Back to Journals » Clinical and Experimental Gastroenterology » Volume 8
Impact of switching from mycophenolate mofetil to enteric-coated mycophenolate sodium on gastrointestinal side effects in patients with autoimmune disease: a Phase III, open-label, single-arm, multicenter study
Authors Manger B, Hiepe F, Schneider M, Worm M, Wimmer P, Paulus E, Schwarting A
Received 30 January 2015
Accepted for publication 1 May 2015
Published 21 July 2015 Volume 2015:8 Pages 205—213
DOI https://doi.org/10.2147/CEG.S81922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Andreas M. Kaiser
Bernhard Manger,1 Falk Hiepe,2 Matthias Schneider,3 Margitta Worm,4 Peter Wimmer,5 Eva-Maria Paulus,5 Andreas Schwarting6
1University Hospital Erlangen, Med Clinic III Polyclinic, Erlangen, 2Rheumatology, University Hospital Charité, Campus Mitte, Berlin, 3Policlinic of Rheumatology, Heinrich-Heine-Universität, Düsseldorf, 4Department of Dermatology, Venereology and Allergology, University Hospital Charité, Campus Mitte, Berlin, 5Novartis Pharma GmbH, Nürnberg, 6Department of Internal Medicine, Johannes Gutenberg University, Mainz, Germany
Background: The purpose of this study was to assess changes in gastrointestinal symptom severity in patients with autoimmune disease who were switched from mycophenolate mofetil to enteric-coated mycophenolate sodium (EC-MPS).
Methods: In this national, explorative, single-arm study, 111 patients were enrolled and switched to equimolar EC-MPS at baseline. The primary endpoint was change in the Gastrointestinal Symptom Rating Scale (GSRS) total score after 6–8 weeks of treatment (Visit 2). The optional follow-up visit was 6–12 weeks after completion of the study (Visit 2). Secondary endpoints were changes in GSRS subscale score; changes in gastrointestinal-related quality of life measured by the Gastrointestinal Quality of Life Index (GIQLI); and general health-related quality of life (HRQoL) measured by Psychological General Well-Being Index and assessment of overall treatment effect (OTE). Change was evaluated by paired t-tests.
Results: At Visit 2, the mean ± standard deviation GSRS total score improved from 2.28±1.13 to 2.02±0.93 points. The change (-0.28±0.92 points, P=0.002) was statistically significant. The change at the follow-up visit (-0.36±0.94 points, P=0.001) was statistically significant and more than the minimal clinical important difference. GSRS subscores showed statistically significant and clinically relevant improvement for abdominal pain (-0.51±1.2 points, P<0.001) and indigestion (-0.42±1.33 points, P=0.002). Overall GIQLI score showed significant improvement from baseline to Visit 2 (-5.8±18.6 points, P=0.002). Per OTE, improvement was reported in 44.1% and 34.2% patients as rated by physicians and patients, respectively. The majority of patients (55%) reported OTE-HRQoL as unchanged. Diarrhea and nausea were the commonly reported adverse events.
Conclusion: Patients switched to EC-MPS experienced less gastrointestinal symptom burden and showed improvement in HRQoL.
