Back to Journals » Research and Reports in Urology » Volume 11
Impact of tumor cytoreduction in metastatic prostate cancer
Authors Sow Y, Sow O, Fall B, Sine B, Sarr A, Zé Ondo C, Diao B, Ndoye AK, Ba M
Received 8 February 2019
Accepted for publication 7 April 2019
Published 7 May 2019 Volume 2019:11 Pages 137—142
DOI https://doi.org/10.2147/RRU.S204507
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Yaya Sow, Ousmane Sow, Boubacar Fall, Babacar Sine, Alioune Sarr, Cyrille Zé Ondo, Babacar Diao, Alain Khassim Ndoye, Mamadou Ba
Urology and Andrology Department at Aristide Le Dantec Hospital, Cheikh Anta Diop University, Dakar, Senegal
Objective: To assess the impact of tumor cytoreduction on cancer outcomes and patient survival in metastatic prostate cancer.
Patients and methods: It is a prospective study spanning a two-year period between October 1st 2015 and March 31st 2017. We enrolled 102 cases of metastatic hormone-sensitive prostate cancer. Fifty-seven (57) patients had exclusively androgen deprivation therapy (ADT) (group 1) and 45 had, in addition, an open prostatectomy or Transurethral resection of the Prostate (group 2). We compared both groups using the total PSA nadir, the time to PSA nadir, the overall survival (OS), and the progression-free survival (PFS).
Results: The average nadir PSA was lower for the tumor cytoreduction group (16.8±1.6 ng/mL (0.01–193.5) versus 110.7±17.9 ng/mL (0.01–1379)). Median time to PSA nadir was shorter in patients in the ADT only group (8 months vs 3 months (p=0.025)). The OS was shorter in patients treated with ADT only compared to the tumor cytoreduction group (median 14 months vs 24 months, respectively (p=0.03)). Similarly, tumor cytoreduction had a positive impact on patient progression (median PFS 20 months (group 1) vs 43 months (group 2)).
Conclusion: Tumor cytoreduction has a positive impact on the oncological results and the survival of patients under ADT.
Keywords: prostate cancer, cytoreduction, androgen suppression, TURP
Introduction
Prostate cancer is the most common cancer in elderly man. It is the third leading cause of cancer death in the male.1 Androgen deprivation therapy (ADT) is the standard treatment for metastatic prostate cancer.
In developing countries, prostate cancer diagnosis is often made at a late stage where, low urinary tract symptoms or urinary retention occur.2 These obstructive disorders must be taken into account in the management to improve the patients’ quality of life. Transurethral resection of the prostate (TURP) is usually indicated in these cases.
Xiao-Jian and al,3 studied the impact of TURP on patients’ survival as a cytoreduction method. They find a better response to ADT for patients who underwent TURP.
The objective of this study was to assess the impact of TURP or open prostatectomy as cytoreductive methods on oncological outcomes and patient survival in metastatic prostate cancer.
Patients and methods
We conducted a prospective study over a two-year period between October 1st 2015 and March 31st 2017. We evaluated the outcomes of 102 patients followed for metastatic hormone-sensitive prostate cancer (mHSPC). Of these patients, 57 are treated with ADT only (group 1) and 45 had, in addition to ADT, open prostatectomy or TURP, which we consider to be tumor cytoreduction (group 2). Patients were included in group 2 when they had bladder outlet obstruction indicating surgery for bladder unblocking. Those who did not need bladder outlet unblocking were included in group 1. ADT was performed by testicular pulpectomy or by LHRH analogs. Open prostatectomy was carried due to lack of endoscopic equipment. Therapeutic protocols are reported in Table 1.
 | Table 1 Therapeutic Protocols |
The pretreatment patient characteristics were evaluated and compared between the two groups. Patients’ general condition was appreciated by ECOG performance status.
