Back to Journals » Cancer Management and Research » Volume 12
Impact of the Activation Status of the Akt/mTOR Signalling Pathway on the Clinical Behaviour of Synovial Sarcoma: Retrospective Analysis of 174 Patients at a Single Institution
Authors Li YX, Ding SS, Wen WJ, Han L, Wang HQ , Shi HY
Received 24 August 2019
Accepted for publication 8 February 2020
Published 9 March 2020 Volume 2020:12 Pages 1759—1769
DOI https://doi.org/10.2147/CMAR.S228578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Eileen O'Reilly
Ying-Xue Li,1,2 Shan-Shan Ding,3 Wen-Juan Wen,2 Lin Han,2 Hong-Qun Wang,1 Huai-Yin Shi1
1Department of Pathology, Medical School of Chinese People’s Liberation Army, Beijing 100853, People’s Republic of China; 2Department of Pathology, Liaocheng People’s Hospital, Liaocheng 252000, Shandong, People’s Republic of China; 3Department of Pathology, PLA Rocket Force Characteristic Medical Center, Beijing 100032, People’s Republic of China
Correspondence: Huai-Yin Shi
Department of Pathology, Medical School of Chinese PLA, Beijing 100853, People’s Republic of China
Tel/fax +86 010 668 873 29
Email [email protected]
Purpose: Phosphoinositide 3-kinase (PI3K) and the downstream Akt/mammalian target of rapamycin (mTOR) pathway are central to the control of cell proliferation and survival. Although abnormal activation of this pathway has been well established in a variety of tumours, limited studies are available on synovial sarcoma. The aim of this study was to investigate the expression of several key proteins of those pathways in synovial sarcomas and to correlate the expression of these proteins with clinicopathologic features and prognosis.
Patients and Methods: A total of 174 patients with synovial sarcomas were recruited for this study. The phosphorylation status of Akt, mTOR, and eukaryotic translation initiation factor 4E binding protein (4E-BP1) was measured by immunohistochemistry assays in formalin-fixed, paraffin-embedded samples. Correlations between the expression levels of these proteins and clinicopathologic features and prognosis were analysed.
Results: The positive rates of phosphorylated (p)Akt, pmTOR, p4E-BP1, and CyclinD1 were 62.7%, 55.6%, 47.1%, and 52.6%, respectively. The positive results of pmTOR, pAkt, and downstream p4E-BP1 were correlated with each other. The positive pAkt, pmTOR, p4E-BP1, and CyclinD1 results were more highly expressed in head and neck and visceral tumours, and positive p4E-BP1 results were correlated with larger size and larger areas of necrosis. In multivariate analysis of clinicopathologic factors, head and neck and visceral location, large tumour size, larger areas of necrosis and frequent mitosis were confirmed as risk factors for shorter overall survival. Positive pAkt, pmTOR and p4E-BP1 results were correlated significantly with shorter overall survival, and CyclinD1 was not in the univariate analysis. The positive pmTOR, pAkt, p4E-BP1, and CyclinD1 results were significantly poor prognostic factors for overall survival, and only positive p4E-BP1 results were significantly associated with shorter event-free survival in multivariate analysis.
Conclusion: This study demonstrated the high expression of pAkt, pmTOR, and p4E-BP1 associated with aggressive clinical behaviour in synovial sarcomas and provided evidence for prognostic evaluation and targeted therapy.
Keywords: synovial sarcoma, pAkt, pmTOR, p4E-BP1, clinicopathologic features, prognosis
Introduction
Synovial sarcoma (SS) is a translocation-associated mesenchymal malignant tumour that accounts for approximately 10% of all soft tissue sarcomas. The characteristic chromosomal translocation t (18; X) (p11; q11), which leads to the formation of a fusion protein, is thought to be the cause of this disease.1–3 Three main histological subtypes of SS have been recognised: biphasic, monophasic and poorly differentiated subtypes.4 The most common treatment for SS is wide surgical resection with or without chemotherapy and radiotherapy.5 Even with multimodal treatments, the prognosis is still poor once distant metastasis occurs.6,7 Therefore, it is important to investigate the prognostic indicator of disease progression and overall survival in SS.
