Back to Journals » Infection and Drug Resistance » Volume 16
Impact of Treatment with Anti-CD20 Monoclonal Antibody on the Production of Neutralizing Antibody Against Anti–SARS-CoV-2 Vaccination in Mature B-Cell Neoplasms
Authors Onishi A , Matsumura-Kimoto Y, Mizutani S , Tsukamoto T, Fujino T , Miyashita A, Nishiyama D, Shimura K, Kaneko H, Kawata E, Takahashi R, Kobayashi T , Uchiyama H, Uoshima N, Nukui Y, Shimura Y , Inaba T , Kuroda J
Received 9 November 2022
Accepted for publication 18 January 2023
Published 25 January 2023 Volume 2023:16 Pages 509—519
DOI https://doi.org/10.2147/IDR.S396271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Akio Onishi,1 Yayoi Matsumura-Kimoto,1,2 Shinsuke Mizutani,1 Taku Tsukamoto,1 Takahiro Fujino,1 Akihiro Miyashita,1,3 Daichi Nishiyama,4 Kazuho Shimura,5 Hiroto Kaneko,5 Eri Kawata,6 Ryoichi Takahashi,7 Tsutomu Kobayashi,1,8 Hitoji Uchiyama,8 Nobuhiko Uoshima,3 Yoko Nukui,9 Yuji Shimura,1 Tohru Inaba,9 Junya Kuroda1
1Division of Hematology and Oncology, Department of Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan; 2Department of Hematology, Japan Community Health Care Organization Kyoto Kuramaguchi Medical Center, Kyoto, Japan; 3Department of Hematology, Japanese Red Cross Kyoto Daini Hospital, Kyoto, Japan; 4Department of Hematology, Fukuchiyama City Hospital, Fukuchiyama, Japan; 5Department of Hematology, Aiseikai Yamashina Hospital, Kyoto, Japan; 6Department of Hematology, Matsushita Memorial Hospital, Moriguchi, Japan; 7Department of Hematology, Omihachiman Community Medical Center, Omihachiman, Japan; 8Department of Hematology, Japanese Red Cross Kyoto Daiichi Hospital, Kyoto, Japan; 9Division of Infection Control & Molecular Laboratory Medicine, Department of Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan
Correspondence: Junya Kuroda, Division of Hematology and Oncology, Department of Medicine, Kyoto Prefectural University of Medicine, 465 Kajii-cho, Kamigyo-ku, Kyoto, 602-8566, Japan, Tel +81-75-251-5740, Fax +81-75-251-5743, Email [email protected]
Background and Purpose: Anti-CD20 monoclonal antibodies (MoAbs), rituximab (RIT), and obinutuzumab (OBZ) are the central components of immunochemotherapy for B-cell lymphoma (BCL). However, these agents potentially cause B-cell depletion, resulting in the impairment of antibody (Ab) production. During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, the optimal prediction of Ab response against anti–SARS-CoV-2 vaccination is critically important in patients with BCL treated by B-cell depletion therapeutics to prevent coronavirus disease 2019 (COVID-19).
Patients and Methods: We investigated the effect of using RIT and/or OBZ on the Ab response in 131 patients with various types of BCL who received the second SARS-CoV-2 mRNA vaccine either after, during, or before immunochemotherapy containing B-cell–depleting moiety between June and November 2021 at seven institutes belonging to the Kyoto Clinical Hematology Study Group. The SARS-Cov-2 neutralizing Ab (nAb) was measured from 14 to 207 days after the second vaccination dose using the iFlash3000 automatic analyzer and the iFlash-2019-nCoV Nab kit.
Results: Among 86 patients who received the vaccine within 12 months after B-cell depletion therapy, 8 (9.3%) were seropositive. In 30 patients who received the vaccine after 12 months from B-cell depletion therapy, 22 (73%) were seropositive. In 15 patients who were subjected to B-cell depletion therapy after vaccination, 2 (13%) were seropositive. The multivariate analysis indicated that an interval of 12 months between B-cell depletion therapy and the subsequent vaccination was significantly associated with effective Ab production. Receiver operating characteristic curve analysis identified the optimal threshold period after anti-CD20 MoAb treatment, which determines the seropositivity against SARS-CoV-2, to be 342 days.