Keywords: mycophenolate mofetil, enteric-coated mycophenolate sodium, autoimmune disease, patient-reported outcome, health-related quality of life
Introduction
The pharmacological activity of mycophenolate mofetil (MMF) and enteric-coated mycophenolate sodium (EC-MPS) is derived entirely from mycophenolic acid (MPA).1,2 MPA is a potent, selective, and reversible inhibitor of inosine monophosphate dehydrogenase and inhibits the de novo pathway of guanosine nucleotide synthesis. MPA predominantly inhibits lymphocyte proliferation, because T-lymphocytes and B-lymphocytes are critically dependent on de novo synthesis of purines.3,4 MMF is rapidly and completely cleaved by gastrointestinal (GI) and liver esterases to yield MPA and morpholino ethanol.3,5
Although MMF is a highly potent agent that has contributed to improvement of immunosuppressive regimens, the efficacy has been limited by unwanted side effects, such as GI complications, including vomiting, diarrhea, esophagitis, gastritis, and bleeding.6 EC-MPS has been designed to reduce MPA-related upper GI adverse events (AEs) by allowing for delivery of the active substance into the small intestine without compromising safety and tolerability.7
The efficacy and safety of EC-MPS has been shown for rejection prophylaxis in de novo8 and maintenance9 renal transplant patients. The pivotal trials were designed to demonstrate therapeutic equivalence between EC-MPS and MMF. EC-MPS 720 mg has been shown to be bioequivalent10 to MMF 1,000 mg in terms of area under the curve, besides being shown as therapeutically equivalent.8 The maintenance study showed that conversion from MMF to EC-MPS did not compromise safety, indicating patients could be converted safely and effectively.9
A recent study in MMF-treated renal transplant patients with mild, moderate, or severe GI complaints showed that conversion from MMF to EC-MPS significantly reduced the GI-related symptom burden and improved patient quality of life.11 In particular, evidence from trials with patient-reported outcomes showed significant and consistent reduction of GI complaints.12–14
In autoimmune diseases, GI manifestations are common and lead to significant impairment of patients’ quality of life.15 It is important to reduce GI symptom burden and consider treatments also in view of their effects on patient-reported outcomes and health-related quality of life (HRQoL). A meta-analysis found that MMF was efficacious in the treatment of proliferative lupus nephritis.16 MMF, although efficacious in the treatment of systemic lupus erythematosus, was associated with GI intolerance.17 There is limited evidence on the benefits of switching from MMF to EC-MPS in terms of GI complaints in patients with autoimmune diseases overall. The objective of this explorative study was to investigate if patients with autoimmune disease could benefit from conversion from MMF to EC-MPS in terms of reduced GI symptoms and improved HRQoL.
Materials and methods
This Phase III, open-label, single-arm study was conducted at 19 centers in Germany from June 2006 to June 2009 (ClinicalTrials.gov identifier NCT00351377, http://clinicaltrials.gov). The study comprised a baseline visit followed by an end of study visit after 6–8 weeks (Visit 2) and an optional follow-up visit 6–12 weeks after Visit 2 (Figure S1). The study was designed and conducted in compliance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the ethical principles defined in the Declaration of Helsinki. The study was approved by the leading local independent ethical review committee at Friedrich-Alexander-University Erlangen-Nuremberg. Written informed consent was obtained from all eligible patients before any study-related procedures.
Patients
Patients aged ≥18 years with an autoimmune disease and who received immunosuppressive therapy including MMF at time of enrolment, required MPA treatment for the study duration, and were willing to switch to EC-MPS were included. MMF and any concomitant drugs that might cause GI symptoms (bisphosphonates, minerals, vitamins, antibiotics, or proton pump inhibitors) had to be administered at stable doses for at least 1 month before enrolment and during the study. Females of child-bearing age had to present a negative pregnancy test at baseline, agree to repeat this test every 4 weeks, and practice an approved method of birth control for the study duration plus 6 weeks.
Key exclusion criteria were: known hypersensitivity to the formulations of MPA and other drug components; GI symptoms not caused by MPA therapy; and acute medical intervention or hospitalization or conditions not related to GI events which required immediate medical intervention.
Treatment
EC-MPS, supplied as 180 mg and 360 mg tablets, was administered for 6–8 weeks according to the investigator’s instructions, preferably twice daily in the morning and in the evening. The dose of EC-MPS had to be equimolar to the dose of MMF the patient took at study entry.
Patients who discontinued study drug before Visit 2 (6–8 weeks after conversion) were considered withdrawn. If an AE was ongoing after discontinuation of the study drug, the patient was observed for safety evaluation for up to 30 days after the last dose of study drug. After study completion/withdrawal, patients could continue therapy with commercially available EC-MPS or an alternative immunosuppressive therapy at the discretion of the investigator.