The phosphoinositide 3-kinase (PI3K) and the downstream Akt/mammalian target of rapamycin (mTOR) pathway are central to the control of cell transcription, translation, metabolism, proliferation, migration and survival.8 Preclinical and epidemiological studies have confirmed that the PI3K/AKT/mTOR pathway plays an essential role in tumour progression and that it is a pivotal factor in regulating tumour cell metabolism and tumour angiogenesis.9,10 Although abnormal activation of the PI3K/Akt/mTOR pathway has been well established in a variety of tumours, including lung cancer, colorectal cancer, breast cancer, endometrial carcinoma and glioblastoma, limited studies are available in synovial sarcoma.11–15 CyclinD1 is a key regulator of the G1/S transition of the cell cycle and is overexpressed in a variety of malignancies. Umekita Y and Ikehara M showed that overexpression of CyclinD1 predicted poor prognosis in breast cancer and adenocarcinomas of the lung.16,17 J Averous observed that 4E-BP1 played a key role in coupling CyclinD1 expression to mTORC1 signalling.18 In this study, we focused on the phosphorylation status of Akt, mTOR, 4E-BP1, and CyclinD1 in a large series of synovial sarcomas and then analysed the relationship of pAkt, pmTOR, p4E-BP1, and CyclinD1 with clinicopathologic features and prognosis.
Materials and Methods
Patients and Materials
We collected 554 consecutive cases from the database of the Department of Pathology, Chinese PLA General Hospital, with a histological diagnosis of synovial sarcoma who were treated between January 2006 and December 2016. Pathology reports and clinical charts were reviewed, and all the useful information was collected. In order to avoid the diagnosis deviation from SS, sections of all patients were re-evaluated independently by two experienced pathologists (Huai-Yin Shi and Hong-Qun Wang). When required, a molecular confirmation was performed by fluorescence in situ hybridisation (FISH) or reverse transcription polymerase chain reaction (RT-PCR) to detect the presence of the specific (X;18)(p11;q11) translocations. The material for the immunohistochemistry was inadequate in 318 cases, and data on outcome were incomplete in 52 cases; therefore, only 174 patients could be included in this study and retrospectively analysed for survival.
Written informed consent was obtained from all patients or their legal guardians and with ethics committee approval of Chinese PLA General Hospital (NO. S2019-314-01). This study was conducted in accordance with the Declaration of Helsinki.
Tumour location was classified as head and neck, distal extremities, proximal extremities and trunk or viscera. Tumours were classified histologically into biphasic, monophasic, or poorly differentiated by pathologists using the World Health Organization (WHO) classification. The French Federation of Cancer Centers (FNCLCC) grading system was used to evaluate the extent of necrosis and mitosis.
Immunohistochemistry
The immunohistochemical study was performed for phospho-Akt (pAkt) (Ser473) (D9E) XPRabbit mAb (Cell Signaling Technology), rabbit anti-mTOR (phospho S2448) antibody (pmTOR) (Abcam), phospho-4E-BP1 (p4E-BP1) (Thr37/46) (236B4) (Cell Signaling Technology), CyclinD1 (clone DSC6, DAKO) and the mouse monoclonal antibody for Ki-67 (MIB-1) (DAKO). Four-micrometre-thick sections of formalin-fixed paraffin-embedded samples were stained according to laboratory standard operating procedures. The sections were pre-treated using pressure cooker antigen retrieval (citrate buffer; pH 6.0) for 2 min, using pAkt (1:50 dilution), pmTOR (1:400 dilution), p4E-BP1 (1:400 dilution), CyclinD1 (1:300 dilution) and Ki-67 (1:100 dilution) as primary antibodies and the EnVision Plus detection system (DAKO).19 Immunostaining of pAkt, pmTOR and p4E-BP1 was recognised in both the cytoplasm and nuclei, and staining of CyclinD1 and Ki-67 showed a nuclear staining pattern. The staining intensity and percentage of positive tumour cells were evaluated by two independent pathologists, and the cases were counted as “positive” or “high expression” when >10% of tumour cells showed moderate or strong staining intensity. PBS was used instead of the primary antibody to serve as a negative control, and slides of tissues known to express pAkt, pmTOR, p4E-BP1, CyclinD1 and Ki-67 were used as positive controls in each staining.
