Back to Journals » Journal of Inflammation Research » Volume 15
Impaired Circulating Antibody-Secreting Cells Generation Predicts the Dismal Outcome in the Elderly Septic Shock Patients
Authors Xu H, Li T, Zhang X, Li H, Lv D, Wang Y, Huo F, Bai J, Wang C
Received 3 June 2022
Accepted for publication 13 August 2022
Published 13 September 2022 Volume 2022:15 Pages 5293—5308
DOI https://doi.org/10.2147/JIR.S376962
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Huihui Xu,1,2,* Teng Li,1,2,* Xiaoming Zhang,1,3 Hongqiang Li,4 Diyu Lv,4 Yiyuan Wang,4 Fangjie Huo,5 Jianwen Bai,4,6 Chunmei Wang4,6
1Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai, 200031, People’s Republic of China; 2University of Chinese Academy of Sciences, Beijing, 100000, People’s Republic of China; 3Shanghai Huashen Institute of Microbes and Infections, Shanghai, 200052, People’s Republic of China; 4Department of Emergency Medicine and Critical Care, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, 200120, People’s Republic of China; 5Department of Respiratory Medicine, Xi’an No. 4 hospital, Xi’an, 710004, People’s Republic of China; 6Department of Emergency Medicine and Critical Care, Shanghai East Hospital, Nanjing Medical University, Nanjing, Jiangsu Province, 211166, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunmei Wang; Jianwen Bai, Department of Emergency Medicine and Critical Care, Shanghai East Hospital, Tongji University School of Medicine, 150 Jimo Road, Shanghai, 200120, People’s Republic of China, Tel +8613761004402 ; +8613386057150, Email [email protected]; [email protected]
Purpose: Sepsis is a condition that derives from a dysregulated host response to infection. Although B lymphocytes play a pivotal role in immune response, little is known about status of their terminally differentiated cells, antibody-secreting cells (ASCs) during immunosuppressive phase of sepsis, especially in elderly patients. Our aim was to extensively characterize the immune functions of ASCs in elderly septic patients.
Patients and Methods: Clinical and laboratory data were collected on days 1, 3, and 7 of hospitalization. Circulating ASCs were evaluated by flow cytometry from fresh whole blood in elderly septic patients at the onset of disease. RNA sequencing analyzed ASCs gene expression profile. Receiver operating characteristic (ROC) curve analysis and logistic regression predicted the survival rate of 28-day mortality.
Results: A total of 103 septic patients were enrolled. The number and proportion of ASCs among total lymphocytes dramatically increased in septic patients, and RNA sequencing analysis showed that ASCs from septic patients exhibited a different gene expression profile. Furthermore, we found these ASCs could promote the function of T cells. Logistic regression analysis showed ASCs population was an independent outcome predictor in septic shock patients.
Conclusion: Our study revealed the complex nature of immune disorders in sepsis and identified circulating ASCs population as a useful biomarker for predicting mortality in elderly septic patients, which provided a novel clue to combat this severe disease.
Keywords: biomarker, antibody-secreting cells, prognosis, sepsis, septic shock
Introduction
Among the common causes of death in ICUs, sepsis is the most highly heterogeneous and elusive syndrome caused by unbalanced host responses to infection.1,2 Introduced since the 4th century, Hippocrates described it as “decay or decomposition of organic matter”.3 As time went by, the clinical criteria for the definition of sepsis became more and more specific. Nowadays, sepsis is classified into two layers, one is “sepsis”, which is defined as a life-threatening condition that occurs when host response to infection injures its own tissues and organs; the other is “septic shock”, in which circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than sepsis alone.4–7 Accordingly, more than half of septic shock arises in patients over 65 years of age,5,8 and the number of elders will double to 756–1400 million by 2030, which may lead to more septic patients.9 Even worse, elderly septic patients do not usually have typical clinical responses, which makes the accepted thresholds for biomarker levels not accurate for diagnostic and prognostic prediction of outcomes.10 Although sequential organ failure assessment (SOFA) score is one of the most popular scoring systems for sepsis prediction, it still has acknowledged limitations in terms of parameters used and mortality discrimination.11 Thus, identification of elderly patients at high mortality risk in sepsis may improve resource allocation and provide a rational way for clinical trials.
Normal immune and physiologic responses eradicate pathogens, and that’s why an effective circulation requires an inflammation-free bloodstream.12 As soldiers in immune system, many types of cells were investigated in sepsis. For example, circulating leukocyte activation is with resultant organ dysfunction and failure.13,14 Moreover, decreased monocyte HLA-DR expression associates with an increased risk of nosocomial infections and death.13 Therefore, inappropriate regulation aggravates the illness which is complicated by a mixture of immune over-activation and suppression, and poses a great challenge for both clinical and basic research.15
Here, we applied “immune cellomics”, a novel systemic strategy, to study the immune status of sepsis and focused on antibody-secreting cells (ASCs), a unique subset differentiated from B cells. It’s already known that B cells have multi-functions in immune response, in addition, early alteration or exhausted-like profiles of these cells have been reported in septic patients.16,17 However, compared to B cells, ASCs which are recognized as the basis of humoral immunity by generating antibodies,18 are less heard of. In this paper, we reported that ASCs were greatly elevated in elderly septic patients. Additionally, ASCs from these patients also exhibited a different gene expression profile compared to healthy donors. Furthermore, we measured the immune functions of ASCs from elderly septic patients by co-culturing with healthy donor-born CD4+ T cells, which showed these ASCs could promote immune function of T cells. Finally, by comparing with other important clinical indicators in sepsis prognosis, circulating ASCs population acted as a prognostic biomarker in the more severe type of disease, septic shock.