Assessments
The primary endpoint was change in GI symptom severity measured by the Gastrointestinal Symptom Rating Scale (GSRS)18,19 total score at Visit 2 compared with baseline (6–8 weeks after the switch from MMF to EC-MPS). The secondary endpoints were changes at Visit 2 and the follow-up visit in GSRS total and subscale scores; changes in GI HRQoL measured by the Gastrointestinal Quality of Life Index (GIQLI);20 and general HRQoL measured by the Psychological General Well-Being Index (PGWB).21 Assessment of overall treatment effect (OTE)22 at Visit 2 was performed by both physicians (OTE for GI symptoms) and patients (OTE for GI symptoms and OTE for HRQoL).
At enrolment, all patients answered three questionnaires, ie, GSRS, GIQLI, and PGWB, and were then converted from MMF to EC-MPS. At Visit 2, all patients completed the GSRS, GIQLI, and PGWB, along with two additional questionnaires, ie, the OTE for symptoms and for HRQoL. The investigators and the patients completed the OTE for symptoms. In an optional follow-up visit, 6–12 weeks after Visit 2, the GSRS, GIQLI, and PGWB questionnaires were administered again. Valid and reliable self-administered measures with good psychometric characteristics23,24 that allow comparison with the results from related clinical studies were used to assess GI symptoms, their impact on daily life, and the well-being of the patients. All questionnaires for the patients were translated into German.
Gastrointestinal Symptom Rating Scale
The most common GI disorders were evaluated with the self-administered version of the GSRS. Within five subdimensions of this disease-specific 15-item measure, ie, reflux, diarrhea, constipation, abdominal pain, and indigestion, the present state of GI complaints is evaluated on a 7-point Likert scale ranging from 1 (absence of burden) to 7 (severe discomfort), producing a mean subscale score. The total score was calculated as the average of the 15 single items, reaching from 1 to 7 score points each, and thus also had a range from 1 to 7 score points. The psychometric characteristics of the German translation of the GSRS were found to be satisfactorily reliable and valid.25
Gastrointestinal Quality of Life Index
Patients’ perception of the impact of disease on their lives was determined using the GIQLI,20 a generic, self-administered 36-item questionnaire for digestive system diseases with five subscales (GI symptoms, emotional status, physical function, social function, stress of medical treatment). With each item ranging from 0 to 4, the total score was produced by summing the single items, leading to a hypothetical range of 0–144 score points. Lower scores represented poorer quality of life.
Psychological General Well-Being Index
Subjective well-being or distress was estimated with the interviewer-administered or self-administered PGWB.21 This generic 22-item instrument has six subscales, ie, anxiety, depressed mood, positive well-being, self-control, general health, and vitality, and one dimension for the total score. From each item ranging from 0 to 5, the total score with a theoretical range from 0 to 110 was computed by summing the items. It was further transformed using the formula (raw score/110) ×100 to fit a range from 0 to 100. Higher scores represented improved well-being.
Overall treatment effect
The change in symptoms or HRQoL was examined with the OTE,22 a 15-point scale ranging from −7 to −1 (worse) and 1 to 7 (better). The first question was whether the symptoms or HRQoL had improved, remained the same, or worsened. In case of betterment or deterioration, the patients were asked to rate the degree of change with possible answers as “almost the same, hardly better/worse at all” (1, −1) to “a very great deal better/worse” (7, −7). Safety was assessed mainly by the number of patients with treatment-emergent AEs.
Statistical analysis
The working hypothesis of this explorative study was that patients would demonstrate reduced GI symptom severity more similar to patients without GI complaints after conversion from MMF to EC-MPS. For all patient-reported outcomes, descriptive statistics, including the mean, standard deviation (SD), and range, were calculated at each time point. A paired t-test with a significance level of 0.05 (two-sided) was used to determine P-values for changes between visits. If patients discontinued due to lack of efficacy, their last value was carried forward for the analysis. The influence of sex on the primary variable was analyzed with a two-way analysis of variance model.