Statistical Analysis
All statistical analyses were performed using SPSS statistical software (version 17.0, Inc., Chicago). Associations between clinicopathologic factors and immunohistochemical staining results for pmTOR, pAkt, p4E-BP1, and CyclinD1 were analysed with the χ2 test or Fisher’s exact test. Overall survival (OS) and event-free survival (EFS) were estimated using the Kaplan-Meier method. OS time was defined as the time between histologic diagnosis and death or the last follow-up visit. EFS time was defined as the time between histologic diagnosis and the local recurrence, distant recurrence, or death. Correlations between clinicopathologic factors and immunopositivity for pAkt, pmTOR, and p4E-BP1 with survival were analysed by the Log rank test. Multivariable analysis was performed using Cox regression models. P values < 0.05 were considered statistically significant.
Results
Patients and Tumour Clinicopathologic Parameters
The clinicopathologic parameters and the survival analysis results of all 174 patients are summarised in Table 1 and Figures 1 and 2. The follow-up ranged from 10 months to 231 months (median, 63 months) for OS in 174 patients with an OS rate of 68.4%. The follow-up ranged from 1 month to 195 months (median, 24 months) for EFS in 174 patients with a 5-year EFS rate of 40.5%. Forty-eight percent of synovial sarcoma occurred in the proximal extremities, with significant differences in tumour location between the <30 age group and the ≥30 age group (P = 0.0027). Proximal tumours were more likely to undergo chemotherapy (P < 0.001) and radiation (P = 0.0004) than were other tumours, and younger age was significantly associated with chemotherapy (<30 are group 79.37%, ≥30 age group 64.86%) (P = 0.0277). In terms of surgical margin, wide resection was adopted for distal and proximal tumours, while marginal resection or intralesional resection was more common for head and neck and visceral tumours (P < 0.001). Large tumours tended to adopt wide resection (P = 0.006). Poorly differentiated SS exhibited larger areas of necrosis (P < 0.001) and more frequent mitosis (P = 0.007) than those of the other (biphasic or monophasic) histologic subtypes. Visceral tumours had larger areas of necrosis (P = 0.046) and more frequent mitosis (P = 0.038) than those of the other (head and neck, distal extremities, proximal extremities and trunk) locations (data not shown).
 |
Table 1 Clinicopathologic Parameters and Survival Analysis |
 |
Figure 1 Overall survival according to representative clinicopathologic parameters (P < 0.05; log-rank test). |
 |
Figure 2 Event-free survival according to location and surgical margin (P < 0.05; log-rank test). |
Head and neck and visceral tumours (P < 0.001), large tumour size (P = 0.045), larger areas of necrosis (P = 0.039) and more frequent mitosis (P < 0.001) were factors found to be significantly associated with poor overall survival in patients by univariate analysis. In addition, head and neck and visceral tumours were more prone to relapse or metastasis (EFS, P < 0.001). We did not observe a different prognosis according to the histologic subtype (EFS, P = 0.587; OS, P = 0.102).
Chemotherapy, Radiation and Surgical Margin
One hundred fifteen patients received adjuvant chemotherapy (74 preoperative and 41 postoperative). The most common adjuvant chemotherapy regimens were a combination of ifosfamide (at a dose of 9 g/m2) and doxorubicin (at a dose of 80 mg/m2) or epirubicin (at a dose of 120 mg/m2). The patients who had received adjuvant chemotherapy (preoperative and/or postoperative) had a better prognosis than those without chemotherapy (OS, P < 0.001; EFS, P = 0.068). Furthermore, patients who received more than three preoperative chemotherapy sessions had a better prognosis than patients with only postoperative chemotherapy (OS, P = 0.019; EFS, P = 0.036) (data not shown). There was no significant difference in the use of radiation. In terms of surgical margin, the prognosis of patients undergoing extensive tumour resection was significantly better than that of patients undergoing marginal resection or internal resection (OS, P < 0.001; EFS, P < 0.001).