Materials and Methods
Patient Eligibility
The study was performed at Institut Pasteur of Shanghai from February 2016 to January 2018. Taken together, 32 healthy controls with age-and sex-matched were enrolled as healthy donors (HD) group. The enrolled criteria were people with 1) no chronic cardiac, liver, renal, lung, immunodeficiency disease; 2) no infection in the last three months; 3) normal peripheral blood test, routine urine test and chest X-ray.
30 patients with community acquired pneumonia (CAP), identified in accordance with guidelines for the diagnosis and treatment of adult CAP in China (2016 Edition),19 were enrolled as infection controls (Inf-Ctrl) group.
103 patients enrolled septic patients (Sep-Pt) group including sepsis and septic shock, who were identified in compliance with the diagnostic criteria from 2016 consensus definitions of American College of Chest Physicians and Society of Critical Care Medicine.5 The exclusion criteria were patients 1) with more than 48 hours of antibiotic treatment; 2) with Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD); 3) with aspiration pneumonia; 4) with hypostatic pneumonia; 5) with bronchial exaggeration; 6) under palliative care; 7) with bone marrow disease; 8) with immunodeficiency or who were treated with any form of prednisone.
Approval was obtained from Shanghai East Hospital institutional review board affiliated to Tongji University (2015–028). Each participant included in the study had received written informed consent. In this study, all processes comply with the Declaration of Helsinki.
Blood Samples
Heparin anti-coagulated blood samples were collected from patients at the onset of disease during a routine blood sampling procedure. Clinical and biological parameters were collected, including demographic characteristics, date, and cause of admission to ICU, status at day 28 after inclusion and type of infection. Severity scores were recorded: Acute Physiology and Chronic Health Evaluation (APACHE II, range 0–71) and SOFA score (range 0–24). Data were not censured if death occurred after the time point analyzed. Concomitantly, heparin anti-coagulated blood samples collected from healthy donors were obtained from volunteers over the age of 65 y.20 Buffy coat blood was obtained as five RNA sequence samples of healthy donors. Sample storage was systematically shorter than 3 h. Blood samples were prepared within 2 h for flow cytometry after collection.
Cells Isolation
Peripheral blood mononuclear cells (PBMC) were purified from blood by Lymphoprep (Axis-Shield) density gradient centrifugation of heparinized or buffy coat blood.21 Cells were resuspended (106 cells/mL) in complete medium consisting of RPMI-1640 containing L-alanyl-L-glutamine dipeptide supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 5×10−5 M of 2-mercaptoethanol (Sigma) and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL, Gibco BRL).
Cells Purification and Culture
In some experiments, cells stained with fluorochrome-labeled antibodies against CD20 (SJ25C1), CD3 (UCHT1) and CD14 (M5E2), CD27 (O323) and CD38 (HIT2) from BD Biosciences or Biolegend. Cells were sorted with moflo astrios EQ (Beckman). Despite heterogeneity across the ASCs spectrum, ASCs were collectively identified as brightly positive for CD27 and CD38 in blood.22 After doublet and debris exclusion, B cells were selected on a biparametric CD20/SSC dot plot and ASCs were selected on CD20−CD3−CD14−CD27hiCD38hi. Before cells culture, PBMC suspension was adjusted to 2×106 cells per mL in complete RPMI-1640 medium. The purity of isolated B cells and ASCs were typically higher than 95%.
ASCs Stimulation of T Cells Assay
ASCs were isolated following the standard procedure. Isolated CD4+ T cells from healthy donors were thawed from liquid nitrogen status at 37 °C for 2 hours before experiment. Thereafter, CD4+ T cells were assessed using standard carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Molecular Probe) dilution methods. To coat the 96-well U-bottom plates with Abs, anti-human CD3 (1 μg/mL, clone: OKT3, Biolegend) were added in 50 μL of phosphate-buffered saline per well and incubated for 2–3 hours at 37 °C. Wells were washed twice with complete RPMI-1640 before addition of cells. CFSE-labeled CD4+ T cells were mixed with ASCs in a 2:1 ratio and co-cultured in CD3-coated plates for 5 days in complete RPMI-1640 medium. The CFSE signal was analyzed by flow cytometry on gated live CD4+ lymphocytes.