Secondary variables were explorative. No correction for multiplicity was made. Descriptive data were produced on all safety variables, including GI complaints. All analyses were based on the intent-to-treat population. Statistical analysis was performed using SAS version 8.2 (SAS Institute, Cary, NC, USA). The minimal clinical important difference26 for the GSRS total score was assumed to be 0.33 score points. Calculating further an SD of the GSRS total score of 1.8, an alpha level of 0.05, and a power of 90%, then a sample size of 165 evaluable patients would be needed.
Results
A total of 200 patients were planned to be enrolled. The study was discontinued prematurely due to slow recruitment, and the analysis was performed with 111 patients. Of the 111 patients screened, enrolled, and subsequently exposed to EC-MPS, 104 patients completed the study. Major protocol violations were noted in 27 patients (24.3%). The most common reasons for these major protocol violations were “dose of EC-MPS not equimolar to MMF dose at study entry” (14, 12.6%), “missing value in primary efficacy parameter” (9, 8.1%), and “too short a treatment duration” (7, 6.3%).
The mean ± SD age of the patient population was 49.5±14.1 years, with predominantly women (73.9%) and Caucasians (94.6%). The three most common autoimmune diseases were systemic lupus erythematosus (51, 45.9%), Basedow’s disease (17, 15.3%), and granulomatosis with polyangiitis (10, 9.0%). The median overall study drug exposure was 51 (range 4–120) days. The median daily dose of EC-MPS was 1,185.6 mg (range 180–2,487.3).
The majority of patients continued the initially chosen dose of the study medication. Dose changes occurred in 8% of patients due to dosing error (n=3), AEs or laboratory/test abnormalities (n=3), unsatisfactory therapeutic effect (n=1), or for other reasons (n=1).
The majority of patients were treated with additional immunosuppressants during the treatment (73%) and follow-up period (69.4%) in addition to the study drug. Eighty patients (72.1%) received systemic corticosteroids during the study and follow-up periods. During the follow-up period, 101 (91.8%) patients received further treatment; of these, the majority stayed on EC-MPS (65, 58.6%), whereas 14 patients (12.6%) switched back to MMF.
To evaluate their GI complaints at baseline, the patients could choose multiple dimensions in the GSRS questionnaire. Seventy-one patients (64.0%) indicated any GI complications. Three patients indicated their condition as severe, 32 (29%) as moderate, and 37 (33%) as mild. Abdominal pain, bloating, and fullness were the most frequently reported complaints, occurring in 41.4% of patients, followed by dyspepsia, diarrhea, and nausea, each occurring in approximately one-third of patients.
Patient-reported GI and HRQoL outcomes
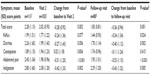
A statistically significant decrease (improvement) in mean ± SD GSRS total score was observed from 2.28±1.13 score points at baseline to 2.02±0.93 points at Visit 2. The mean ± SD change from baseline to Visit 2 was −0.28±0.92 points, which was statistically significant (P=0.002) and close to the minimal clinically important difference (0.33). The mean ± SD score at the follow-up visit was 1.83±0.81 points. However, the mean ± SD change from baseline to the follow-up visit was −0.36±0.94 points, which was both statistically significant (P=0.001) and clinically relevant.
In the treatment period, from baseline to Visit 2, mean ± SD GSRS subscores showed statistically significant and clinically relevant improvement for abdominal pain (−0.51±1.20 points; P<0.001) and indigestion (−0.42±1.33 points; P=0.002). The improvement in GSRS subscores for reflux and diarrhea was close to statistical significance and the minimal clinically important difference. However, the mean ± SD subscore for constipation showed a slight increase (worsening) by 0.02±1.18 points (P=0.836). Consistent with the total score, from Visit 2 to the follow-up visit, improvement in mean ± SD subscores occurred for reflux (−0.18±0.77 points; P=0.049), diarrhea (−0.18±0.87 points; P=0.069), and constipation (−0.12±0.93 points; P=0.249). Abdominal pain and indigestion showed a slight increase of 0.05±0.81 and 0.03±0.82 points, respectively (Table 1).