Conclusion: The use of anti-CD20 MoAb within 12 months before vaccination is a critical risk for poor Ab response against anti–SARS-CoV-2 vaccination in patients with BCL.
Keywords: COVID-19, vaccine, B-cell lymphoma, anti-CD20 monoclonal antibody
Introduction
The anti–coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccines, such as BNT162b2 (BioNTech/Pfizer) and mRNA-1273 (Moderna), have been widely used to prevent coronavirus disease 2019 (COVID-19), and their promising efficacies have been reported in Japan.1,2 Once the mRNA vaccine encapsulated in lipid nanoparticles is taken up by human cells, such as dendritic cells or myocytes, it is translated into a viral spike protein antigen that stimulates B cells to produce a neutralizing antibody (nAb) against the spike protein, which prevents viral infection and also induces cytotoxic T cells that acquire the ability of cellular immune responses against infected cells.3,4 Therefore, immunocompromised patients and patients receiving treatment with an immunosuppressive moiety are generally at high risk for impaired antibody response against anti–SARS-CoV-2 vaccination.5,6 Among the various settings of immunosuppressive treatment, therapeutic approaches that deplete B cells or impair B-cell function, such as an anti-CD20 monoclonal antibody (MoAb) or inhibitors for B-cell receptor signaling, have especially attracted attention as risk factors for severe COVID-19 in patients with B-cell malignancies, including B-cell lymphomas (BCLs) and chronic lymphocytic leukemia.7–15 However, most previous studies investigated combinations of patients with various types of B-cell and non–B-cell lymphomas using various types of treatment strategies, sometimes including watch-and-wait without treatment. To establish an optimal management strategy to prevent COVID-19 that is more specific for patients with BCL who have a history of anti-CD20 MoAb treatment, we conducted this multi-institutional study to investigate the efficacy of the anti–SARS-CoV-2 vaccine and the risk factors for Ab production impairment following anti–SARS-CoV-2 vaccination in this particular population of patients treated in seven institutes belonging to the Kyoto Clinical Hematology Study Group (KOTOSG).
Materials and Methods
Study Design
Patients with any disease subtype of BCL who were older than 20 years and received the second dose of the anti–SARS-Cov-2 vaccine, either the BNT162b2 mRNA COVID-19 or mRNA1273 SARS-CoV-2 vaccine, within 2 years from the latest immunochemotherapy containing anti-CD20 MoAb were eligible for this study. Serum samples were collected from patients between 2 and 36 weeks after the second dose of COVID-19 vaccination as routine clinical practice and were preserved at −80°C until analysis. All patients received vaccination according to the national Japanese vaccination program. Patients were excluded from this study in case of having past SARS-Cov-2 infection or receiving prophylactic/therapeutic nAb for SARS-Cov-2. To investigate the kinetic/change of Ab levels after vaccination in immunocompetent individuals, sera were obtained from healthy volunteers at least twice in most participants at various time points between 1 and 44 weeks after the second dose of COVID-19 vaccination. We measured the serum level of SARS-Cov-2 nAb using the iFlash3000 automatic analyzer and the iFlash-2019-nCoV Nab kit (both manufactured by YHLO, China), which quantify nAbs against SARS-CoV-2 in the blood using the principle of chemiluminescent immunoassay.16 Measuring nAb allows us to identify antibodies that provide long-term immunity against COVID-19.17–19 The concentration of nAb over 10 AU/mL was considered to be positive with this system. From the patient’s case report form, we collected patient data including age, gender, BCL subtype, disease status, white blood cell count, neutrophil count, lymphocyte count, monocyte count, platelet count, type and number of prior chemotherapy regimens, history of prior autograft, and time between vaccination and treatment with anti-CD20 MoAb at the point of vaccination.