Flow Cytometric Analysis
Whole blood or PBMC was surface stained with fluorochrome-labeled antibodies against CD45 (HI30), CD3 (UCHT1), HLA-DR (L243), CD20 (SJ25C1), CD27 (O323) and CD38 (HIT2) from eBioscience, BD Biosciences, Biolegend or Miltenyi. B cells were selected on a biparametric CD20/HLA-DR dot plot and ASCs were selected on CD3−CD20−CD27hiCD38hi.
For intracellular cytokine staining, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) (Sigma) and 1 μg/mL Ionomycin (Sigma) in the presence of 5 μg/mL Brefeldin A (Biolegend) for 5 h at 37 °C. Then cells were washed twice with phosphate-buffered saline and stained with zombie yellowTM dye (Biolegend) to eliminate dead cells. After surface staining with antibodies against CD3 and CD20, cells were fixed, permeabilized using the cytofix/cytoperm kit (BD Biosciences) according to the manufacturer’s instructions and subsequently stained for the detection of intracellular cytokines Interferon (IFN)-γ, Interleukin (IL)-4 and IL-17. Labeled cells were analyzed on an LSR Fortessa flow cytometer (BD Biosciences), and data were analyzed by Flowjo software (Tree Star).
Absolute numbers of CD45+ lymphocytes per microliter were calculated. RBCs were lysed by lysis (BD Biosciences). B cells were selected based on a biparametric CD20/HLA-DR dot plot and the percentage of CD20+ cells in CD45+/PBMC was calculated. ASCs were gated based on CD20−CD3−CD27hiCD38hi and the percentage of ASCs in CD45+/PBMC was calculated. For an adequate experimental staining control, the appropriate irrelevant anti-mouse isotype controls IgG1-FITC, APCs and PE were used. The absolute numbers of B cells subsets were calculated following standard flow cytometry criteria for lymphocyte subsets identification. Firstly, we calculated the percentage of cells expressing CD20 in the lymphocyte gate defined by forward and side scatter in PBMC. The absolute number of circulatory B cells was determined by multiplying the percentage of CD20+ cells with total number of lymphocytes per microliter measured using URIT-2900. Next, we obtained the absolute number of ASCs by multiplying the total number of B cells previously calculated by the percentage of ASCs: B. All absolute numbers were expressed as cells per milliliter.
Statistical Analyses Pathway Analysis, Clustering, and Visualization
Statistical tests were performed using SPSS (SPSS, Chicago, IL, USA, version 24.0) or Prism (GraphPad software, version 7.0) for MAC. Data were expressed as means ± SEM. Because most variables did not always fulfill the normality hypothesis, differences between groups were analyzed using Mann-Whitney U-test for nonparametric data, and analysis of variance followed by a Wilcoxon signed-rank test was used for within-group analyses. The reliability of the use of different phenotype markers concentrations or of main clinical variables to predict death due to septic shock was calculated by plotting receiver operating characteristic curves. The level of significance was set at p < 0.05. Gene set enrichment analysis (GSEA) is widely applied to determine whether a predefined gene set shows statistically significant difference. Hierarchical clustering using Ward’s linkage and Euclidean distance was performed on healthy and septic ASCs using R. Visualizations of the cluster hierarchy plots, and histograms were created in R software environment. Heat map was created using the pheatmap package in R.
Results
Circulating ASCs Increase in Elderly Septic Patients
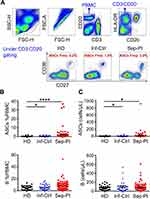
Circulating ASCs were analyzed from PBMC of 32 elderly healthy donors (HD), 30 community-acquired pneumonia infectious controls (Inf-Ctrl), and 103 septic patients (Sep-Pt) at hospitalization. For all controls and patients involved, age, gender, clinical features, and laboratory data are summarized in Table 1. No significant differences could be determined based on age or gender. Among Sep-Pt group, pneumonia represented the initiation of infection (73/103); significant higher levels of routinely used biomarkers for inflammation like C-reactive protein (CRP), procalcitonin (PCT), white blood cells (WBC) count was found, as well as serum lactic acid and clinical evaluation, such as SOFA and APACHE II score. By applying flow cytometry, ASCs were distinguished by CD3−CD20−CD27hiCD38hi (Figure 1A). The absolute number and percentage of ASCs in PBMC increased significantly in Sep-Pt group compared with HD group (Figure 1B and C).
 |
Table 1 Clinical and Laboratory Data Among Elderly Healthy Donors, Infectious Controls and Septic Patients |
Septic ASCs Exhibit Unique Molecular Features
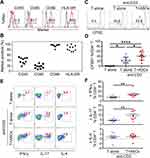
To assess the gene expression landscape of these elevated ASCs in elderly septic patients, we isolated healthy (n = 5) and septic (n = 5) ASCs from peripheral blood samples by cell sorting. The polyadenylated RNA fractions extracted from isolated ASCs were then analyzed by pair-end RNA sequencing (RNA-seq). We initially performed principal components analysis (PCA), a statistical method for reducing high dimensional data while retaining the drivers of expression variability, to compare the overall transcriptome profile between groups. We observed that the two groups are well segregated, indicating that sepsis elicits a global transcriptional signal in ASCs (Figure 2A). Different gene expression in ASCs was found between elderly healthy donors and septic patients, with 210 high expression and 303 low expression genes (Figure 2B). Furthermore, the normalized enrichment scores for gene ontology biological processes revealed clear differences between two groups which included the most up-regulated genes related to oxidative phosphorylation, aminoacyl tRNA biosynthesis and propanoate metabolism, while down-regulated genes associated with primary immunodeficiency, cell adhesion molecules and hematopoietic cell lineage activities (Figure 2C and D).