Immunohistochemical Reactivity of pmTOR, pAkt, p4E-BP1, and CyclinD1
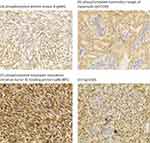
The results of immunohistochemical studies of pmTOR, pAkt, p4E-BP1, and CyclinD1 are summarised in Table 2 and in Figure 3. The positive rates of pmTOR, pAkt, p4E-BP1, and CyclinD1 were 62.7%, 55.6%, 47.1%, and 52.6%, respectively. The expression of pmTOR, pAkt, and p4E-BP1 was mainly cytoplasmic, and in some cases, parallel nuclear staining was visible. In the tumours of biphasic SS, much stronger staining in nuclei than that in the cytoplasm of epithelioid tumour cells was observed, and the positive rate of epithelioid-predominant staining pattern was pAkt (62.5%, 10/16, P = 0.359), pmTOR (70.0%, 7/10, P = 0.559) and p4E-BP1 (53.3%, 8/15, P = 0.013). In poorly differentiated SS, the positive rates of pAkt, pmTOR and p4E-BP1 were likely to be higher than those of other tissue subtypes and were 80.0% (4/5, P = 0.359), 80.0% (4/5, P = 0.559) and 90% (9/10, P = 0.013), respectively. CyclinD1 expression was nuclear and displayed an epithelioid-predominant staining pattern in biphasic SS. The positive rate of CyclinD1 in monophasic, biphasic and poorly differentiated SS was 45%, 84.6%, and 50%, respectively (P = 0.030).
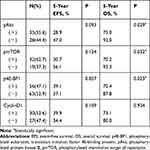 |
Table 2 Immunohistochemical Results and Survival Analysis |
The statistical analysis for possible correlations of the immunohistochemical results and clinicopathologic parameters was performed, showing several significant associations (Table 3). The positive results of pmTOR, pAkt, and downstream p4E-BP1 were correlated with each other, whereas CyclinD1 expression did not show a significant correlation with other molecules in the Akt/mTOR signalling pathway. Tumours with pAKT expression usually showed pmTOR positivity (P < 0.001) and p4E-BP1 (P < 0.001) positivity. Furthermore, pmTOR positivity was significantly associated with p4E-BP1 positivity (P = 0.038). pAkt, pmTOR, p4E-BP1, and CyclinD1 were more highly expressed in head and neck and visceral tumours than in distal extremities, proximal extremities and the trunk (P = 0.042, P = 0.027, and P = 0.025, respectively). There was no difference in the expression of pAkt, pmTOR and p4E-BP1 between the preoperative chemotherapy group and the postoperative chemotherapy group, but the expression of CyclinD1 in the preoperative chemotherapy group was lower than that in the postoperative chemotherapy group (P = 0.013). The positive results of p4E-BP1 tended to occur in larger size tumours (P = 0.026) and in those with larger areas of necrosis (P = 0.023).
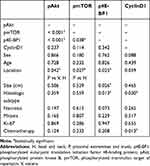 |
Table 3 Statistical Analysis of the Immunohistochemical Results and Clinicopathologic Parameters |
The survival analysis of the immunohistochemical results is summarised in Table 2 and in Figure 4. Immunopositivity of pAkt, pmTOR and p4E-BP1 was a significant risk factor for a poorer prognosis (OS: P = 0.042, P = 0.027, and P = 0.025, respectively). The Kaplan-Meier survival curves for overall survival rates according to the immunohistochemical results of pAkt, pmTOR and p4E-BP1 are displayed in Figure 4. We did not observe a different prognosis according to the immunohistochemical results of CyclinD1.