Statistical Analysis
We compared the relationship between the response to anti–SARS-CoV-2 vaccination and the time since the last chemotherapy using the Kruskal–Wallis test. Univariate and multivariate analyses were performed with logistic regression for factors correlated with seropositivity. The confidence interval was 95%, and p < 0.05 was considered statistically significant. We used the receiver operating characteristic curve (ROC) analysis to investigate the relationship between seropositivity after COVID-19 vaccination and the time from the last dose of anti-CD20 MoAb. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).20
Results
Patients’ Background and Information About the Healthy Volunteers
In this study, we analyzed 131 patients with BCL, including 116 patients who received a second COVID-19 vaccine after completing immunochemotherapy with anti-CD20 MoAb and 15 patients who received a second COVID-19 vaccine within 6 months before starting immunochemotherapy between June and November 2021 at the institutes belonging to the KOTOSG. The median patient age was 72 years (range, 30–96 years). The most prevalent disease subtype was diffuse large B-cell lymphoma, and the second most prevalent was follicular lymphoma, accounting for 53% and 28% of our cohort, respectively. Among the 131 patients with BCL, 97 (74%) received only rituximab-containing immunochemotherapy, 23 (18%) received only obinutuzumab-containing immunochemotherapy, and 9 (6.9%) had a history of both rituximab- and obinutuzumab-containing therapy. Four patients (3%) had a history of both rituximab and polatuzumab vedotin, an antibody-drug conjugate composed of an anti-CD79b MoAb conjugated by a protease-cleavable linker to monomethyl auristatin E. Fourteen patients (11%) had a history of high-dose chemotherapy with autologous peripheral blood stem cell transplantation. Of 116 patients who received the COVID-19 vaccine after being treated with immunochemotherapy containing anti-CD20 MoAb, 86 patients (77%) received the vaccine within 12 months of finishing immunochemotherapy, whereas 30 patients (23%) received the vaccine after >12 months (Table 1). We also recruited 59 healthy volunteers, including 24 (41%) men and 35 (59%) women. The median age of the healthy volunteers was 47 years (range, 24–64 years), and none had a history of COVID-19.
 |
Table 1 Patients Background |
Serum Levels of SARS-CoV-2 nAb in Patients with BCL and Healthy Volunteers
We obtained the patients’ sera at 14 to 207 days after the second COVID-19 vaccination. Sera were also obtained from 56 healthy volunteers at least twice and from three volunteers once. We first examined the serum level and kinetics of SARS-CoV-2 nAb in healthy volunteers. Figure 1 shows that all healthy volunteers became seropositive after vaccination, and the serum level of SARS-CoV-2 nAb decreased gradually with time after vaccination. During the examination period, we observed a decrease in serum SARS-CoV-2 nAb to less than 10 AU/mL (9.38 AU/mL) in only one case at 254 days after vaccination, which indicated a promising long-term efficacy of the vaccination that was generally maintained for more than 6 months in healthy subjects. In 116 BCL patients with a history of anti-CD20 MoAb treatment before vaccination, 30 patients were found to be seropositive (Figure 1). When 7 patients below 49 years old were selected to match their median age with that of healthy volunteers, 3 patients were found to be seropositive. Thus, even between the matched cohorts, the seropositive rate of BCL patients treated by anti-CD20 MoAb was significantly low compared with that of healthy volunteers. In addition, when examined with the Fisher test, the frequencies of seropositive patients below 49 years old and patients over 50 years old were not significantly different (p=0.37).
In 15 BCL patients who received anti–COVID-19 vaccination before undergoing B-cell depletion therapy, we obtained sera between 108 and 200 days after vaccination. Two patients in this subgroup were seropositive.
Association Between Time from Anti-CD20 MoAb Treatment and the Response to the COVID-19 Vaccine
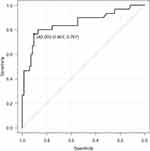
Next, to identify the cutoff period that determines the efficacy of COVID-19 vaccination among the 116 patients with BCL who received the vaccine after undergoing anti-CD20 MoAb treatment, we examined the relationship between seropositivity after COVID-19 vaccination and the time from the last dose of anti-CD20 MoAb using ROC curve analysis. The result showed that, in our cohort, 342 days was the optimal threshold period after B-cell removal therapy that determines seropositivity after COVID-19 vaccination (Figure 2).