Septic ASCs Promote T Cell Function and Correlate with Peripheral T Follicular Helper
The expression of costimulatory molecules, like CD40, CD80, CD86, and HLA-DR were analyzed by flow cytometry on elderly septic ASCs (Figure 3A and B). As it is known that anti-CD3 could bind to CD3 on surface of T cells and activates T cell receptor to induce signals transduction.23 In order to determine whether these ASCs have comparable functions in stimulating T cells as B cells do,24 CFSE labeled healthy donor-born CD4+ T cells were cultured with septic ASCs at ratio of 2:1 under stimulation of anti-CD3. Statistical analysis elucidated that the percentage of proliferating CFSEloCD4+ T cells increased distinctly in the presence of septic ASCs than those cultured alone (Figure 3C and D).
Next, we investigated the capacity of septic ASCs to influence production of pro- and anti-inflammatory cytokines by T cells. On the 5th day after co-culture, cells were re-stimulated with PMA and Ionomycin for 5 h in the presence of Golgi Stop. Later, CD4+ T cells were analyzed for intracellular cytokines IFN-γ, IL-17 and IL-4. We observed addition of septic ASCs raised the production of IFN-γ and IL-17 in CD4+ T cells under co-culture system (Figure 3E and F).
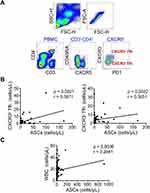
T follicular helper (Tfh) cells are T cells specialized in providing help to B cells.24 On the surface marker level, Tfh cells are generally characterized by expression of CXCR5 and PD-1 with different subsets being identified based on CXCR3.25 In our experiments, we classified Tfh cells into CXCR3+ and CXCR3− subsets (Figure 4A) and found both subsets were positively correlated with ASCs from periphery blood in elderly Sep-Pt group (Figure 4B). To sum up, septic ASCs promoted T cells proliferation, modulated the production of cytokines by CD4+ T cells, in addition, they correlated positively with peripheral Tfh cells.
Prediction of Mortality by Circulating ASCs Concentrations
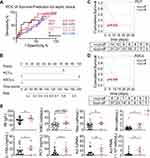
To further confirm the phenomenon of these increased number of cells, we looked deeper into the two layers of 103 septic patients, which included sepsis and septic shock group in line with sepsis classification 3.0. The comparisons of the diagnostic accuracy of ASCs, together with other routine laboratory parameters for diagnosis of the disease are shown in Table 2. 58 patients were diagnosed with sepsis (58/103), the rest with septic shock (45/103). There are statistically significant differences in vasopressor use (p < 0.0001), platelets (p = 0.0001), bilirubin (p = 0.0339), creatinine (p = 0.0084), and lactic acid (p = 0.0043) between sepsis and septic shock groups. Moreover, in septic shock group, the level of ASCs varied between survivors and non-survivors (p = 0.040) (Table 3). However, no such difference existed in sepsis group (Table S1). In receiver operating characteristic (ROC) curve analysis, areas under ROC curve (AUC) of ASCs (AUC = 0.697, p = 0.040) was inferior to PCT (AUC = 0.672, p = 0.073) or CRP (AUC = 0.544, p = 0.643) (Figure 5A), with optimal cut-off value of ASCs and PCT being 6.36 cells/μL and 24.82 ng/mL, respectively. Afterwards, we used the cut-off value to classify ASCs, PCT into ASCs-c and PCT-c, which either is equal to “1” (higher than the optimal cut-off value) or “0” (lower than the optimal cut-off). CRP, lactic acid, and B cells were re-defined in the same way. As a result, univariate analysis revealed that variables of PCT-c (OR 0.144, 95% CI 0.035–0.601, p = 0.008), and ASCs-c (OR 0.078, 95% CI 0.013–0.470, p = 0.005) were markedly correlated with the prognosis of septic shock. Multivariable analysis Hosmer and Lemeshow test revealed that circulating ASCs level (OR 0.107, 95% CI 0.016–0.725, p = 0.022) and PCT (OR 0.196, 95% CI 0.041–0.933, p = 0.041) were both negatively correlated with prognosis of septic shock, indicating these two parameters were independently protective factors of septic shock in elderly patients (Table 4).