Multivariate Survival Analysis
After multivariate analysis of clinicopathologic factors, head and neck and visceral location, large tumour size, larger areas of necrosis and frequent mitosis were confirmed as negative prognostic parameters. The immunohistochemical results of pmTOR, pAkt, p4E-BP1, and CyclinD1 were adjusted by the above 4 clinicopathologic parameters (location, size, tumour necrosis and mitotic activity) (Table 4), and the multivariate analysis indicated that pmTOR, pAkt, p4E-BP1, and CyclinD1 were significantly poor prognostic factors for overall survival (P < 0.001, P = 0.001, P < 0.001, and P < 0.001, respectively), and only positive p4E-BP1 results were significantly associated with shorter event-free survival (P = 0.034).
 |
Table 4 Multivariate Survival Analysis for Immunohistochemical Parameters Adjusted by Location, Size, Tumour Necrosis and Mitotic Activity |
Discussion
To develop effective targeted therapies, several key signalling pathways have attracted interest over the past decade and have been identified as potential treatment targets in sarcoma.1,20 The Akt/mTOR pathway regulates all critical phases of cell growth, including proliferation, differentiation, and apoptosis, and deregulation of this pathway has been reported in several types of tumours.8,13,14,21,22 The oncogenic role of aberrant Akt/mTOR signalling pathway activation has been investigated in sarcoma.11,23 However, few previous studies from the Chinese population have focused on synovial sarcomas. The present study aimed to investigate the mechanism of activation of the Akt/mTOR pathway and its engagement in clinicopathologic features and prognosis in a large series of synovial sarcomas from a Chinese population.
Several adverse clinical prognostic factors of synovial sarcomas have been identified in previous large studies.6,7,24–27 The results of our study demonstrated that large tumour size, head and neck and visceral location, larger areas of necrosis and frequent mitosis were poor prognostic factors for SS. Large tumour size, larger areas of necrosis and high mitotic activity have been consistently associated with poor outcomes for SS in many previous studies.6,28,29 We also observed that head and neck and visceral tumours were more prone to relapse or metastasis. As far as we know, the prognostic implications of head and neck and visceral location have rarely been assessed; however, Deshmukh et al reported that proximal and truncal tumours have a worse survival than tumours of the distal extremities.29 G. Louis et al reported that small and superficial SSs have a better overall survival than larger and deeper-seated synovial sarcomas.30 The role of the histologic subtype was not certain; biphasic histology appears to be associated with better prognoses, but in our study, we demonstrated a statistically significant difference in overall survival when we compared monophasic synovial sarcoma with poorly differentiated synovial sarcoma.
Whether chemotherapy provides a clinically meaningful benefit for patients with SS with localised disease is still debated. In our study, the patients who had received adjuvant chemotherapy (preoperative and/or postoperative) had a better prognosis than those without chemotherapy. Furthermore, patients who received more than three preoperative chemotherapy sessions had a better prognosis than patients with only postoperative chemotherapy. Unlike our SS studies, the current series did not demonstrate better survival for patients with localised disease undergoing chemotherapy.25 In addition, we were not able to further verify the potential role of radiotherapy in SS. Several reports have indicated that adequate surgical excision is the mainstay of treatment for SS.31 We also found that the prognosis of patients undergoing extensive tumour resection was significantly better than that of patients undergoing marginal resection or internal resection.
The phosphorylation of kinases along the Akt/mTOR pathway reflects their activated status. In our study, we used immunohistochemistry with three phosphorylation-specific antibodies to detect Akt, mTOR, and 4E-BP1 phosphorylation status in synovial sarcomas. We found that higher levels of pAKT, pmTOR, and p4E-BP1 were statistically significantly associated with poor OS. The immunopositivity for pAkt, pmTOR, and p4E-BP1 was identified as an adverse prognostic factor in this study. These results support previous studies on patients with gastric carcinomas, breast cancers and sarcomas.23,26,32 We also found that each pair of phosphorylated proteins was highly positively associated in synovial sarcomas, ie, tumours with pAKT expression usually showed pmTOR positivity and p4E-BP1 positivity. Furthermore, pmTOR positivity was significantly associated with p4E-BP1 positivity. This phenomenon confirmed the activation cascade of the Akt/mTOR/4E-BP1 pathway in synovial sarcomas. In previous studies, Zhou et al also reported that the positive expression results of pmTOR, pAkt, and downstream p4E-BP1 were correlated with each other in invasive breast cancers.33 Although we did not observe a different prognosis according to the immunohistochemical results of CyclinD1, the multivariate analysis indicated that CyclinD1 was a significantly poor prognostic factor for overall survival. In contrast, EWA et al reported that pAKT expression was associated with high CyclinD1 labelling in neuroblastoma,21 whereas CyclinD1 expression did not show a significant correlation with other molecules in the Akt/mTOR signalling pathway in our study.
