Back to Journals » Clinical Epidemiology » Volume 14
Impact of Thromboprophylaxis Intensity on Patients’ Mortality Among Hospitalized Patients with COVID-19: A Propensity-Score Matched Study
Authors Almohareb SN , Al Yami MS , Assiri AM, Almohammed OA
Received 19 January 2022
Accepted for publication 11 March 2022
Published 19 March 2022 Volume 2022:14 Pages 361—368
DOI https://doi.org/10.2147/CLEP.S359132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eyal Cohen
Sumaya N Almohareb,1– 3 Majed S Al Yami,1– 3 Ahmed M Assiri,4 Omar A Almohammed5,6
1Department of Pharmacy Practice, College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia; 2Department of Clinical Pharmacy, King Abdulaziz Medical City, Riyadh, Saudi Arabia; 3King Abdullah International Medical Research Center, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia; 4Health Volunteering Center, Ministry of Health, Riyadh, Saudi Arabia; 5Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 6Pharmacoeconomics Research Unit, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
Correspondence: Omar A Almohammed, Department of Clinical Pharmacy, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh, 11451, Saudi Arabia, Tel +966 555104065, Email [email protected]
Purpose: Venous thromboembolism (VTE), a major complication that has been reported in patients with COVID-19, is associated with an increased risk of mortality. The purpose of this study was to compare in-hospital mortality among hospitalized patients with COVID-19 who received high-intensity versus standard-intensity thromboprophylactic anticoagulation.
Patients and Methods: A secondary database analysis was conducted using data for adult patients who were hospitalized for COVID-19 in Saudi Arabia and received enoxaparin for thromboprophylaxis during their hospitalization. While enoxaparin 40 mg daily is considered the standard-intensity, doses higher than the standard but not to reach the therapeutic dose were considered as high-intensity. The primary outcome in the study was in-hospital mortality, and the secondary outcomes included intensive care unit (ICU) and hospital length of stay. Chi-square and t-tests were used to assess the difference between the two independent groups, and propensity score matching was performed to adjust for baseline characteristics.
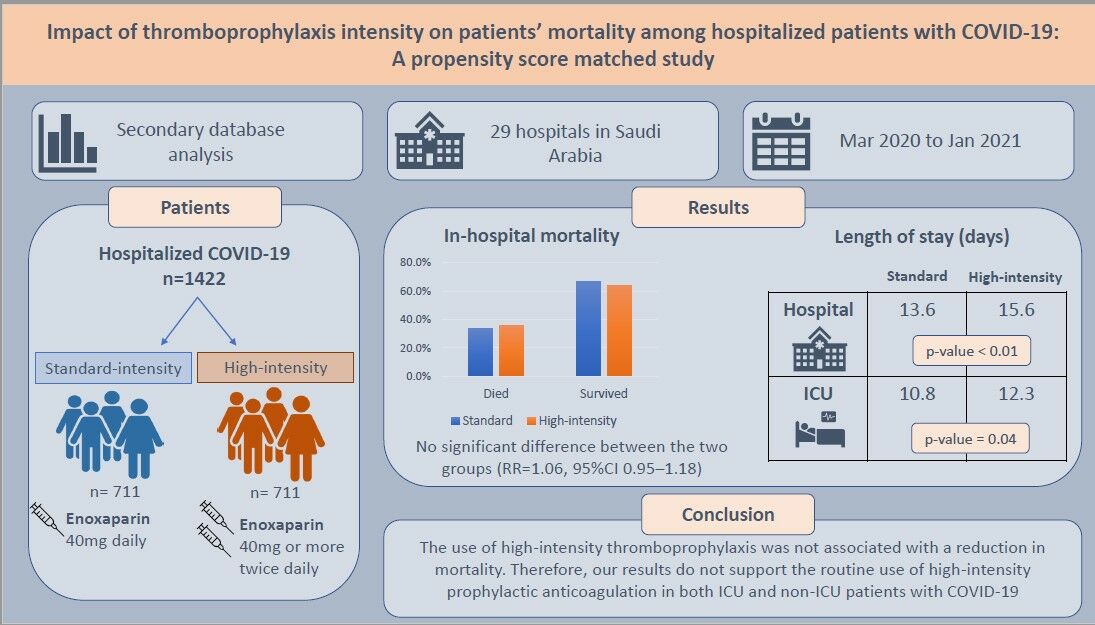
Results: From 3508 patients who received high- or standard-intensity enoxaparin, 1422 patients, 711 in each group, were included in the analyses after propensity score matching. The mean age of the participants was 57.2 years, and around 30% of them were female. About 72% of the patients were admitted to the ICU. No difference was observed between the two groups in the in-hospital mortality outcome (36% vs 33.5% in the high-intensity and the standard group, respectively; RR=1.06, 95% CI 0.95– 1.18). However, patients who received high-intensity thromboprophylaxis had a significantly longer duration of hospitalization (15.6 days vs 13.6 days; p=0.003) and ICU stay (12.3 days vs 10.8 days; p=0.039) compared to patients who received the standard dose.