We further examined more precisely the impact of the time from the last dose of anti-CD20 MoAb treatment on the efficacy of nAb production by the COVID-19 vaccine. The seropositivity rates of SARS-CoV-2 nAb in patients with a time interval between the last dose of anti-CD20 MoAb and vaccination <3 months, 3 to 6 months, 6 to 9 months, 9 to 12 months, and >12 months were 6.4%, 9.5%, 7.7%, 40.0%, and 73.0%, respectively. The median serum levels of SARS-CoV-2 nAb (AU/mL) in patients with a time interval between the last dose of anti-CD20 MoAb and vaccination <3 months, 3 to 6 months, 6 to 9 months, 9 to 12 months, and >12 months were 0.00, 0.95, 4.49, 0.33, and 25.50 AU/mL, respectively, and the difference was significant between patients who received the vaccination 0–3, 3–6, and 6–9 months after the last dose of B-cell depletion therapy and those who received it 12 months after the last dose of B-cell depletion therapy (Figure 3). These results collectively show that the use of anti-CD20 MoAb treatment is strongly associated with the impairment of antibody production by the anti–SARS-CoV2 vaccine and its negative impact is sustained for at least 6 months and generally up to approximately 12 months after completion of anti-CD20 MoAb treatment.
 |
Figure 3 Serum level of SARS-CoV-2 nAb and time from the last dose of anti-CD20 MoAb to vaccination. Abbreviation: M, month. |
In contrast, seropositivity was not associated in our cohort with the duration between vaccination and the start of anti-CD20 MoAb treatment in 15 BCL patients who received anti–COVID-19 vaccination before B-cell depletion therapy (Figure 4).
 |
Figure 4 Serum level of SARS-CoV-2 nAb and time from vaccination to the start of anti-CD20 MoAb to vaccination. |
Investigation of the Factors Associated with nAb Production After COVID-19 Vaccination in Patients with BCL with a History of Anti-CD20 MoAb Treatment
We then attempted to identify factors associated with nAb production in response to COVID-19 vaccination in patients with BCL who received previous treatment with anti-CD20 MoAb. The univariate analysis demonstrated that, although low white blood cell count, low lymphocyte count, and low hemoglobin level were significantly associated with poor response to vaccination, the DLBCL disease subtype, lack of history of autologous stem cell transplantation, and time from the last dose of anti-CD20 MoAb to vaccination longer than 9 months were significantly associated with a favorable response. Among the factors identified by the univariate analysis as significant, the multivariate analysis revealed that time from the last dose of anti-CD20 MoAb to vaccination longer than 12 months was the only factor significantly associated with a favorable nAb response to COVID-19 vaccination (Table 2).
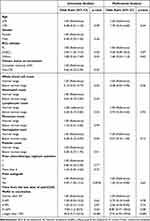 |
Table 2 Uni and Multivariate Analyses for the Risk Factors for the Impairment of Antibody Production After COVID-19 Vaccination |
Discussion
The COVID-19 vaccine has been available in Japan since February 2021, and as of September 2022, 80.4% of the total population had received two doses of the vaccine, resulting in a relatively high vaccination rate among developed countries.21 As the spread of COVID-19 infection continues, the COVID-19 vaccine, primarily the mRNA vaccine, contributes greatly to infection prevention. However, some evidence has suggested that the effectiveness of the COVID-19 vaccine is limited in immunocompromised patients with hematologic diseases, especially patients with BCL who receive B-cell depletion therapeutics within 1 year before vaccination.7–15 Such a situation is similar to what has been observed with vaccinations against various types of pathogens, such as influenza virus, or Pneumococcal bacteria, which has been reported to be less effective in lymphoma patients during or within 6 to 12 months of treatment containing rituximab,22,23 whereas more serious is an increased risk of documented COVID-19 with related hospitalization and even death in vaccinated patients with hematological neoplasms compared with vaccinated matched healthy controls with an intact immune system.24