In poorly differentiated SS, the positive rate of pAkt, pmTOR and p4E-BP1 was likely to be higher than that in other tissue subtypes. pAkt, pmTOR, and p4E-BP1 were more highly expressed in head and neck and visceral tumours than in distal extremities, proximal extremities and the trunk. Kazuki et al reported that pAkt tended to increase with low differentiation and deep invasion in oral cancer.34 These features suggested that pAkt, pmTOR and p4E-BP1 might correlate with low differentiation and could be prognostic factors. The univariate prognostic factor p4E-BP1 was correlated with a larger size and a larger extent of necrosis. Because p4E-BP1 is considered a funnelling factor through which the transforming signals converge, it channels the oncogenic proliferative signalling pathways regardless of the upstream specific oncogenic alteration. EWA et al reported that high expression of p-4E-BP1 was significantly associated with lower tumour differentiation.21
In our study, we demonstrated that the expression of pmTOR, pAkt, and p4E-BP1 was mainly cytoplasmic, and in some cases, parallel nuclear staining was visible. Kazuki et al showed that pAkt and pmTOR were localised to the nuclei and cytoplasm of epithelial or carcinoma cells in oral squamous cell carcinoma.34 EWA et al observed concurrent cytoplasmic and nuclear pmTOR expression in neuroblastoma and p4E-BP1 in most cases of neuroblastoma in the cytoplasm and often within the nucleus.21 In addition, we found that much stronger staining in nuclei than in cytoplasm of epithelioid tumour cells was observed in the biphasic SS tumours. Setsu et al have also reported that some tumours had extensive staining in the cytoplasm of epithelioid SS cells.23 This epithelioid-dominant staining pattern was reported in some previous studies, but its significance remains unclear.35
As a downstream target of phosphorylated Akt, inhibition of mTOR would also be a potential therapeutic approach to reduce the effects of constitutively activated Akt in sarcomas. Previous studies have demonstrated that mTOR inhibition could decrease the proliferation, migration and invasion of multiple synovial sarcoma cell lines in vitro.36 Moreover, clinical trials of molecular targeting drugs have also been undertaken in soft-tissue sarcoma. Ridaforolimus, a small-molecule kinase inhibitor of mTOR, exhibited significant antitumor activity in preclinical and clinical studies and prolonged progression-free survival in a clinical trial targeting advanced soft-tissue sarcoma, although there was no significant difference in OS.37,38 Pazopanib, a multitarget kinase inhibitor, also exhibited potential efficacy against soft-tissue sarcoma, especially in a group of patients with SS.39,40 In our study, frequent deregulation of the Akt/mTOR signalling pathway and its prognostic role in synovial sarcomas support the notion of using mTOR inhibitors as an additional synovial sarcoma treatment. Moreover, the detection of pAkt, pmTOR, or p4E-BP1 through the simplicity and reproducibility of immunohistochemical staining might be very helpful for identifying and predicting which patients are most likely to derive the most benefit from treatment with an mTOR inhibitor.
Conclusion
We analysed the expression of key proteins of the Akt/mTOR pathway, including pAkt, pmTOR, and p4E-BP1, in synovial sarcomas, and this was compared to relevant clinicopathologic features and prognosis of synovial sarcomas. We demonstrated the high expression of pAkt, pmTOR, and p4E-BP1 associated with aggressive clinical behaviour in SS. Further study of the Akt/mTOR pathway will be helpful in determining better markers for prognostic evaluation and effective therapeutic targets for synovial sarcomas.
Ethics Approval
These studies were performed in accordance with ethical guidelines under the protocols approved by the Institutional Medical Ethics Review Board of Chinese PLA General Hospital, Beijing, China, and the reference number was S2019-314-01.