Conclusion: The use of high-intensity thromboprophylaxis was not associated with a reduction in mortality. Therefore, our results do not support the routine use of high-intensity prophylactic anticoagulation in both ICU and non-ICU patients with COVID-19.
Keywords: COVID-19, venous thromboembolism, anticoagulation, thromboprophylaxis, mortality
Graphical Abstract:
Introduction
Venous thromboembolism (VTE) has been reported as a common complication of COVID-19 infection. Among hospitalized patients, the overall incidence of VTE was found to be elevated, with rates of 12% to 26%.1–4 Moreover, patients admitted to intensive care units (ICUs) have a higher risk of developing thrombosis compared with non-ICU patients.5 Several proposed mechanisms for the hypercoagulability state associated with COVID-19 have been suggested, including direct endothelial injury, complement-system activation, the release of inflammatory mediators and cytokine storm, and thromboinflammation caused by coagulation abnormalities.6–9
Early initiation of thromboprophylaxis in hospitalized COVID-19 patients was associated with a reduced risk of mortality and the need for intubation.10,11 However, some controversy has arisen regarding the optimal dosing strategies for these patients. Results from observational studies comparing intermediate or full doses of anticoagulant versus the standard thromboprophylactic dose have been mixed. Some studies reported better outcomes with escalated intensities of anticoagulation, while others found no difference or worse outcomes.12–19 These conflicting results from observational studies, along with the higher incidence of VTE early in the pandemic, have led to variability in experts’ opinions, institutional protocols, and clinicians’ decisions regarding the optimal thromboprophylaxis regimen. However, the current guidelines recommend a standard thrombophylactic dose for all hospitalized patients with COVID-19 due to the lack of high-quality evidence to recommend against or support the use of a higher dose of anticoagulant.20–23
Results from the INSPIRATION trial evaluating the use of an intermediate dose of anticoagulant for thromboprophylaxis found no benefit over the standard dose in preventing thrombosis or mortality in ICU patients.24 Another small, randomized clinical trial that assessed the use of intermediate-dose versus standard-dose enoxaparin in patients with severe COVID-19 reported no significant differences between the two regimens.25 Besides that, several clinical trials showed better outcomes when therapeutic doses of anticoagulant were used in moderately ill patients,26–28 but not in critically ill patients.26,29 To date, large-scale studies evaluating the use of intermediate versus standard thromboprophylaxis doses in patients with COVID-19 are lacking. Therefore, the aim of this study was to compare the use of high-intensity versus standard-intensity of enoxaparin for thromboprophylaxis among hospitalized patients with COVID-19 in terms of in-hospital mortality using real-world data as well as identify any difference in the duration of hospitalization or length of stay (LOS) in the ICU.
Methods
Study Design and Setting
Secondary database analysis was conducted to evaluate the effect of using high-intensity versus standard-dose thromboprophylaxis in hospitalized patients with COVID-19. The database was obtained from the Saudi Ministry of Health (MOH) and included data for hospitalized COVID-19 patients from 29 hospitals in Saudi Arabia (SA) that were designated to receive patients with COVID-19. The study design and protocol were revised and approved by the Saudi MOH Central Institutional Review Board (IRB Log No: 21-96 M).