Patients were reviewed every three months with total PSA control. Assessing results criteria were: total PSA nadir, time to reach nadir (the time from the start of treatment to the lowest PSA), overall survival (OS), and progression-free survival (PFS). The origin date was the date when ADT was initiated.
After progression, the evaluation criteria for second-line treatment were the PSA kinetics and OS. The second-line treatment included either estrogen therapy or chemotherapy.
Data processing is performed using SPSS 20.0 software. The comparative analysis of the data is carried out by the Student’s t-test and chi-square to compare patient’s pre and post-therapeutic features. OS and PFS were assessed using the Kaplan–Meier method and the log-rank method to determine survival differences between groups. Statistical tests were deemed significant at p<0.05.
Results
Patients’ average was 71.1±8.6 years (54–88). The pre-therapeutic clinical characteristics of the patients are reported in Table 2. No difference was noted between the two groups.
 | Table 2 Patients’ pre-therapeutic characteristics |
The average PSA nadir was lower for the tumor cytoreduction group compared to the group ADT only (16.8±1.6 ng/mL (0.01–193.5) vs 110.7±17.9 ng/mL (0.01–1379) p=0.025). Also, the median time to PSA nadir was longer in group 2 (8 months vs 3 months; p=0.025).
The median OS of the patients in the series was 16 months (95% CI 12.9–19.1). It was shorter in group 1: 14 months vs 24 months; p=0.03 (Figure 1).
The median PFS of the series was 36 months. It increased from 20 months for group 1 to 43 months for group 2 (p=0.025). Tumor cytoreduction had a positive impact on delaying patient progression (Figure 2).
After a median follow-up period of 16 months, 61 patients (59.8%) had progression clinically or biologically (36 patients (35.3%) in group 1 and 25 patients (24.5%) in group 2).
The average total PSA level after progression of the patients in the series was 778.3 ng/mL (20–7889) and 985 ng/mL (20–7889) for the group 1 vs 480.5 ng/mL (98.3–1860 ng/mL) for the group 2 (p=0.08).
After second-line treatment, the average total PSA level was 807.5 ng/mL (57.8–8923). It was lower in the group 2; 454.4 ng/mL (57.8–1389) vs 1052.7 ng/mL (88.2–8923) p=0.069. The median OS was 6 months (95% CI 5.4–6.5) for the series, 6 months for group 1 and 8 months for group 2 (Figure 3). OS was similar for both groups at the castration resistance stage. Outcomes after cancer progression were not significantly different between the two groups. Table 3 summarizes the therapeutic results of the series and both groups.
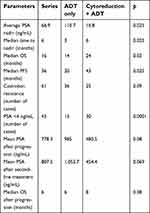 | Table 3 Outcomes according to the different parameters studied |
 | Figure 3 Overall survival curve of group 1 and 2 patients after disease progression. |
Discussion
ADT has been the standard treatment for metastatic prostate cancer ever since the work of Huggins and Hodges, which helped understand the hormone-sensitivity of prostate cancer cells.4 ADT, can stop cancer progression. It was performed on both metastatic and N+ prostate cancer before it was expanded to locally advanced and localized forms.5 This ADT might be medical, using LHRH analogs or antagonists and/or anti-androgens. Also, it might be surgical (orchiectomy or bilateral pulpectomy).5
ADT is clinically and biologically effective in the majority of metastatic prostate cancers resulting in a significant decrease in total PSA level and clinical symptoms. However, disease progression is expected within 18–24 months. It is hormone refractory prostate cancer stage.6
The time to progression depends on several factors. Prognostic factors of progression are the initial PSA, the tumor volume (extension and number of sites and volume of metastases), and the Gleason’s score.6 During treatment, nadir PSA, time to nadir (TTN), and ratio TTN/Nadir are powerful prognostic factors.6,7 Indeed a low PSA nadir and a long TTN, are associated with better survival.