Acknowledgment
This study was supported by the Medical and Health Science and Technology Development Plan project of Shandong Province (2017WSA15057).
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Stacchiotti S, Van Tine BA. Synovial sarcoma: current concepts and future perspectives. J Clin Oncol off J Am Soc Clin Oncol. 2017;36(2):JCO2017751941.
2. Su L, Sampaio Arthur V, Jones Kevin B, et al. Deconstruction of the SS18-SSX fusion oncoprotein complex: insights into disease etiology and therapeutics. Cancer Cell. 2012;21(3):333–347. doi:10.1016/j.ccr.2012.01.010
3. Svejstrup Jesper Q. Synovial sarcoma mechanisms: a series of unfortunate events. Cell. 2013;153(1):11–12. doi:10.1016/j.cell.2013.03.015
4. Spiguel A. Soft tissue sarcomas. Cancer Treat Res. 2014;162:203–223. doi:10.1007/978-3-319-07323-1_10
5. Palmerini E, Staals EL, Alberghini M, et al. Synovial sarcoma. Cancer. 2009;115(13):2988–2998. doi:10.1002/cncr.v115:13
6. Bianchi G, Sambri A, Righi A, Dei Tos AP, Picci P, Donati D. Histology and grading are important prognostic factors in synovial sarcoma. Eur J Surg Oncol. 2017;43(9):1733–1739. doi:10.1016/j.ejso.2017.05.020
7. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627–634. doi:10.1002/(ISSN)1097-0142
8. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature Rev Drug Discovery. 2014;13(2):140–156. doi:10.1038/nrd4204
9. Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA a Cancer J Clin. 2010;59(2):111–137. doi:10.3322/caac.20003
10. Parker VER, Knox RG, Zhang Q, Wakelam MJO, Semple RK. Phosphoinositide 3-kinase-related overgrowth: cellular phenotype and future therapeutic options. Lancet. 2015;385:S77. doi:10.1016/S0140-6736(15)60392-0
11. Hernando E, Charytonowicz E, Me MS, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13(6):748–753. doi:10.1038/nm1560
12. Turner NC, Neven P, Loibl S, Andre F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet. 2017;389(10087):2403–2414. doi:10.1016/S0140-6736(16)32419-9
13. Bahrami A, Hasanzadeh M, Hassanian SM, et al. The potential value of the PI3K/Akt/mTOR signaling pathway for assessing prognosis in cervical cancer and as a target for therapy. J Cell Biochem. 2017;118(12):4163–4169. doi:10.1002/jcb.v118.12
14. Bahrami A, Khazaei M, Shahidsales S, et al. The therapeutic potential of PI3K/Akt/mTOR inhibitors in breast cancer: rational and progress. J Cell Biochem. 2018;119(1):213–222. doi:10.1002/jcb.26136
15. Li X, Wu C, Chen N, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7(22):33440–33450. doi:10.18632/oncotarget.7961
16. Umekita Y, Ohi Y, Sagara Y, Yoshida H. Overexpression of cyclinD1 predicts for poor prognosis in estrogen receptor-negative breast cancer patients. Int J Cancer. 2002;98(3):415–418. doi:10.1002/ijc.10151
17. Ikehara M, Oshita F, Ito H, Ohgane N, Kameda Y. Expression of cyclin D1 but not of cyclin E is an indicator of poor prognosis in small adenocarcinomas of the lung. Oncol Rep. 2003;10(1):137–139.
18. Averous J, Fonseca BD, Proud CG. Regulation of Cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene. 2007;27:1106–1113.
19. Li Y, Zhang X, Wang A, et al. Transducer-like enhancer of split 1 (TLE1) as a novel biomarker for diagnosis of synovial sarcoma correlates with translocation t(X;18): a study of 155 cases in China. Int J Clin Exp Pathol. 2019;12(1):251–258.
20. Hoang NT, Acevedo LA, Mann MJ, Tolani B. A review of soft-tissue sarcomas: translation of biological advances into treatment measures. Cancer Manag Res. 2018;10:1089–1114. doi:10.2147/CMAR.S159641
21. Ewa IYW, El Bieta DY, Robert R, et al. Analysis of PI3K/AKT/mTOR signalling pathway in high risk neuroblastic tumours. Pol J Pathol off J Pol Soc Pathologists. 2010;61(4):192–198.