 |
Table 2 Clinical and Laboratory Data Between Sepsis and Septic Shock Groups |
 |
Table 3 Clinical Data and Laboratory Data in Survivors and Non-Survivors of Septic Shock Group at Day 1 |
 |
Table 4 Univariate and Multivariate Analysis of Septic Shock Group |
Circulating ASCs Levels in Predicting the Survival Rate of 28-Day Mortality in Septic Shock
Subsequently, we used the two parameters mentioned above to develop a predictive nomogram for septic shock in elderly patients. The total points corresponded to a predicted unfavorable outcome in septic shock (Figure 5B). What’s more, septic shock patients with a higher PCT level (> 24.82 ng/mL) had obviously better outcomes than those with lower level (≤ 24.82 ng/mL) (Log rank test, p = 0.032) in 28-day mortality (Figure 5C). Meantime, circulating ASCs levels > 6.36 cells/μL even presented a better prognosis than those ≤ 6.36 cells/μL (Log rank test, p = 0.006) in septic shock patients (Figure 5D). Moreover, some laboratory indicators and inflammatory mediators, such as hemoglobin, neutrophil, and monocytes, showed visible differences between ASCs highly and lowly produced groups in septic shock patients (Figure 5E). Thus, we identified ASCs population as a best model for prediction of 28-day mortality risk for septic shock.
Kinetic Analysis of IgA+ ASCs and Immunoglobulins in Septic Shock Patients
Since circulating ASCs population had a good ability to predict mortality in septic shock, we wondered how these cells displayed in different time points. In accordance with the outcome of disease, we further evaluated ASCs level between survived and deceased septic shock patients on day 1, 3 and 7, among which 13 survived patients were involved. No significant difference was found in absolute number of ASCs in survived group (Figure 6A), however, it did slightly increase on day 7 compared to day 1 in deceased group (Figure 6A). It’s noteworthy that there was a visible decrease of ASCs counts on day 1 in deceased than survived group (Figure 6A).
ASCs are critical for secreting immunoglobulins (Ig), so we detected the level of Ig in plasma from these patients. Similar tendency seemed to be found among IgG, IgM and IgA in septic shock patients, a significant increase of IgA appeared in survived group on day 7 compared to day 3, meanwhile, IgA in deceased group decreased on day 3 (Figure 6B). What’s more, IgA expression in circulating ASCs was measured. From results shown in Figure 6C, we observed a lowest point of IgA+ ASCs in deceased patients on day 3, with expression lower than those in survived group at the same time point. We concluded that antibody production activity of IgA+ ASCs was grossly different between survived and deceased septic shock patients on day 3. Kinetic changes of ASCs, IgA+ ASCs and Ig in sepsis group were shown in Figure S1, with similar patterns of ASCs counts except one patient in deceased group peaking unexpectedly at the onset of disease.
Discussion
Sepsis is a disease that seriously endangers life and health; septic shock, the more severe type is with rapid onset, high mortality, and limit of effective prognostic indicators. Immunosuppression is the main cause of death in patients with sepsis in hospitals, and how to reduce the mortality in this stage is of critical difficulty for current management. At present, the prognostic indicators, especially good immune index for septic shock cannot meet their needs in clinical practice. Here, we estimated immune index in peripheral blood from elderly septic patients (Sep-Pt) and found ASCs were significantly increased in elderly Sep-Pt compared to controls (HD and Inf-Ctrl) groups. Besides, these ASCs had particularly co-stimulatory molecules, like CD40, CD80, CD86 and HLA-DR. They could promote T cells proliferation, as well as the production of cytokines, and correlate positively with peripheral Tfh cells in elderly Sep-Pt group. Within Sep-Pt group, we divided the disease into two layers, which are sepsis and septic shock; and found circulating ASCs decreased in septic shock patients at day 1 predicted an unfavorable outcome. Taken together, these results would imply that elevated circulating ASCs population could be expected as a precise prognostic marker to distinguish septic patients and associated with a dismal outcome.
ASCs (also named plasma cells, PCs) are vital for a functional adaptive immune system, responsible for production of antibodies to eliminate foreign antigens.26 A study demonstrated the frequency of CD19+CD27hi ASCs was dramatically increased in acute phase of Kawasaki disease (KD).27 Ashenafi et al also revealed a greater proportion of circulating plasmablasts (PB), which is CD3−CD19+CD20−CD27hiCD38hi, was found in adult participants with active TB.28 In contrast, Sealy et al showed that ASCs were fractionally reduced, but nonetheless observed in respiratory tract tissues in the absence of eosinophils by evaluating virus-specific responses 1 month and 4 months following an intranasal virus infection of eosinophil-null mice.29 In our data, the absolute number and the percentage of ASCs in Sep-Pt group remarkably increased compared to HD group. It has been reported that the inflammatory environment of different infectious or autoimmune diseases promotes the expansion of this compartment, especially CD38+ population.30 Meanwhile, CD27 expression is vital for memory B cells development, as the ligand of CD27 yields critical signals that control the entry of B cells into the pathway to develop into ASCs.31 These facts are comparable in our finding that CD27+CD38+ cells expanded in Inf-Ctrl and Sep-Pt groups, indicating these increased proportion are most likely associated with infection. ASCs responses are more rapid and shorter-lived than antibody responses,32 nevertheless, the relevance of abnormally ASCs numbers in response to infection presentation, such as sepsis, is unclear. Further studies will be needed to clarify this mechanism in septic patients.