Subjects and Database
The database records from March 2020 to January 2021 of all hospitalized patients with a confirmed COVID-19 diagnosis were screened for inclusion. Hospitalized patients were included if they were adults (>18 years) and received enoxaparin for thromboprophylaxis during their hospitalization. Data for pregnant women were excluded from the analysis. The data, which were obtained from the Saudi MOH database, included the patients’ demographics, comorbidities, severity stage of COVID-19, type of oxygen therapy, ICU admission, inpatient complications, enoxaparin dose, in-hospital mortality, and dates of admission to and discharge from the hospital and ICU (if admitted to ICU) or decease (if the patient died during hospitalization).
Study Groups and Outcomes
The standard dose of thromboprophylaxis was defined as using enoxaparin 40 mg daily; meanwhile, high-intensity dosage was defined as administering enoxaparin 40 mg, 60 mg, 80 mg, or 120 mg twice daily. The use of either approach among hospitalized patients was based on a national protocol, released early in the pandemic, which was developed by the Saudi MOH for patients with COVID-19. The dose of thromboprophylaxis depends on the patient’s weight and D-dimer level (see Supplement Material for the thromboprophylaxis protocol). The main outcome of the study was the incidence of in-hospital mortality. Other outcomes included the duration of hospitalization and LOS in ICU, determined from the dates of admission to and discharge from the hospital or date of decease.
Statistical Analysis
The baseline characteristics for patients were reported using frequencies and percentages for categorical variables and mean ± standard deviation (SD) for the continuous variables, as appropriate. A chi-square test for categorical variables and student’s t-tests for continuous variables were used to assess the difference between the two independent groups (high-intensity vs standard dose) in terms of demographics, comorbidities, severity stage of COVID-19, the type of oxygen therapy needed, ICU admission, and inpatient complications as well as patients’ outcomes in the study: specifically, in-hospital mortality, duration of hospitalization, and LOS in ICU.
The two groups were made comparable by matching in terms of baseline demographics, comorbidities, severity stage of COVID-19, and the type of oxygen therapy needed. The groups were matched via propensity score (PS) matching using the greedy nearest neighborhood matching method (1:1) with a caliber of 0.2 SD for the logit of the estimated PS. The two groups were matched using the Proc PSMATCH procedure for propensity score,30,31 and all data were analyzed using SAS® software, version 9.4 (SAS Institute Inc., Cary, NC). For sensitivity analysis, the PS matching was repeated adding ICU admission to the list of variables in the matching to control for its effect on the study outcomes. The PS matched groups from the sensitivity analysis and their related results were reported in the Supplemental Materials.
Results
Patients’ Characteristics
The study included a total of 3508 hospitalized patients with COVID-19. Among those, 43.2% received high-intensity thromboprophylaxis, whereas 56.8% received the standard dose for thromboprophylaxis. After the matching, a total of 1422 patients, 711 in each group, were included in the analyses of the outcomes. The patients’ mean age was around 57 years, and about one-third of them were elderly (≥ 65 years). The mean body mass index (BMI) in the high-intensity group was 29.6 (± 7.2) versus 28.6 (± 5.8) in the standard-dose group, and more than one-third of the patients were obese (BMI>30). Table 1 presents details of these data.
 |
Table 1 Patients’ Characteristics Before and After Propensity Score Matching |
The most common comorbidity was diabetes, followed by hypertension and cardiovascular diseases. After matching, no difference was found between the groups in terms of the proportion of patients who were hospitalized at a critical COVID-19 stage; however, more patients needed ICU admission in the high-intensity compared to the standard group (77.1% vs 67.8%; p<0.01). In terms of oxygen therapy, most of the included patients were on oxygen (96%), and no differences were noted between the two groups regarding the type of oxygen therapy used. In the area of inpatient complications, the two groups were comparable, except in the case of acute respiratory distress syndrome (ARDS), which was more prevalent in the high-intensity group (55.7% vs 39%; p<0.01). A summary of these results can be found in Table 1. The most common dosing strategies used in the high-intensity group were enoxaparin 40 mg twice daily, followed by enoxaparin 60 mg twice daily (Table 2).