22. Corti F, Nichetti F, Raimondi A, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: a review of current evidences and future perspectives. Cancer Treat Rev. 2019;72:45–55. doi:10.1016/j.ctrv.2018.11.001
23. Setsu N, Kohashi K, Fushimi F, et al. Prognostic impact of the activation status of the Akt/mTOR pathway in synovial sarcoma. Cancer. 2013;119(19):3504–3513. doi:10.1002/cncr.28255
24. Marc L, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002;62(1):135–140.
25. Outani H, Nakamura T, Murata H, et al. Localized synovial sarcoma: a single institutional study of 191 patients with a minimum follow-up of 5 years for survivors. J Surg Oncol. 2019;119(7):850–855. doi:10.1002/jso.v119.7
26. Scheer M, Dantonello T, Hallmen E, et al. Synovial sarcoma recurrence in children and young adults. Ann Surg Oncol. 2016;23(S5):618–626. doi:10.1245/s10434-016-5535-2
27. Stegmaier S, Leuschner I, Poremba C, et al. The prognostic impact of SYT-SSX fusion type and histological grade in pediatric patients with synovial sarcoma treated according to the CWS (Cooperative Weichteilsarkom Studie) trials. Pediatr Blood Cancer. 2017;64(1):89–95. doi:10.1002/pbc.v64.1
28. Trassard M, Doussal V, Le Hacène K, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol off J Am Soc Clin Oncol. 2001;19(2):525. doi:10.1200/JCO.2001.19.2.525
29. Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clinorthoprelatres. 2004;419(419):155–161.
30. Louis G, Jean B, Fran Oise B, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol. 2004;22(20):4040–4050. doi:10.1200/JCO.2004.11.093
31. Randall RL, Schabel KLS, Ying H, Joyner DE, Albritton KH. Diagnosis and management of synovial sarcoma. J Surg Oncol. 2010;97(4):314–320.
32. Soares P, Deng L, Chen J, et al. Correlation between activation of PI3K/AKT/mTOR pathway and prognosis of breast cancer in Chinese women. PLoS One. 2015;10(3):e0120511. doi:10.1371/journal.pone.0120511
33. Xiaoyan Z, Ming T, Valerie SH, et al. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004;10(20):6779. doi:10.1158/1078-0432.CCR-04-0112
34. Tashiro K, Oikawa M, Miki Y, Takahashi T, Kumamoto H. Immunohistochemical assessment of growth factor signaling molecules: MAPK, Akt, and STAT3 pathways in oral epithelial precursor lesions and squamous cell carcinoma. Odontology. 2019;108:91–101.
35. Nicolaus F, Marcel T, Elmar E, et al. Phosphatidylinositol-3ʹ-kinase/AKT signaling is essential in synovial sarcoma. Int J Cancer. 2011;129(7):1564–1575. doi:10.1002/ijc.25829
36. Hui JL, Wang X, Crowe P, Goldstein D, Yang J. Targeting the PI3K/PTEN/AKT/mTOR pathway in treatment of sarcoma cell lines. Anticancer Res. 2016;36(11):5765–5771. doi:10.21873/anticanres.11160
37. Dancey JE, Jose M. Ridaforolimus: a promising drug in the treatment of soft-tissue sarcoma and other malignancies. Future Oncol. 2011;7(7):827–839. doi:10.2217/fon.11.57
38. Mita MM, Gong J, Chawla SP. Ridaforolimus in advanced or metastatic soft tissue and bone sarcomas. Expert Rev Clin Pharmacol. 2014;6(5):465–482. doi:10.1586/17512433.2013.827397
39. Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol (Madr). 2016;56(1):88–92. doi:10.1080/0284186X.2016.1234068
40. Wilky BA, Meyer CF, Trent JC. Pazopanib in sarcomas. Curr Opin Oncol. 2013;25(4):373–378. doi:10.1097/CCO.0b013e3283622d3a
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.