This study focused especially on the efficacy of the COVID-19 vaccine in patients with BCL who received anti-CD20 MoAb treatment, as several studies have suggested that patients with this particular disease group are at especially high risk of inappropriate antibody production.7–15 Because it is critically important to identify factors associated with a poor response to COVID-19 vaccination, we investigated the impact of the history of autologous stem cell transplantation and the number of prior treatment regimens in addition to the use of anti-CD20 MoAb; however, among the treatment-related factors, the duration from the last dose of anti-CD20 MoAb and vaccination was the only factor significantly associated with the response to COVID-19 vaccine in our cohort. In addition, we found that no other factors examined, including patient age and blood cell counts, were associated with seropositivity in our cohort, whereas previous studies suggested the relationship between white blood cell count; lymphocyte counts, including CD4-positive T cells and CD19-positive B cells; and the efficacy of COVID-19 vaccine in patients with lymphoma.10,12,15 One of the conceivable reasons for these discrepancies might be the difference in the patient population analyzed, as the study by Narita included not only BCLs but also T-cell lymphomas and Hodgkin lymphoma, whereas the study by Perry included patients who were subjected to watch and wait without receiving anti-CD20 MoAb treatment. Tanguay et al pointed out that a peripheral B-cell count of <50/µL was a risk factor for poor antibody production after the COVID-19 vaccine, although this finding was not always related to prior anti-CD20 MoAb treatment. Nevertheless, there is consensus regarding the importance of the duration from completion of B-cell depletion therapy to vaccination within 6 to 12 months, which significantly impairs nAb production against the SARS-Cov-2 RNA vaccine.10,12,13,15 In this regard, although data about the precise lymphocyte subset were unfortunately unavailable in our cohort, our study may preclude the necessity of precise blood cell/lymphocyte profiling, which is both labor-intensive and sometimes expensive, for predicting prompt nAb production after COVID-19 vaccination in a specific population of patients with BCL who were previously treated with B-cell depletion therapy.
Booster vaccination by the third vaccination and the administration of COVID-19 nAb agent, such as tixagevimab-cilgavimab, have shown promise,25,26 and those may also increase antibody titers and prevent severe COVID-19 even in high-risk patients, including patients with BCL who previously received B-cell depletion therapy.14,27–29 Considering the results of previous studies as well as those of our study, the administration of the COVID-19 nAb agent may be more promising for BCL patients treated with anti-CD20 MoAb within 12 months. Even in patients with BCL who received anti-CD20 MoAb treatment for longer than 12 months before the second vaccination, approximately 30% of patients were seronegative. Therefore, the serum level of nAb is expected to be measured after vaccination in those patients, and in case of insufficient response, an additional dose of vaccine is recommended or the administration of a nAb agent may be considered. If nAb monitoring is unavailable, the risk of insufficient response should be considered in the decision-making regarding the use of a boost vaccination or nAb agent.
The other important point of our study is the insufficient antibody production in patients with BCL who received vaccination before the initiation of anti-CD20 MoAb treatment. Although this result was concordant with the result reported by Tanguay,15 this was, at the same time, discordant with the findings of a previous study that reported that nAb remained for 4 months after vaccination in patients subsequently treated with B-cell depletion therapy.9 The underlying reason for this discrepancy is currently unclear, although our study and the study by Tanguay et al included only 15 and 22 patients, respectively. To clarify the factors causing insufficient antibody production in this setting, a future study with a larger number of subjects is needed.
One of the limitations of this study is that the blood samples were obtained at the patients’ visits, required for clinical purposes, but to avoid frequent visits to the hospital during the COVID-19 pandemic status, they were not obtained regularly after vaccination for the research purpose. In addition, because of the small number of patients, it was difficult to investigate precise features of patients with a time interval between the last dose of anti-CD20 MoAb and vaccination 9 to 12 months, although they showed incongruent vaccine response. It was also impossible to investigate the relationship between the serum nAb level and the emergence of symptomatic COVID-19. In the future, it would also be important to clarify the risk of insufficient antibody production by boosting vaccination and COVID-19 disease in patients with BCL treated with B-cell depletion therapy.
Conclusions
In this study, we found that the time from the last dose of anti-CD20 MoAb to the vaccination within 12 months is a critical risk factor for poor response to COVID-19 vaccination in patients with BCL who were previously treated with anti-CD20 MoAb.
