Back to Journals » Research Reports in Clinical Cardiology » Volume 7
Impact of stent deployment pressure and poststenting dilatation on the outcome of elective percutaneous coronary intervention
Authors Nour M
Received 10 June 2016
Accepted for publication 1 August 2016
Published 12 September 2016 Volume 2016:7 Pages 109—116
DOI https://doi.org/10.2147/RRCC.S114771
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Kones
Mahmoud K Nour
Department of Critical Care Medicine, Faculty of Medicine, Cairo University, Giza, Cairo, Egypt
Background: High-pressure stent deployment was proposed as a strategy that ensures complete stent apposition but its relation to outcome is markedly debated.
Objective: To verify the influence of stent deployment pressure and poststenting dilatation on the outcome of percutaneous coronary intervention.
Methods: We included 120 patients with single vessel coronary artery disease who were subjected to elective stenting. Patients were divided into two groups: group A, high deployment pressure ≥16 atm and group B, low deployment pressure 8–12 atm. Group B was subdivided into two subgroups according to whether patients underwent poststenting dilatation (subgroup B1) or not (subgroup B2). Clinical and angiographic 6-month follow-up was performed.
Results: The mean stent deployment pressure was higher in group A compared to group B (19±3 vs 10±2, P=0.01). There was no statistically significant difference found between groups A and B regarding major adverse cardiac events (16.7% vs 23.3%, P=0.404) including target lesion revascularization (5% vs 6.7%, P=0.970) and late loss (1.1 mm ±0.9 mm vs 1.22 mm ±0.85 mm, P=0.454), yet the restenosis rate was significantly lower in group A (23.3% vs 43.3%, P=0.032). Subgroup B1 showed a significantly lower restenosis rate compared to subgroup B2 (26.7% vs 60%, P=0.018), yet there was no significant difference between both subgroups regarding major cardiac adverse events (20% vs 26.7%, P=0.761).
Conclusion: High-pressure stent deployment was associated with significant reduction in the restenosis rate but not in the 6 months major adverse cardiac events. Poststenting dilatation resulted in a significantly lower restenosis rate when applied in the low-pressure stent deployment group.
Keywords: stent deployment pressure, major adverse cardiac events, restenosis
Introduction
Prior to the beginning of the coronary stenting era, coronary balloon angioplasty was a balance between achieving higher luminal diameter at the point of the maximum percent stenosis and some procedure-related complications, such as medial injury or dissection, which occurred more frequently in animal models with increasing balloon inflation pressure.1 Following the stent era, some authors reported that using high inflation pressure would result in better stent expansion, as confirmed by intravascular ultrasound,2 and lower incidence of stent thrombosis.3,4 Using a noncompliant (NC) balloon for postdilatation stenting was proposed as a method that could achieve better uniform stent expansion when compared to stent-mounted semicompliant balloon.5 However, findings are still debatable and others report better optimization of stent expansion with stent balloons than NC balloons.6 Colombo et al7,8 proposed that applying a high-pressure dilatation would achieve appropriate stent apposition and lower the incidence of stent thrombosis. This practice was widely accepted by cardiologists and was integrated into recommendations for coronary stenting.9 Large well-designed studies are needed to highlight the major role of antiplatelet therapy after coronary stenting in decreasing the incidence of stent thrombosis,10,11 to clarify the added benefit of dual antiplatelet therapy when compared to anticoagulation in preventing stent thrombosis,12,13 and to assure the independent role of high-pressure stent dilatation in reducing the incidence of in-stent restenosis in the follow-up angiograms. Some authors postulated that high-pressure stent dilatation may result in decreasing the incidence of in-stent restenosis as a result of better achievements in luminal diameter14 and inflow characteristics.15
Aim of the study
To study the impact of stent deployment pressure and poststenting dilatation on the outcome of elective percutaneous coronary intervention (PCI).
Patients and methods
After obtaining written informed consent from the patient or the first-degree relatives, the current work was conducted as a prospective cohort study involving 120 patients with coronary artery disease admitted to the Critical Care Department, Cairo University, for elective PCI between October 2010 and June 2013. Ethical approval was obtained from the Critical Care Medicine Department Committee, Faculty of Medicine, Cairo University. Eighty-eight patients were males and 32 were females with a mean age of 56±8.6 years and age range of 30–72 years. We enrolled patients with known significant single vessel de novo coronary artery disease who were candidates for elective PCI. We excluded patients with known significant in-stent restenosis admitted for repeat PCI. The studied patients were divided into two groups (equal in number) according to the applied stent deployment pressure. Group A, those who received high inflation pressure (≥16 atm) during stent deployment, and group B, who received low inflation pressure (8–12 atm) during stent deployment. Group B was further subdivided into two equal subgroups according to whether poststenting NC dilatation balloon was used or not: B1 (postdilatation balloon applied and B2 (postdilation balloon not applied). Postdilatation was not done in those patients whose nominal stent deployment pressure resulted in an acceptable stent expansion and so included in subgroup B2; however, if the nominal deployment pressure did not result in good expansion, postdilatation NC balloon was used and such patients were included in subgroup B1.