As a more severe case of sepsis, septic shock is typically characterized by an initial cytokine-mediated hyper-inflammation to an immunosuppressive phase.33–35 The numbers of CD4, CD8, and HLA-DR positive cells (such as dendritic cells, macrophages, and B cells) were notably reduced in sepsis as reported.34 Considering ASCs are developed from antigen-activated B cells which go through several essential stages before maturity, the changes on their surface are easy to be found. So, we investigated the co-stimulatory molecules of CD40, CD80, CD86 and HLA-DR on ASCs isolated from septic patients. CD86 is reported of a high level in circulating CD19+ B cells in septic shock patients than healthy donors, whereas CD80 was lower in survivors than non-survivors at ICU admission.16 What’s more, most circulating ASCs do express high levels of HLA-DR and CD86 in MS.36 In our study, we also revealed the distinct expression of these co-stimulators in Sep-Pt group, and increased expression of these circulating antigens suggests apoptosis susceptibility may be involved in this immune disorder of disease.
By using anti-CD3 antibody, which binds CD3 on surface of T cells, could later stimulate signal and activate T cells.23 In our data, we found anti-CD3 promoted slight production of cytokines like IL-17 and IFN-γ; regardless of that, in the presence of septic ASCs, this phenomenon was distinctly enlarged, which showed the same tendency as B cells did (data not showed). Therefore, it suggests these septic ASCs might have similar functions in stimulating T cells.
As a key T helper cells in GCs, T follicular helper (Tfh) cells contribute to B cells clonal expansion, somatic hypermutation, and high-affinity antibody maturation.37,38 They help with memory B cells development and are associated with production of ASCs together with antibody responses.39–41 We observed that circulating septic ASCs were positively correlated with both types of Tfh cells which are CD3+CD4+CXCR5+PD1hiCXCR3+ and CD3+CD4+CXCR5+PD1hiCXCR3−. Similarly, the numbers of memory B cells and ASCs, but not naive B cells, are reported to positively correlate with Tfh cells at the onset and after 24 h of sepsis.42
Immunoglobulins, which are products of mature B cells, help eradicate pathogens during sepsis, and many studies have indicated that low Ig concentrations predict high mortality.43–46 In our results, septic shock patients showed lower IgA expression at day 3 than day 1 in non-survival group, and it raised at day 7 in survival group; meanwhile IgA+ proportion in circulating ASCs was lower at day 3 in non-survival compared to survival group. Comparably, Venet et al observed low levels of IgG concentrations in septic shock patients, with no apparent association with mortality.44 Despite that, we still noticed a slight decreased tendency of IgG and IgM as time went by in non-survival septic shock patients, which conforms to research that indicate Ig play important roles in the outcome of patients. In a study, within 254 children patients received IgM-enriched IVIg treatment for sepsis or septic shock, 150 of them who received for 5 days had a lower mortality rate than those who received only for 3 days.47 The protective relationship between endogenous IVIg levels and sepsis mortality has also been clarified; and patients with moderate (SOFA < 8) but not severe (SOFA ≥ 8) sepsis had a risk level of Ig for mortality.47 In consequence, future research should pay more attention to investigating the relationship between value of Ig and prognosis of disease.
Currently, CRP, PCT, and SOFA scores are widely used in clinical diagnosis of sepsis. PCT is generally believed to have higher specificity than CRP, however, both can be inaccurate in non-infectious conditions.48 Other molecules such as troponin and cell-free DNA have also been reported.49,50 In our results, we showed that circulating ASCs decreased in septic shock group at day 1, predicted an unfavorable outcome; and patients with low ASCs and PCT levels had higher 28-day mortality in septic shock. On the contrary, a report proved ASCs-rich infiltration tended to occur later in kidney allografts and resulted in an adverse outcome.51 Considering septic shock with a more severe period of immune dysfunction, already set the body in the situation of immunosuppression, in which an active immune status should be inspired; and that may be why in septic shock group, the more ASCs patients produced, the better outcome they obtained. However, in allografts condition, when body already prepares normal immune environment, the appearance of too many ASCs may associate with cell-mediated and/or antibody-mediated rejection, thus leading to harmful endings.
We are aware there are limitations in this research. Firstly, the number cohort is restricted for elderly sepsis study; besides, since ASCs are age dependent, we should enlarge population of patients in future. Secondly, we did not measure the length of incubation period for disease, and unfortunately some patients had already injected with hydrocortisone, which could influence ASCs frequency. Thirdly, some enrolled patients were treated with sepsis management including vasopressors or antibiotics, which could also affect some of results in this finding.
Highlights: initially, we performed a prospective controlled study of ASCs among ICU elderly patients with sepsis and septic shock. Besides, the researches were conducted conforming to the latest Sepsis 3.0 definitions. Next, this is the first report examining prognostic value of ASCs in sepsis, especially for elderly septic shock patients. Through a strict clinical design, the value of ASCs and their related immunological indicators would be used as new immunological biomarkers for prognosis of elderly septic shock, which is expected to provide important clues for effective treatment and management of this disease. Sepsis impacts immunity after developing into the “immunosuppression stage”, which actively makes the body produce large numbers of ASCs. Exploring the phenotypes and functions of these cells is another important innovation point in this work. ASCs themselves and related immune molecules would hopefully be used as novel prognostic biomarkers, and the pathway may be a potential therapeutic target.