 |
Table 2 Dosing Strategies for Thromboprophylaxis in the High-Intensity Group |
Patients’ Outcomes
The number of patients who died during hospitalization was 494 (34.7%). Among these, 256 patients were in the high-intensity thromboprophylaxis group, compared to 238 patients in the standard thromboprophylaxis group. However, this difference between the groups in terms of their mortality outcome was not statistically significant (36% vs 33.5%; p=0.32; Table 3). The sub-group analysis of the primary outcome for patients admitted to ICU found no difference in mortality between the two groups (46.2% vs 47.7%; OR=1.06; 95% CI 0.83–1.36; Table 4). Moreover, when mortality data were stratified by the patients’ BMI to adjust for the effect of weight on the study outcome, the odds of mortality were similar between patients who received high-intensity thromboprophylaxis and the standard dose among patients with BMI ≥ 30 (35.4% vs 33.8%; OR=0.93; 95% CI 0.65–1.34; Table 4).
 |
Table 3 Primary and Secondary Outcomes for the Matched Cohorts |
 |
Table 4 In-Hospital Mortality Stratified by Patients’ BMI and ICU Admission for the Matched Cohorts |
Patients who received high-intensity thromboprophylaxis had a significantly longer duration of hospitalization, with a mean difference of around two days, compared to patients who received standard thromboprophylaxis (15.6 days vs 13.6 days; p<0.01; MD=1.97, 95% CI 0.66–3.28). Moreover, the high-intensity thromboprophylaxis was associated with a longer stay in ICU compared to patients who received standard thromboprophylaxis (12.3 days vs 10.8 days; p=0.04; MD=1.58, 95% CI 0.08–3.09). These results are presented in Table 3.
Sensitivity Analysis
The sensitivity analysis was conducted to control for the effect of the difference between the groups in terms of the number of patients admitted to the ICU. The results from the sensitivity analysis confirmed the robustness of the findings in the main analysis, as the results from the sensitivity analysis of the outcomes in the study were comparable to that in the main analysis. The results from the sensitivity analysis were presented in Tables S1–S4.
Discussion
The benefit of using a higher intensity of anticoagulant for thromboprophylaxis in patients with COVID-19 remains unclear. This large propensity score-matched study, which evaluated the effect of using high-intensity doses compared to the standard dose of enoxaparin for thromboprophylaxis in hospitalized patients with COVID-19, found no difference in the incidence of in-hospital mortality between the two groups. In addition, this study found a significant increase in the duration of hospitalization and ICU LOS in the high-intensity group compared to the standard-dose group.
The results of our study were consistent with those reported in previous randomized clinical trials comparing an intermediate prophylactic dose to the standard dose of enoxaparin in patients with severe or critical cases of COVID-19.24,25 The INSPIRATION trial reported that using an intermediate dose did not result in any statistical difference in the composite outcome of venous or arterial thrombosis, extracorporeal membrane oxygenation, or mortality within 30 days; furthermore, the rates of all-cause mortality were comparable between the two groups.24 Likewise, the Perepu et al study found no significant differences in thrombosis at 30 days or all-cause mortality between the two groups; meanwhile, the cumulative incidence of death was similar in both groups for patients who were admitted to the ICU or medical wards at the time of enrollment.25 These results are similar to what we found in the sub-group analysis of the current study.