All patients were subjected to
History taking, clinical examination, 12-lead electrocardiogram, diagnostic coronary angiography, PCI, and quantitative coronary angiography analysis (QCA). All used stents were cobalt chromium bare metal stents (BMS). Procedural success was defined as postprocedural residual stenosis ≤30% before removal of the guiding catheter.
Follow-up after 6 months
- Clinical follow-up: defined as occurrence of major adverse cardiac events (MACE): (death, myocardial infarction, or target lesion revascularization during follow-up). All deaths were considered cardiac unless an unequivocal noncardiac cause could be established.
- Angiographic follow-up: the studied patients were subjected to follow-up coronary angiography after 6 months. After nitroglycerin administration, angiograms were obtained in the same views as baseline (pre-PCI) and after the procedure. Procedural and 6-month views were calculated by QCA. Reference vessel diameter, minimal luminal diameter (MLD), and percent degree of stenosis were measured. Late lumen loss (post-PCI MLD – follow-up MLD) and late loss index (expressed as calculated ratio of late loss and acute gain) were calculated. Binary restenosis was defined as ≥50% diameter stenosis on the follow-up angiogram.
Statistical analysis
The Statistical Package for Social Sciences (version 20; IBM Corporation, Armonk, NY, USA) software was used for data entry and analysis. Categorical variables were expressed as frequency tables and compared with chi-square statistics test. Continuous variables were expressed as mean and standard deviation (SD). The paired Student’s t-test was used to compare any two measurements made for the study patients. A probability level of P ≤0.05 was chosen to be significant.
Results
- Demographic data
- Procedural data
- QCA parameters: there was no statistically significant difference between the two groups as regards baseline (preprocedural) or postprocedural QCA parameters (Table 4).
- Thrombolysis in myocardial infarction flow: preprocedural, thrombolysis in myocardial infarction flow was comparable in both groups despite different lesion characteristics (P-value: 0.99) (Table 5).
- PCI data (Table 6)
- Direct stenting was done in 53.3% patients of group A, while it was done in 41.6% patients of group B (P=0.272; there was no statistically significant difference between the study groups regarding the incidence of direct stenting). Other lesions in both groups were dilated first before stenting.
- The stent deployment pressure in group a was significantly higher than that of group B (P=0.01).
- Immediate procedural results and complications (Table 7)
- Immediate procedure success with resulting diameter stenosis ≤30% was achieved in all patients of both the groups.
- Procedural complications: there was no statistically significant difference between both groups as regards procedural complications (Table 7). Also we found no statistically significant difference between the subgroups of studied group B as regards procedural data or postprocedural complications apart from the use of postdilatation NC balloon, which was only used in subgroup B1 (P-value <0.001) (Table 8).
- Follow-up
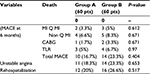
- Clinical follow-up: there was no statistically significant difference between the two study groups as regards MACE (death, myocardial infarction, or target lesion revascularization), unstable angina, or rehospitalization rate after 6 months (Table 9).
- Angiographic follow-up: there was no statistically significant difference between follow-up angiographic data in both studied groups apart from restenosis rate that was significantly lower in the high-pressure stent deployment group (23.3% vs 43.3%, P-value: 0.032) (Table 10).
- Relations between poststenting dilatation and the occurrence of major cardiac events and restenosis in group B patients
Patients in group B who were subjected to poststenting balloon dilation exhibited insignificantly lower incidence of MACE (20% vs 26.7%, P=0.761) and insignificantly lower rehospitalization rate (23.3% vs 30% P=0.77) with similar incidence of unstable angina (23.3% vs 23.3%, P=0.99) when compared to those patients in group B who were not subjected to poststenting dilatation (Table 11). Angiographic binary restenosis at the follow-up angiograms was significantly different between both subgroups (B1 and B2 26.6% vs 60%, respectively; P-value: 0.018).