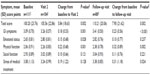
Mean ± SD overall GIQLI scores showed a significant improvement from 100.2±23.8 points at baseline to 105.6±22.9 points at Visit 2 (change, 5.8±18.6 points; P=0.002). The mean ± SD overall GIQLI score was 110.2±20.1 points at the follow-up visit (change from baseline, 7.9±21.6 points; P=0.002). Consistent with the GIQLI subscores, from baseline to Visit 2, there were significant improvements (ie, increases) in the subscores for GI symptoms (P=0.001), emotional status (P=0.028), and physical function (P=0.001). The increase in subscores for social function and stress of medical treatment was not significant. From baseline to the follow-up visit, there was a significant improvement in the GIQLI subscores for GI symptoms (P<0.001) and physical function (P=0.002; Table 2).
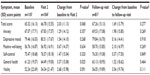
PGWB assessment showed a non-significant mean ± SD increase (improvement) by 2.0±11.1 points from baseline to Visit 2 and a subsequent non-significant decrease (worsening) by −0.8±9.7 points from Visit 2 to the follow-up visit (Table 3).
OTE yielded clinically significant results for all assessments (Figure 1). The improvements in OTE symptoms were rated slightly higher by the investigators than by the patients (44.1% versus 34.2%). The impact on HRQoL was not as marked because the majority of patients (55%) evaluated their condition as “about the same”. More patients evaluated their condition as “improved” (27.9%) than “worsened” (9.0%, Figure 1).
Safety
Of the 111 patients in the safety population, 76 (68.5%) experienced treatment-emergent AEs, with 44 (39.6%) experiencing GI-related complications and 60 (54.1%) experiencing other AEs. Most frequently “GI disorders” (40.5%), “infections and infestations” (23.4%), and “musculoskeletal and connective tissue disorders” (10.8%) occurred. Of the GI disorders, diarrhea and nausea (14.4% and 11.7%, respectively) were the most common (Table 4). Most of the AEs were classified as mild (34, 30.6%) or moderate (39, 35.1%). Only three patients suffered from AEs that were regarded as severe (one case each of thrombocytopenia, pneumonia, and diarrhea). Moderate GI complications occurred in 18 patients (16.2%) and moderate other AEs occurred in 25 patients (22.5%). Twenty-three patients (20.7%) had GI complications that were suspected to be drug-related compared with 13 patients (11.7%) who reported other drug-related AEs. Seven serious AEs were reported; one non-fatal and one fatal, both of which were not GI-related. One serious AE and six non-serious AEs led to discontinuation of the study drug.
  | Table 4 Number (%) of patients with most frequent adverse events (2.5% or more in the total population) |
Discussion
The results of this explorative study show a decline in the GI symptom burden and improvement in HRQoL after switching patients with autoimmune disease from MMF to EC-MPS. The GI symptom severity in terms of the GSRS total score as the primary objective significantly declined. This corresponds to the GSRS subscale results, the outcomes of the GIQLI, the improvement in general health reported in the PGWB, and the clinically significant decrease of symptoms observed with the OTE reported by patients and investigators. Nearly one third of the patients evaluated their HRQoL as “improved”, more than half as “about the same”, and only a small group (9%) as “worsened”. The subscale “general health” improved when assessing the PGWB. This result is consistent with the subscale results of the GIQLI concerning social and stress aspects and reflects the general impairment26–28 of the patients’ life due to the autoimmune disease. Further, significant improvements in GSRS and GIQLI observed at the follow-up visit indicate that EC-MPS provides sustained effects with respect to reducing GI symptom severity and improving quality of life.