Ethical Approval and Consent to Participate
All participants provided written informed consent. The study was conducted in compliance with the Declaration of Helsinki, and the study protocol was approved by the institutional review board (IRB) of Kyoto Prefectural University of Medicine (protocol code ERB-C-2180-2) and IRBs of the participating institute.
Acknowledgment
The authors greatly thank all the participants and their families. We also thank all of the medical, nursing, data-processing, laboratory, and clinical staff members in KOTOSG, especially, Dr. Isa R, Dr. Hirakawa K, Dr. Kiyota M, Mr. Okumura K, and Mr. Kosaka T for their contributions to this study and their dedicated patient care.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Y. M-K. has received honoraria from Bristol-Myers Squibb (BMS) and Janssen Pharmaceutical. S.M. has received honoraria from Sanofi and Ono Pharmaceutical. T.T. has received honoraria from BMS, Janssen Pharmaceutical, Sanofi, Kyowa Kirin, and Chugai Pharmaceutical. D.N. has received honoraria from Janssen Pharmaceutical and Chugai Pharmaceutical. K.S. has received honoraria from Chugai Pharmaceutical and Sanofi. T.K. has received honoraria from BMS, Ono Pharmaceutical, Chugai Pharmaceutical, Sanofi, and Janssen Pharmaceutical. N.U. has received honoraria from BMS, Janssen Pharmaceutical and Takeda. Y.S. has received honoraria from Ono Pharmaceutical, BMS, Janssen Pharmaceutical, Sanofi, Kyowa Kirin, Takeda Pharmaceutical, and Chugai Pharmaceutical. J.K. is a consultant for Janssen Pharmaceutical and BMS and has received research funding from Kyowa Kirin, Chugai Pharmaceutical, Ono Pharmaceutical, Sanofi, Takeda Pharmaceutical, and Mochida Pharmaceutical and has received honoraria from Janssen Pharmaceutical, Kyowa Kirin, Chugai Pharmaceutical, Ono Pharmaceutical, Sanofi, BMS, and Takeda Pharmaceutical. All other authors have no conflicts of interest in this work.
References
1. Sugiyama A, Kurisu A, Nagashima S, et al. Seroepidemiological study of factors affecting anti-spike IgG antibody titers after a two-dose mRNA COVID-19 vaccination in 3744 healthy Japanese volunteers. Sci Rep. 2022;12(1):16294. doi:10.1038/s41598-022-20747-x
2. Maeda H, Saito N, Igarashi A, et al. Effectiveness of mRNA COVID-19 vaccines against symptomatic SARS-CoV-2 infections during the delta variant epidemic in Japan: vaccine effectiveness real-time surveillance for SARS-CoV-2 (VERSUS). Clin Infect Dis. 2022;2022:ciac292.
3. Bellamkonda N, Lambe UP, Sawant S, Nandi SS, Chakraborty C, Shukla D. Immune response to SARS-CoV-2 vaccines. Biomedicines. 2022;10(7):1464. doi:10.3390/biomedicines10071464
4. Federico M. How do anti-SARS-CoV-2 mRNA vaccines protect from severe disease? Int J Mol Sci. 2022;23(18):10374. doi:10.3390/ijms231810374
5. Oyaert M, De Scheerder MA, Van Herrewege S, et al. Evaluation of humoral and cellular responses in SARS-CoV-2 mRNA vaccinated immunocompromised patients. Front Immunol. 2022;13:858399. doi:10.3389/fimmu.2022.858399
6. Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. doi:10.1136/bmj-2021-068632
7. Okamoto A, Fujigaki H, Iriyama C, et al. CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma. Blood Adv. 2022;6(11):3230–3233. doi:10.1182/bloodadvances.2021006302
8. Gurion R, Rozovski U, Itchaki G, et al. Humoral serological response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica. 2022;107(3):715–720. doi:10.3324/haematol.2021.279216
9. Shree T, Shankar V, Lohmeyer JJK, et al. CD20-targeted therapy ablates de novo antibody response to vaccination but spares preestablished immunity. Blood Cancer Discov. 2022;3(2):95–102. doi:10.1158/2643-3230.BCD-21-0222