The overall mortality rate in our study was high (34.7%), driven mainly by the high proportion of ICU patients, representing about 70% of the patients in the study. However, this rate is comparable to the rate reported in a meta-analysis of ICU patients with COVID-19 (41.6%; 95% CI 34.0–49.7%).32 About two-thirds of the patients considered in this study were admitted to the ICU, making our sample similar to the sample in Moll et al’s retrospective study, which included 94 ICU patients and compared the use of intermediate versus standard doses of heparin for thromboprophylaxis. Specifically, Moll et al found no significant difference in in-hospital mortality or symptomatic VTE between the two groups.18 In another retrospective study that investigated hospitalized patients with COVID-19, a lower rate of in-hospital mortality was observed in the group that received an intermediate dose compared to the standard dose for thromboprophylaxis. Although the study had a large sample size (2785 patients), only 382 patients that emerged from PS matching (191 patients in each group) were included in the mortality analysis.19 This variation in the mortality rates from our study might be attributed to differences in the severity status of the patients considered in these studies and the use of different anticoagulation doses.
Using a therapeutic dose of anticoagulation in COVID-19 patients has been evaluated in several randomized controlled trials.26–29 While the benefit of using a therapeutic dose appears to be dependent on the phase of COVID-19 illness and the patient’s setting, these trials failed to show any superiority in using a therapeutic anticoagulant dose over the standard dose in ICU patients. In contrast, for non-ICU patients with moderate illness, using therapeutic anticoagulation was associated with better outcomes, represented by a lower requirement for cardiovascular or respiratory organ support and less occurrence of major thromboembolism and death. These results suggest that the early initiation of therapeutic anticoagulation during the early stages of the illness might be an important factor in the patients’ outcomes. In our study, no beneficial effect was observed on mortality for both ICU and non-ICU patients which could be related to the differences in the used anticoagulant dosing in comparison with these trials.
Although this study is one of the largest to compare the use of high-intensity thromboprophylaxis to the standard dose using real-world data, several study limitations must be taken into consideration when interpreting the results. First, this was a secondary database analysis, which limited our ability to control or adjust for confounders to the available variables in the database. Also, information about the time of initiation of anticoagulant and patients’ laboratory data, such as the level of D-Dimer, were lacking. Furthermore, other essential outcomes could not be assessed, like the incidence of VTE, bleeding events, and other long-term outcomes occurring post-discharge. Another limitation is the large number of ICU patients in our population, which may restrict the generalizability of the study findings to non-ICU patients.
Conclusion
In hospitalized patients with COVID-19, using high-intensity thromboprophylaxis was not associated with a reduction in the primary outcome, in-hospital mortality, compared to standard-intensity thromboprophylaxis. Thus, these results do not support the routine use of high-intensity prophylactic anticoagulation in either ICU or non-ICU patients. However, more data from prospective clinical trials are still needed, especially in the case of non-ICU patients.
Data Sharing Statement
The datasets used and/or analyzed during the current study are not publicly available due the presence of patients’ specific information and the limitation enforced by the IRB office in the MOH.
Ethics Approval and Consent to Participate
Ethical approval was received from the Ministry of Health (MOH), Kingdom of Saudi Arabia (IRB No: 21-96 M/14-09-2021), with the need for written consent waived by the ethical committee due to the retrospective nature of the study. All methods were carried out in accordance with relevant guidelines and regulations.