The overall safety profile was favorable. Only six patients (5.5%) discontinued the study drug, and in three patients, doses were changed because of AEs or an unsatisfactory therapeutic effect. In one-fifth of patients, drug-related treatment-emergent GI disorders occurred. These effects of EC-MPS have also been observed in other studies.29,30 Generally, the observed AEs correspond to those outlined in the summary of product characteristics.7 In general, these results support the findings of other clinical studies with comparable design11,31–34 as well as the outcomes of randomized controlled trials.14,35,36
Early termination due to slow recruitment lead to a comparatively small sample size, and technical problems with measurement lead to unreliable or uninterpretable results. The latter complication is more common in trials utilizing patient-reported outcomes with self-administered measures37 as opposed to interviewer-administered instruments, and might be a reason for a high intervariability of results adding to known intra- and interpatient variabilty of GI reactions on MPA.38–41 The choice of tools and endpoints was reasonable in terms of the aim of the study, ie, to obtain patient-reported assessments.
The lack of a control group might bias trial results. Nevertheless, this was an important explorative study. Up until now, the evidence for the potential benefit of EC-MPS has been based on specific indications, such as lupus nephritis,42,43 cutaneous lupus,44,45 or uveitis,46 but not on a broader range of prevalent patterns within autoimmune diseases. The original plan of the study also included application of disease-specific measures as a secondary objective to identify specific indications that can profit most from conversion, but these evaluations were not performed because of the decreased sample size. These study results therefore, need to be interpreted with caution due to the decreased sample size.
Although new treatments for autoimmune disease are on the horizon,47 and have already been approved for systemic lupus erythematosus,48 MPA is important and widely accepted as a treatment of autoimmune diseases.49,50 Therefore, considering all limitations, this study makes a valuable contribution to the existing knowledge of treatment options for autoimmune diseases with an inherent high GI symptom burden and may also support decision-making regarding a better quality of life.
Conclusion
Patient-reported outcomes showed a clinically significant decrease in GI symptom severity. The safety profile was favorable and in accordance with the summary of product characteristics. Patients with autoimmune disease and GI complaints may benefit from switching from MMF to EC-MPS.
Acknowledgments
We thank Vinay Pagadala (Novartis Healthcare Pvt Ltd, India) and Bettina Trantow (freelance medical writer) for their editorial assistance with this paper.
Disclosure
This study was sponsored by Novartis Pharma GmbH. Financial support for editorial assistance was also provided by Novartis Pharma GmbH. Potential conflicts of interest are as follows: BM, FH, MS, and MW have received research funding from Novartis; AS has received research funding and consultancy fees from Novartis; and E-MP and PW are employees of Novartis Pharma GmbH.
References
Allison AC, Eugui EM. Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev. 1993;136:5–28. | |
Wu JC. Mycophenolate mofetil: molecular mechanisms of action. Perspectives in Drug Discovery and Design. 1994;2:185–204. | |
Bullingham RE, Nicholls A, Hale M. Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant Proc. 1996;28:925–929. | |
Zizzo G. De Santis M, Ferraccioli GF. Mycophenolic acid in rheumatology: mechanisms of action and severe adverse events. Reumatismo. 2010;62:91–100. | |
Lee WA, Gu L, Miksztal AR, Chu N, Leung K, Nelson PH. Bioavailability improvement of mycophenolic acid through amino ester derivatization. Pharm Res. 1990;7:161–166. | |
Behrend M. Adverse gastrointestinal effects of mycophenolate mofetil: aetiology, incidence and management. Drug Saf. 2001;24:645–663. | |
