;132(11):3422–3427. | |
Ma J, Betts NM. Zinc and copper intakes and their major food sources for older adults in the 1994–1996 continuing survey of food intakes by individuals (CSFII). J Nutr. 2000;130(11):2838–2843. | |
Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20(1):3–18. | |
Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am J Clin Nutr. 1998;68(2):499S–508S. | |
Krebs NF. Zinc supplementation during lactation. Am J Clin Nutr. 1998;68(2):509S–512S. | |
Hunt JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr. 2003;78(3):633S–639S. | |
Sakai T, Wariishi M, Nishiyama K. Changes in trace element concentrations in hair of growing children. Biol Trace Elem Res. 2000;77(1):43–51. | |
Yamaguchi M. Role of zinc in bone formation and bone resorption. J Trace Elem Exp Med. 1998;11(2–3):119–135. | |
Sun J, Wang J, Zi N, Jing M, Weng X. Effects of zinc supplementation and deficiency on bone metabolism and related gene expression in rat. Biol Trace Elem Res. 2011;143(1):394–402. | |
Cerovic A, Miletic I, Sobajic S, Blagojevic D, Radusinovic M, El-Sohemy A. Effects of zinc on the mineralization of bone nodules from human osteoblast-like cells. Biol Trace Elem Res. 2007;116(1):61–71. | |
Fernandez-Madrid F, Prasad AS, Oberleas D. Effect of zinc deficiency on nucleic acids, collagen, and noncollagenous protein of the connective tissue. J Lab Clin Med. 1973;82(6):951–961. | |
Hadley KB, Newman SM, Hunt JR. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J Nutr Biochem. 2010;21(4):297–303. | |
Scrimgeour AG, Stahl CH, McClung JP, Marchitelli LJ, Young AJ. Moderate zinc deficiency negatively affects biomechanical properties of rat tibiae independently of body composition. J Nutr Biochem. 2007;18(12):813–819. | |
Hosea HJ, Taylor CG, Wood T, Mollard R, Weiler HA. Zinc-deficient rats have more limited bone recovery during repletion than diet-restricted rats. Exp Biol Med (Maywood). 2004;229(4):303–311. | |
Rossi L, Migliaccio S, Corsi A, et al. Reduced growth and skeletal changes in zinc-deficient growing rats are due to impaired growth plate activity and inanition. J Nutr. 2001;131(4):1142–1146. | |
Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43(5):370–377. | |
Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. | |
Osati-Ashtiani F, King LE, Fraker PJ. Variance in the resistance of murine early bone marrow B cells to a deficiency in zinc. Immunology. 1998;94(1):94–100. | |
Keen CL, Gershwin ME. Zinc deficiency and immune function. Annu Rev Nutr. 1990;10(1):415–431. | |
Kahmann L, Uciechowski P, Warmuth S, Malavolta M, Mocchegiani E, Rink L. Effect of improved zinc status on T helper cell activation and TH1/TH2 ratio in healthy elderly individuals. Biogerontology. 2006;7(5–6):429–435. | |
Bao B, Prasad AS, Beck FWJ, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab. 2003;285(5):E1095–E1102. | |
Lacativa PGS, de Farias MLF. Osteoporosis and inflammation. Arq Bras Endocrinol Metabol. 2010;54(2):123–132. | |
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000; 106(12):1481–1488. | |
Yang X, Karsenty G. Transcription factors in bone: developmental and pathological aspects. Trends Mol Med. 2002;8(7):340–345. | |
Bu SY, Lerner M, Stoecker BJ, et al. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif Tissue Int. 2008;82(6):475–488. | |
Ding J, Ghali O, Lencel P, et al. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84(15):499–504. | |
Krum SA, Chang J, Miranda-Carboni G, Wang C-Y. Novel functions for NFκB: inhibition of bone formation. Nat Rev Rheumatol. 2010;6(10):607–611. | |
Droke EA, Hager KA, Lerner MR, et al. Soy isoflavones avert chronic inflammation-induced bone loss and vascular disease. J Inflamm (Lond). 2007;4:17. | |
Smith B, Lerner M, Bu S, et al. Systemic bone loss and induction of coronary vessel disease in a rat model of chronic inflammation. Bone. 2006;38(3):378–386. | |
Smith BJ, Lightfoot SA, Lerner MR, et al. Induction of cardiovascular pathology in a novel model of low-grade chronic inflammation. Cardiovasc Pathol. 2009;18(1):1–10. | |
Hill AD, Patterson KY, Veillon C, Morris ER. Digestion of biological materials for mineral analyses using a combination of wet and dry ashing. Anal Chem. 1986;58(11):2340–2342. | |
Mitchell W, Meng I, Nicholson S, Aspinall R. Thymic output, ageing and zinc. Biogerontology. 2006;7(5):461–470. | |
King JC. Assessment of zinc status. J Nutr. 1990;120:1474–1479. | |
Mercalli ME, Seri S, Aquilio E, et al. Zinc deficiency and thymus ultrastructure in rats. Nutr Res. 1984;4(4):665–671. | |
Beach RS, Gershwin ME, Hurley LS. Altered thymic structure and mitogen responsiveness in postnatally zinc-deprived mice. Dev Comp Immunol. 1979;3:725–738. | |
Bremner I. Nutritional and physiological significance of metallothionein. Experientia Suppl. 1986;52:81–107. | |
Pfaffl MW, Windisch W. Influence of zinc deficiency on the mRNA expression of zinc transporters in adult rats. J Trace Elem Med Biol. 2003;17(2):97–106. | |
Rezaei KA, Chen Y, Cai J, Sternberg P. Modulation of Nrf2-dependent antioxidant functions in the RPE by Zip2, a zinc transporter protein. Invest Ophthalmol Vis Sci. 2008;49(4):1665–1670. | |
Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. | |
Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281(34):24085–24089. | |
Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102(19):6843–6848. | |
Blalock TL, Dunn MA, Cousins RJ. Metallothionein gene expression in rats: tissue-specific regulation by dietary copper and zinc. J Nutr. 1988;118(2):222–228. | |
Szczurek EI, Bjornsson CS, Taylor CG. Dietary zinc deficiency and repletion modulate metallothionein immunolocalization and concentration in small intestine and liver of rats. J Nutr. 2001;131(8):2132–2138. | |
Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2):447S–463S. | |
Kong Y-Y, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. | |
Yun TJ, Tallquist MD, Aicher A, et al. Osteoprotegerin, a crucial regulator of bone metabolism, also regulates B cell development and function. J Immunol. 2001;166(3):1482–1491. | |
Erben RG, Lausmann K, Roschger P, et al. Long-term marginal zinc supply is not detrimental to the skeleton of aged female rats. J Nutr. 2009;139(4):703–709. | |
Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26(10):1175–1186. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.