Conclusion
Our study revealed the complex nature of immune disorders in sepsis and identified circulating ASCs population as a useful biomarker for predicting mortality in elderly septic patients, which provided a novel clue to combat this severe disease.
Abbreviations
AECOPD, Acute Exacerbation of Chronic Obstructive Pulmonary Disease; APACHE II, Acute Physiology and Chronic Health Evaluation; ASCs, antibody-secreting cells; AUC, Areas under the ROC curve; CAP, community acquired pneumonia; CFSE, carboxyfluorescein succinimidyl ester; CRP, C-reactive protein; ED, emergency department; GCs, germinal centers; GSEA, Gene set enrichment analysis; Hb, hemoglobin; HD, healthy donors; Inf-Ctrl, infectious controls; IFN, interferon; IL, interleukin; Ig, immunoglobulins; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; PB, plasmablasts; PBMC, Peripheral blood mononuclear cells; PCA, principal components analysis; PCs, plasma cells; PCT, procalcitonin; PMA, phorbol 12-myristate 13-acetate; RNA-seq, RNA sequencing; ROC, receiver operating characteristic; Sep-Pt, septic patients; SLE, systemic lupus erythematosus; SOFA, Sepsis Organ Failure Assessment score; Tfh, T follicular helper; WBC, white blood cells.
Acknowledgments
The authors thank ICU (Shanghai East Hospital, Tongji University School of Medicine, China) staff for their great work and cooperation. The authors also thank Key Laboratory of Molecular Virology & Immunology for technical support. Huihui Xu and Teng Li contributed equally to this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. doi:10.1038/nri.2017.36
2. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi:10.1056/NEJMra1208623
3. Marshall JC. Sepsis: rethinking the approach to clinical research. J Leukoc Biol. 2008;83(3):471–482. doi:10.1189/jlb.0607380
4. Henry KE, Hager DN, Pronovost PJ, Saria S. A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med. 2015;7(299):299ra122. doi:10.1126/scitranslmed.aab3719
5. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
6. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–774. doi:10.1001/jama.2016.0288
7. Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):775–787. doi:10.1001/jama.2016.0289
8. Mayr FB, Yende S, Linde-Zwirble WT, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303(24):2495–2503. doi:10.1001/jama.2010.851
9. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi:10.1056/NEJMoa022139
10. Banerjee D, Opal SM. Age, exercise, and the outcome of sepsis. Crit Care. 2017;21(1):286. doi:10.1186/s13054-017-1840-9
11. Zygun DA, Laupland KB, Fick GH, Sandham JD, Doig CJ. Limited ability of SOFA and MOD scores to discriminate outcome: a prospective evaluation in 1436 patients. Can J Anaesth. 2005;52(3):302–308. doi:10.1007/BF03016068
12. Munford RS. Severe sepsis and septic shock: the role of gram-negative bacteremia. Annu Rev Pathol. 2006;1:467–496. doi:10.1146/annurev.pathol.1.110304.100200
13. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi:10.1038/nri3552
14. Brown KA, Brain SD, Pearson JD, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157–169. doi:10.1016/S0140-6736(06)69005-3
15. Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi:10.1146/annurev-pathol-011110-130327
16. Monserrat J, de Pablo R, Diaz-Martin D, et al. Early alterations of B cells in patients with septic shock. Crit Care. 2013;17(3):R105. doi:10.1186/cc12750
17. Gustave CA, Gossez M, Demaret J, et al. Septic shock shapes B cell response toward an exhausted-like/immunoregulatory profile in patients. J Immunol. 2018;200(7):2418–2425. doi:10.4049/jimmunol.1700929
18. Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. Plasma cell development and survival. Immunol Rev. 2010;237(1):140–159. doi:10.1111/j.1600-065X.2010.00940.x
19. Qu JM, Cao B. 中国成人社区获得性肺炎诊断和治疗指南(2016年版)修订要点. 中华结核和呼吸杂志, 39(04): 241-242. The original language title is 中国成人社区获得性肺炎诊断和治疗指南(2016年版)修订要点 [Guidelines for the diagnosis and treatment of adult community acquired pneumonia in China (2016 Edition)]. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39(4):241–242. Chinese. doi:10.3760/cma.j.issn.1001-0939.2016.04.001瞿介明, 曹彬, 2016.