Budde K, Glander P, Diekmann F. Review of the immunosuppressant enteric-coated mycophenolate sodium. Expert Opin Pharmacother. 2004;5:1333–1345. | |
Salvadori M, Holzer H, de Mattos A, et al; ERL B301 Study Groups. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2004;4:231–236. | |
Budde K, Curtis J, Knoll G, et al; ERL B302 Study Group. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant. 2004;4:237–243. | |
Budde K, Bauer S, Hambach P, et al. Pharmacokinetic and pharmacodynamic comparison of enteric-coated mycophenolate sodium and mycophenolate mofetil in maintenance renal transplant patients. Am J Transplant. 2007;7:888–898. | |
Chan L, Mulgaonkar S, Walker R, Arns W, Ambühl P, Schiavelli R. Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplantation. 2006;81:1290–1297. | |
Budde K, Dürr M, Liefeldt L, Neumayer HH, Glander P. Enteric-coated mycophenolate sodium. Expert Opin Drug Saf. 2010;9:981–994. | |
Manitpisitkul W, Lee S, Cooper M. Mycophenolic acid agents: is enteric coating the answer? Transplant Research and Risk Management. 2011;3:45–53. | |
Bolin P Jr, Gohh R, Kandaswamy R, et al. Mycophenolic acid in kidney transplant patients with diabetes mellitus: does the formulation matter? Transplant Rev (Orlando). 2011;25:117–123. | |
Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Gastrointestinal manifestations in systemic autoimmune diseases. Maedica (Buchar). 2011;6:45–51. | |
Mak A, Cheak AA, Tan JY, Su HC, Ho RC, Lau CS. Mycophenolate mofetil is as efficacious as, but safer than, cyclophosphamide in the treatment of proliferative lupus nephritis: a meta-analysis and meta-regression. Rheumatology (Oxford). 2009;48:944–952. | |
Pisoni CN, Sanchez FJ, Karim Y, et al. Mycophenolate mofetil in systemic lupus erythematosus: efficacy and tolerability in 86 patients. J Rheumatol. 2005;32:1047–1052. | |
Dimenäs E, Glise H, Hallerbäck B, Hernqvist H, Svedlund J, Wiklund I. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand J Gastroenterol. 1995;30:1046–1052. | |
Dimenäs E, Carlsson G, Glise H, Israelsson B, Wiklund I. Relevance of norm values as part of the documentation of quality of life Instruments for use in upper gastrointestinal disease. Scand J Gastroenterol Suppl. 1996;221:8–13. | |
Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. | |
Dupuy HJ. The Psychological General Well-Being (PGWB) Index. In: Wenger NK, Mattson ME, Furberg CD, Elinson J, editors. Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. New York, NY, USA: Le Jacq Publishing; 1984. | |
Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. | |
Kleinman L, Kilburg A, Machnicki G, et al. Using GI-specific patient outcome measures in renal transplant patients: validation of the GSRS and GIQLI. Qual Life Res. 2006;15:1223–1232. | |
Machnicki G, Pefaur J, Gaite L, et al. Gastrointestinal (GI)-specific patient reported outcomes instruments differentiate between renal transplant patients with or without GI symptoms: results from a South American cohort. Health Qual Life Outcomes. 2008;6:53. | |
Kulich KR, Malfertheiner P, Madisch A, et al. Psychometric validation of the German translation of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in patients with reflux disease. Health Qual Life Outcomes. 2003;1:62. | |
Cook CE. Clinimetrics corner: the minimal clinically important change score (MCID): a necessary pretense. J Man Manip Ther. 2008;16:E82–E83. | |
Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol. 2005;32:1706–1708. | |
Yeatts RP. Quality of life in patients with Graves ophthalmopathy. Trans Am Ophthalmol Soc. 2005;103:368–411. | |
Brister K, Yau CL, Slakey D. Enteric coating of mycophenolate reduces dosage adjustments. Transplant Proc. 2009;41:1657–1659. | |
Sommerer C, Glander P, Arns W, et al. Safety and efficacy of intensified versus standard dosing regimens of enteric-coated mycophenolate sodium in de novo renal transplant patients. Transplantation. 2011;91:779–785. | |
Bolin P, Tanriover B, Zibari GB, et al. Improvement in 3-month patient-reported gastrointestinal symptoms after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in renal transplant patients. Transplantation. 2007;84:1443–1451. | |