In our center, a progression rate of 20.2% with a median PFS of 10.5 months (6–25) and an OS of 52.3% at 2 years were noticed in an earlier study assessing bilateral pulpectomy outcomes.2
Tumor cytoreduction in metastatic cancers corresponds to treatments aimed at local tumor control or a decrease in tumor volume with the aim of improving survival and/or treating local complications in patients undergoing systemic treatment. We used in our series open prostatectomy or TURP to decrease tumor volume.
In metastatic kidney cancer, it has been shown that cytoreductive nephrectomy provides a benefit in survival in selected patients in combination with systemic therapy.8 In metastatic prostate cancer, cytoreduction is a new area of exploration.9 It can be performed for local tumor control: radical prostatectomy (RP) or radiotherapy (RT) or to treat local complications (TURP). Several studies have reported a benefit on the survival of metastatic patients with cytoreduction by local treatment.10,11
Like TURP or open prostatectomy, other tumor local treatments offer beneficial effect to the patients by improving oncological outcomes. The other local treatments studied were RP, RT, and cryotherapy.
Heidenreich and al,12 investigated the feasibility of RP in patients with metastatic prostate cancer associated with ADT by comparing them to a control group of metastatic patients on ADT only. RP significantly improved both the time to progression (40 months vs 29 months, p=0.04), and the median PFS (38.6 vs 26.5 months, p=0.032). On the other hand, global survivals were similar.
Previously, Culp et al,13 report overall and specific survival rates of 67.4% and 75.8%, respectively, for metastatic patients with RP vs 22.5% and 48.7% for those without local treatment. Similarly, Fossati and al,14 after an analysis of the SEER data (Surveillance Epidemiology and End Results), noted that the local treatment (RP or brachytherapy) offered a higher specific survival rate in metastatic patients in whom specific mortality by cancer (SMC) predicted was less than 40%. On the other hand, no benefit was noted when the predicted SMC was greater than 50%.
Cytoreduction by RP, in addition to improving the survival of metastatic patients, provides a better comfort by reducing the risk of local complications such as urinary retentions, hematuria, rectal invasion, ureteral obstruction and risk of renal failure, and pains related to pelvic nerve infiltration.9 Cytoreductive RP was associated with greater blood loss, longer intervention time, and higher incontinence rate compared to RP in patients with localized cancer.15 However, Kateralis and al,9 report a good rate of continence after cytoreduction by RP-assisted robot.
Other methods of tumor cytoreduction such as RT and cryotherapy are also studied in mHSPC. Local RT or brachytherapy treatment improves patient survival compared to patients without local treatment.10,11,13 This benefit of RT was lower than that of cytoreduction RP.10
Ming-Xiong and al,16 reported a reduction in the risk of progression of 79.3% and an increase in the time to castration resistance after cryosurgery of the prostate in metastatic patients compared to patients without local treatment.
Regarding TURP, the study of Xiao-Jian et al3 is the only one we know that assesses the impact of TURP on patient survival by considering it as a cytoreduction method. These authors reported a better response to ADT for the group with TURP with a lower median PSA nadir (0.15 ng/mL vs 0.82 ng/mL p=0.015) and a longer time to reach PSA nadir. The rate of progression was higher for the group without TURP. There is also a positive influence on overall and specific survival, however, without statistical significance.
Our study confirms the benefit of tumor cytoreduction on both PSA kinetics and patient survival. Compared to ADT alone, TURP or open prostatectomy cytoreduction offered lower PSA nadir and longer time to PSA nadir. It also significantly improves PFS and OS.
This study, although prospective, has some limits including the smaller number of patient, the short patient follow-up time and patients selection that depended on the need for bladder outlet unblocking and not randomly. Further randomized studies with larger numbers are needed to confirm the benefit noted in order to allow routine use of TURP as a cytoreduction method.