Acknowledgments
The authors would like to extend their appreciation to King Saud University for funding this work through the Researcher Supporting Project (RSP-2021/77), King Saud University, Riyadh, Saudi Arabia. In addition, authors would also thank the General Directorate of Clinical Excellence at the Saudi Ministry of Health for providing the data collected from hospitals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The author (OAA) received a fund from the Researcher Supporting Project number (RSP-2021/77), King Saud University, Riyadh, Saudi Arabia, to support the publication of this article. The funding agency had no role in designing the study, conducting the analysis, interpreting the data or writing the manuscript.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sridharan GK, Vegunta R, Rokkam VRP, et al. Venous thromboembolism in hospitalized COVID-19 patients. Am J Ther. 2020;27(6):e599–e610. doi:10.1097/mjt.0000000000001295
2. Jiménez D, García-Sanchez A, Rali P, et al. Incidence of VTE and bleeding among hospitalized patients with Coronavirus Disease 2019: a systematic review and meta-analysis. Chest. 2021;159(3):1182–1196. doi:10.1016/j.chest.2020.11.005
3. Porfidia A, Valeriani E, Pola R, et al. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res. 2020;196:67–74. doi:10.1016/j.thromres.2020.08.020
4. Nopp S, Moik F, Jilma B, et al. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4(7):1178–1191. doi:10.1002/rth2.12439
5. Mansory EM, Srigunapalan S, Lazo-Langner A. Venous thromboembolism in hospitalized critical and noncritical COVID-19 patients: a systematic review and meta-analysis. TH Open. 2021;5(3):e286–e294. doi:10.1055/s-0041-1730967
6. Spyropoulos AC, Bonaca MP. Studying the coagulopathy of COVID-19. Lancet. 2021. doi:10.1016/S0140-6736(21)01906-1
7. Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation. 2020;142(17):1609–1611. doi:10.1161/CIRCULATIONAHA.120.050354
8. Ma L, Sahu SK, Cano M, et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci Immunol. 2021;6(59). doi:10.1126/sciimmunol.abh2259
9. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18(7):1559–1561. doi:10.1111/jth.14849
10. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi:10.1016/j.jacc.2020.08.041
11. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
12. Ferguson J, Volk S, Vondracek T, et al. Empiric therapeutic anticoagulation and mortality in critically ill patients with respiratory failure from SARS-CoV-2: a retrospective cohort study. J Clin Pharmacol. 2020;60(11):1411–1415. doi:10.1002/jcph.1749
13. Motta JK, Ogunnaike RO, Shah R, et al. Clinical outcomes with the use of prophylactic versus therapeutic anticoagulation in Coronavirus Disease 2019. Crit Care Explor. 2020;2(12):e0309. doi:10.1097/cce.0000000000000309
14. Helms J, Severac F, Merdji H, et al. Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: bi-Center cohort study. Ann Intensive Care. 2021;11(1):14. doi:10.1186/s13613-021-00809-5
15. Hsu A, Liu Y, Zayac AS, et al. Intensity of anticoagulation and survival in patients hospitalized with COVID-19 pneumonia. Thromb Res. 2020;196:375–378. doi:10.1016/j.thromres.2020.09.030
16. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi:10.1016/j.jacc.2020.05.001
17. Al-Samkari H, Gupta S, Leaf RK, et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann Intern Med. 2021;174(5):622–632. doi:10.7326/m20-6739
18. Moll M, Zon RL, Sylvester KW, et al. Intermediate versus standard dose heparin prophylaxis in COVID-19 ICU patients: a propensity score-matched analysis. Thromb Res. 2021;203:57–60. doi:10.1016/j.thromres.2021.04.009
19. Meizlish ML, Goshua G, Liu Y, et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. Am J Hematol. 2021;96(4):471–479. doi:10.1002/ajh.26102
20. Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872–888. doi:10.1182/bloodadvances.2020003763
21. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with Coronavirus Disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi:10.1016/j.chest.2020.05.559
22. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859–1865. doi:10.1111/jth.14929
23. National Institutes of Health: COVID-19 Treatment Guidelines Panel. COVID-19 treatment guidelines: antithrombotic therapy in patients with COVID-19; 2021. Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/.
24. Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–1630. doi:10.1001/jama.2021.4152
25. Perepu US, Chambers I, Wahab A, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost. 2021;19(9):2225–2234. doi:10.1111/jth.15450
26. Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612–1620. doi:10.1001/jamainternmed.2021.6203
27. Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi:10.1136/bmj.n2400
28. Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802. doi:10.1056/NEJMoa2105911
29. Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385(9):777–789. doi:10.1056/NEJMoa2103417
30. SAS Institute Inc. The PSMATCH Procedure. SAS/STAT® 142 User’s Guide. Cary, NC.: SAS Institute Inc.; 2016.
31. Pan W, Bai H. Propensity score methods for causal inference: an overview. Behaviormetrika. 2018;45(2):317–334. doi:10.1007/s41237-018-0058-8
32. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75(10):1340–1349. doi:10.1111/anae.15201
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