10. Narita K, Nakaji S, Tabata R, et al. Antibody response to COVID-19 vaccination in patients with lymphoma. Int J Hematol. 2022;115(5):728–736. doi:10.1007/s12185-022-03305-z
11. Pascale SP, Nuccorini R, Pierri T, et al. Evaluation of serological response to anti-SARS-CoV-2 mRNA vaccination in hematological patients. Front Immunol. 2022;13:892331. doi:10.3389/fimmu.2022.892331
12. Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi:10.1182/bloodadvances.2021005094
13. St-Pierre F, Doukas P, Boyer J, Nieves M. CLL-211 humoral immune response following COVID-19 vaccination in patients with Chronic Lymphocytic Leukemia (CLL) and Indolent Non-Hodgkin Lymphoma (NHL): results from a large, single-center observational study. Clin Lymphoma Myeloma Leuk. 2022;22(Suppl 2):S273. doi:10.1016/S2152-2650(22)01335-0
14. Ollila TA, Masel RH, Reagan JL, et al. Seroconversion and outcomes after initial and booster COVID-19 vaccination in adults with hematologic malignancies. Cancer. 2022;128(18):3319–3329. doi:10.1002/cncr.34354
15. Tanguay M, Boutin M, Laumaea A, et al. B-cell cytopenia and time to last B-cell-depleting therapy predict response to SARS-COV-2 vaccines in patients with lymphoproliferative disorders. Vaccine. 2022;40(9):1203–1207. doi:10.1016/j.vaccine.2022.01.040
16. Kaneko Y, Sugiyama A, Tanaka T, et al. The serological diversity of serum IgG/IgA/IgM against SARS-CoV-2 nucleoprotein, spike, and receptor-binding domain and neutralizing antibodies in patients with COVID-19 in Japan. Health Sci Rep. 2022;5(3):e572. doi:10.1002/hsr2.572
17. Lau EHY, Tsang OTY, Hui DSC, et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat Commun. 2021;12(1):63. doi:10.1038/s41467-020-20247-4
18. Jeewandara C, Jayathilaka D, Gomes L, et al. SARS-CoV-2 neutralizing antibodies in patients with varying severity of acute COVID-19 illness. Sci Rep. 2021;11(1):2062. doi:10.1038/s41598-021-81629-2
19. Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18(2):318–327. doi:10.1038/s41423-020-00588-2
20. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi:10.1038/bmt.2012.244
21. Global Change Data Lab. Oxford martin school. Coronavirus (COVID-19) vaccinations-our world in data. Available from: https://ourworldindata.org/covid-vaccinations.
22. Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118(26)::6769–6771. doi:10.1182/blood-2011-08-372649
23. Makatsori M, Kiani-Alikhan S, Manson AL, et al. Hypogammaglobulinaemia after rituximab treatment-incidence and outcomes. QJM. 2014;107(10):821–828. doi:10.1093/qjmed/hcu094
24. Mittelman M, Magen O, Barda N, et al. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood. 2022;139(10):1439–1451. doi:10.1182/blood.2021013768
25. Naito T, Tsuchida N, Kusunoki S, et al. Reactogenicity and immunogenicity of BNT162b2 or mRNA-1273 COVID-19 booster vaccinations after two doses of BNT162b2 among healthcare workers in Japan: a prospective observational study. Expert Rev Vaccines. 2022;21(9):1319–1329. doi:10.1080/14760584.2022.2093722
26. Focosi D, Casadevall A, Critical A. Analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (Evusheld™) for COVID-19 prophylaxis and treatment. Viruses. 2022;14(9):1999. doi:10.3390/v14091999
27. Levin MJ, Ustianowski A, Wit SD, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of covid-19. N Engl J Med. 2022;386(23):2188–2200. doi:10.1056/NEJMoa2116620
28. Lim SH, Stuart B, Joseph-Pietras D, et al. Immune responses against SARS-CoV-2 variants after two and three doses of vaccine in B-cell malignancies: UK PROSECO study. Nat Cancer. 2022;3(5):552–564. doi:10.1038/s43018-022-00364-3
29. Nguyen Y, Flahault A, Chavarot N, et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin Microbiol Infect. 2022;22:00383.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.