Conclusion
Tumor cytoreduction (TURP or open prostatectomy) performed in our practice for bladder outlet unblocking has a positive impact on the oncological results of patients with mHSPC. The cytoreduction offers more beneficial effect to the patients, with lower PSA nadir, longer time to PSA nadir, and longer PFS. However, after cancer progression outcomes were not significantly different between the two groups.
Ethics statement
Our Institutional Review Board (the urology pedagogical committee of the Cheikh Anta Diop University of Dakar) approved this study. This study was carried out in accordance with the principles of the Declaration of Helsinki. All patients got information about the study and gave their written informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grosclaude P, Belot A, Marliac LD, Remontet L, Leone N, Bossard N. Prostate cancer incidence and mortality trends in France from 1980 to 2011. Prog Urol. 2015;25:536–542. doi:10.1016/j.purol.2015.04.011
2. Fall B, Tengue K, Sow Y, et al. Place of bilateral pulpectomy as a method of androgen suppression therapy in prostate cancer. Prog Urol. 2012;17(6):1027–3.
3. Qin X-J, Ma C-G, Ye D-W, et al. Tumor cytoreduction results in better response to androgen ablation: a preliminary report of palliative transurethral resection of the prostate in metastatic hormone sensitive prostate cancer. Urol Oncol. 2012;30:145–149. doi:10.1016/j.urolonc.2010.02.010
4. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297.
5. Boccon-Gibod L, Richaud P, Coloby P, et al. First line indications for hormonal therapy in prostate cancer. Prog Urol. 2010;20:109–115. doi:10.1016/j.purol.2009.11.001
6. Gagnat A, Larré S, Fromont G, Pirès C, Doré B, Irani J. Survival is associated with time to reach PSA nadir (DAN) and the ratio DAN/nadir value after androgen deprivation for prostate cancer. Prog Urol. 2011;21(5):341–348. doi:10.1016/j.purol.2010.09.024
7. Choueiri TK, Xie W, D’Amico AV, et al. Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen deprivation therapy. Cancer. 2009;115(5):981–987. doi:10.1002/cncr.24064
8. Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC. Nephrectomy followed by interferon α-2b compared with interferon α-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–1659. doi:10.1056/NEJMoa003013
9. Katelaris N, Murphy D, Lawrentschuk N, Katelaris A, Moon D. Cytoreductive surgery for men with metastatic prostate cancer. Prostate Int. 2016;4(3):103–106. doi:10.1016/j.prnil.2015.11.003
10. Leyh-Bannurah SR, Gazdovich S, Budaus L, et al. Local therapy improves survival in metastatic prostate cancer. Eur Urol. 2017;72(1):118–124. doi:10.1016/j.eururo.2017.03.020
11. Carneiro A, Baccaglini W, Glina FPA, et al. Impact of local treatment on overall survival of patients with metastatic prostate cancer: systematic review and meta-analysis. Ibju. 2017;43(4):588–599.
12. Heidenrich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: result of a feasibility and case-control study. J Urol. 2015;193(3):832–838. doi:10.1016/j.juro.2014.09.089
13. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65(6):1058–1066. doi:10.1016/j.eururo.2013.11.012
14. Fossati N, Trinh QD, Sammon J, et al. Identifying optimal candidates for local treatment of the primary tumor among patients diagnosed with metastatic prostate cancer: a SEER-based study. Eur Urol. 2015;67(1):3–6. doi:10.1016/j.eururo.2014.08.056
15. Kim DK, Parihar JS, Kwon YS, et al. Risk of complications and urinary incontinence following cytoreductive prostatectomy: a multi‑institutional study. Asian J Androl. 2018;20:1–9. doi:10.4103/aja.aja_7_17
16. Sheng M-X, Wan -L-L, Liu C-M, Liu C-X, Chen S-S. Cytoreductive cryosurgery in patients with bone metastatic prostate cancer: a retrospective analysis. Kaohsiung J Med Sc. 2017;33(12):609–615. doi:10.1016/j.kjms.2017.07.002
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