20. Wang RX, Yu CR, Dambuza IM, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20(6):633–641. doi:10.1038/nm.3554
21. Mogensen CE. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi:10.3109/00365516809076979
22. Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I. Advances in human B cell phenotypic profiling. Front Immunol. 2012;3:302. doi:10.3389/fimmu.2012.00302
23. Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003;275(1–2):251–255. doi:10.1016/S0022-1759(03)00010-3
24. Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. 2016;271(1):246–259. doi:10.1111/imr.12411
25. Locci M, Havenar-Daughton C, Landais E, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–769. doi:10.1016/j.immuni.2013.08.031
26. Delves PJ, Roitt IM, Mackay IR, Rosen FS. The immune system. First of two parts. N Engl J Med. 2000;343(1):37–49. doi:10.1056/NEJM200007063430107
27. Sugahara-Tobinai A, Inui M, Metoki T, et al. Augmented ILT3/LILRB4 expression of peripheral blood antibody secreting cells in the acute phase of Kawasaki disease. Pediatr Infect Dis J. 2019;38(4):431–438. doi:10.1097/INF.0000000000002259
28. Ashenafi S, Aderaye G, Zewdie M, et al. BCG-specific IgG-secreting peripheral plasmablasts as a potential biomarker of active tuberculosis in HIV negative and HIV positive patients. Thorax. 2013;68(3):269–276. doi:10.1136/thoraxjnl-2012-201817
29. Sealy RE, Surman SL, Vogel P, Hurwitz JL. Antibody-secreting cells in respiratory tract tissues in the absence of eosinophils as supportive partners. Int Immunol. 2016;28(11):559–564. doi:10.1093/intimm/dxw035
30. Amici SA, Young NA, Narvaez-Miranda J, et al. CD38 is robustly induced in human macrophages and monocytes in inflammatory conditions. Front Immunol. 2018;9:1593. doi:10.3389/fimmu.2018.01593
31. Agematsu K. Memory B cells and CD27. Histol Histopathol. 2000;15(2):573–576. doi:10.14670/HH-15.573
32. Carter MJ, Mitchell RM, Meyer SP, Kelly DF, Truck J. The antibody-secreting cell response to infection: kinetics and clinical applications. Front Immunol. 2017;8:630. doi:10.3389/fimmu.2017.00630
33. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi:10.1056/NEJMra021333
34. Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi:10.1001/jama.2011.1829
35. Schefold JC, Hasper D, Reinke P, Monneret G, Volk HD. Consider delayed immunosuppression into the concept of sepsis. Crit Care Med. 2008;36(11):3118. doi:10.1097/CCM.0b013e31818bdd8f
36. Telesford KM, Kaunzner UW, Perumal J, et al. Black African and Latino/a identity correlates with increased plasmablasts in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(1):e634. doi:10.1212/NXI.0000000000000634
37. King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9(11):757–766. doi:10.1038/nri2644
38. Shulman Z, Gitlin AD, Targ S, et al. T follicular helper cell dynamics in germinal centers. Science. 2013;341(6146):673–677. doi:10.1126/science.1241680
39. Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5(176):176ra32. doi:10.1126/scitranslmed.3005191
40. Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509(7502):637–640. doi:10.1038/nature13300
41. Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247(1):52–63. doi:10.1111/j.1600-065X.2012.01124.x
42. Duan S, Jiao Y, Wang J, et al. Impaired B-cell maturation contributes to reduced B cell numbers and poor prognosis in sepsis. Shock. 2020;54(1):70–77. doi:10.1097/SHK.0000000000001478
43. Andaluz-Ojeda D, Iglesias V, Bobillo F, et al. Early natural killer cell counts in blood predict mortality in severe sepsis. Crit Care. 2011;15(5):R243. doi:10.1186/cc10501
44. Venet F, Gebeile R, Bancel J, et al. Assessment of plasmatic immunoglobulin G, A and M levels in septic shock patients. Int Immunopharmacol. 2011;11(12):2086–2090. doi:10.1016/j.intimp.2011.08.024
45. Taccone FS, Stordeur P, De Backer D, Creteur J, Vincent JL. Gamma-globulin levels in patients with community-acquired septic shock. Shock. 2009;32(4):379–385. doi:10.1097/SHK.0b013e3181a2c0b2
46. Bermejo-Martin JF, Rodriguez-Fernandez A, Herran-Monge R, et al. Immunoglobulins IgG1, IgM and IgA: a synergistic team influencing survival in sepsis. J Intern Med. 2014;276(4):404–412. doi:10.1111/joim.12265
47. Martin-Loeches I, Muriel-Bombin A, Ferrer R, et al. The protective association of endogenous immunoglobulins against sepsis mortality is restricted to patients with moderate organ failure. Ann Intensive Care. 2017;7(1):44. doi:10.1186/s13613-017-0268-3
48. Luzzani A, Polati E, Dorizzi R, et al. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741. doi:10.1097/01.CCM.0000063440.19188.ED
49. John J, Woodward DB, Wang Y, et al. Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care. 2010;25(2):270–275. doi:10.1016/j.jcrc.2009.12.001
50. Rannikko J, Seiskari T, Huttunen R, et al. Plasma cell-free DNA and qSOFA score predict 7-day mortality in 481 emergency department bacteraemia patients. J Intern Med. 2018;284(4):418–426. doi:10.1111/joim.12766
51. Filippone EJ, Farber JL. The implications of B-lineage cells in kidney allografts. Transplantation. 2020;104(10):2011–2023. doi:10.1097/TP.0000000000003163
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.