Darji P, Vijayaraghavan R, Thiagarajan CM, et al. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in renal transplant recipients with gastrointestinal tract disorders. Transplant Proc. 2008;40:2262–2267. | |
Toledo AH, Hendrix L, Buchholz V, et al. Improvement of gastrointestinal symptoms after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in liver transplant patients. Clin Transplant. 2012;26:156–163. | |
Reinke P, Budde K, Hugo C, et al. Reductions of gastrointestinal complications in renal graft recipients after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplant Proc. 2011;43:1641–1646. | |
Langone AJ, Chan L, Bolin P, Cooper M. Enteric-coated mycophenolate sodium versus mycophenolate mofetil in renal transplant recipients experiencing gastrointestinal intolerance: a multicenter, double-blind, randomized study. Transplantation. 2011;91:470–478. | |
Shehata M, Bhandari S, Venkat-Raman G, et al. Effect of conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium on maximum tolerated dose and gastrointestinal symptoms following kidney transplantation. Transpl Int. 2009;22:821–830. | |
Friedman LM, Furberg CD, DeMets DL, et al. Assessment of health-related quality of life. In: Friedman LM, Furberg CD, DeMets DL, editors. Fundamentals of Clinical Trials. New York, NY, USA: Springer; 2010. | |
Roland M, Barbet C, Paintaud G, et al. Mycophenolate mofetil in patients with systemic lupus erythematosus: a prospective pharmacokinetic study. Lupus. 2009;18:441–447. | |
De Winter BCM. Proefschrift. Variability in the Pharmacokinetics of Mycophenolic Acid. Implications for Therapeutic Drug Monitoring. Rotterdam, The Netherlands: Optima Grafische Communicatie; 2010. | |
Neumann I, Haidinger M, Jäger H, et al. Pharmacokinetics of mycophenolate mofetil in patients with autoimmune diseases compared to renal transplant recipients. J Am Soc Nephrol. 2003;14:721–727. | |
Tedesco-Silva H, Felipe CR, Park SI, et al. Randomized crossover study to assess the inter- and intrasubject variability of morning mycophenolic acid concentrations from enteric-coated mycophenolate sodium and mycophenolate mofetil in stable renal transplant recipients. Clin Transplant. 2010;24:E116–E123. | |
Traitanon O, Avihingsanon Y, Kittikovit V, et al. Efficacy of enteric-coated mycophenolate sodium in patients with resistant-type lupus nephritis: a prospective study. Lupus. 2008;17:744–751. | |
Zeher M, Doria A, Lan J, et al. Efficacy and safety of enteric-coated mycophenolate sodium in combination with two glucocorticoid regimens for the treatment of active lupus nephritis. Lupus. 2011;20:1484–1493. | |
Kreuter A, Tomi NS, Weiner SM, et al. Mycophenolate sodium for subacute cutaneous lupus erythematosus resistant to standard therapy. Br J Dermatol. 2007;156:1321–1327. | |
Haeck IM, Knol MJ, Ten Berge O, et al. Enteric-coated mycophenolate sodium versus cyclosporine A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol. 2011;64:1074–1084. | |
Deuter CM, Doycheva D, Stuebiger N, Zierhut M. Mycophenolate sodium for immunosuppressive treatment in uveitis. Ocul Immunol Inflamm. 2009;17:415–419. | |
Karim MY, Pisoni CN, Khamashta MA. Update on immunotherapy for systemic lupus erythematosus – what’s hot and what’s not! Rheumatology (Oxford). 2009;48:332–341. | |
European Medicines Agency. Assessment report. Benlysta. International non-proprietary name: belimumab. Procedure No EMEA/H/C/002015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002015/WC500110152.pdf. Accessed July 22, 2014. | |
Bertias G, Ioannidis JP, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195–205. | |
Fischer-Betz R, Hiepe F; Kommission Pharmakotherapie der DGRh. [Revision of the recommendations of the Commission on Pharmacotherapy of the German Society for Rheumatology. Comment on the use of mycophenolic acid for systemic lupus erythematosus]. Z Rheumatol. 2007;66:78–82. German. |
Supplementary material
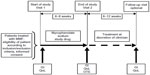  | Figure S1 Study flow chart. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.