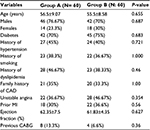  | Table 1 Baseline patient’s clinical characteristics Abbreviations: CAD, coronary artery disease; CABG, coronary artery bypass grafting; MI, myocardial infarction. |
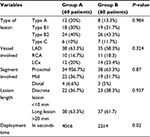  | Table 2 Different vessels, lesion types, and length treated in both groups Abbreviations: LAD, left anterior descending; LCx, left circumflex artery; RCA, right coronary artery. |
  | Table 3 Lesion length in group B patients |
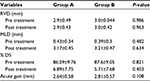  | Table 4 QCA parameters in both groups Abbreviations: % DS, percent degree of stenosis; MLD, minimal luminal diameter; QCA, quantitative coronary angiography analysis; RVD, reference vessel diameter. |
  | Table 5 Preprocedural TIMI flow in both groups Abbreviation: TIMI, thrombolysis in myocardial infarction. |
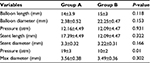  | Table 6 Balloon and stent characteristics in both groups Abbreviation: max, maximum. |
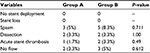  | Table 7 Procedure-related complications |
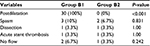  | Table 8 Procedural data and procedure-related complications in subgroups B1 and B2 |
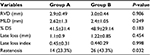  | Table 10 Angiographic data at 6-month follow-up Abbreviations: % DS, percent degree of stenosis; MLD, minimal luminal diameter; RVD, reference vessel diameter. |
  | Table 11 Relation between postdilatation and the occurrence of major cardiac events in group B patients Abbreviations: pts, patients; MACE, major adverse cardiac events. |
Discussion
In-stent restenosis is related to different clinical, angiographic, and procedural variables. Among them, some authors stated that the influence of stent deployment pressure is remarkable.16–19 High pressure is mandatory to ensure optimal stent apposition and expansion, but it can cause excessive neointimal growth.20–24 Studies that have compared the different pressures of balloon inflations used for coronary stenting are minimal and gave controversial results.25–31 Many studies stated that stent underexpansion could be considered a predictor for future in-stent restenosis,32 and this view has been confirmed using different imaging techniques33,34 and a case–control study using intravascular ultrasound.35 In-stent restenosis is classically described to occur 6 months after PCI and stent implantation.36,37 This is the time duration required for neointimal formation and reepithelialization.38 After 6 months, the instent restenosis rate is less likely to increase.39 Accordingly, most of the studies done to evaluate in-stent restenosis – like ours – included follow-up, either clinical or angiographic, 6 months after stent deployment. The objective of our work was to evaluate the impact of stent deployment pressure and poststenting dilatation on the outcome of elective PCI. We found no statistically significant difference between the low- and high-pressure stent deployment groups regarding the immediate postinterventional complications (edge dissection, coronary vasospasm, acute stent thrombosis, and no reflow). Similar to our results, Dirschinger et al did not show any significant difference between high- and low-pressure deployment groups as regards residual dissection after stenting or stent thrombosis.30 Contrary to our results, Uretsky et al showed that the systematic use of very high deployment pressure (20 atm) increases the procedural complications rate.27
It is well known that the luminal gain achieved after stenting could predict the in-stent restenosis at follow-up.16 Applying high-pressure inflation is expected to afford a better lumen diameter at the end of the intervention. Colombo et al7,8 proposed that applying high-pressure dilatation would achieve appropriate stent apposition and lower the incidence of stent thrombosis.7,8 This was not observed in our study where there was no statistically significant difference in the acute lumen gain between the high- and low-pressure groups (2.64±0.58 vs 2.81±0.57, respectively, P=0.108). Similar to our results, Mattos et al conducted a study involving 82 patients to study the effect of balloon pressure inflation (yet in patients undergoing primary PCI for acute myocardial infarction) and stated that the acute lumen gain was similar between the high- and low-pressure deployment groups (0.8±0.1 vs 0.9±0.1, P=0.13).40
We found no statistically significant difference between the two study groups as regards MACE, unstable angina, or rehospitalization rate after 6 months. Similar to our results, Dirschinger et al found no statistically significant difference between the high- and low-pressure deployment groups as regards clinical outcome at 1-year follow-up.30 Also, our results go hand in hand with that published by deQuadros et al. They state that at 1-year follow-up, the hyperexpansion and the control groups had similar rates of MACE (10.8% vs 10.7%), including target vessel revascularization (8.2% vs 6.5%).39
In this study, in-stent restenosis rate was statistically significantly lower in the high-pressure stent deployment group (group A) compared to the low-pressure stent deployment group (group B) (23.3% vs 43.3%, P=0.032). This result goes hand in hand with the common concept “the more the expansion of the stent during deployment, the better is the response and angiographic follow-up”. Despite this approach gaining same acceptance, some studies have stated that more stent expansion during deployment may result in higher arterial wall injury, thus more aggressive neointimal hyperplasia and, consequently, higher in-stent restenosis rate.41,42
Similar to our results, Hoffmann et al, who in their study involved 120 multilink HP stents randomized to deployment at higher pressure (16–20 atm) or low pressure (8–10 atm), stated that in-stent restenosis rate was lower in the high-pressure group (21.7%) compared to the low-pressure group (26.7%; yet with no statistical significance).43 Our explanation for the lack of statistical significance in the study by Hoffman et al (as opposed to the statistical difference in our studied patients) could be related to the fact that, in their study, the lesions were short (low-pressure group mean length 10 mm vs high-pressure group mean length 9.7 mm), while in our study, long lesions (>20 mm) were present in 63.3% of the high-pressure group and 61.7% of the low-pressure group. Hoffmann et al might have found more difference between the two groups as regards restenosis rate had they included long lesions (as in our study), thus reaching statistical significance. Contrary to our results, Dirschinger et al found nearly similar rates of in-stent restenosis in the high- and low-pressure stent deployment groups (30.4% vs 31.4%, respectively).30 Direct stenting was done in 53.3% of patients in group A, while in group B, it was done in 41.6% of patients(P=0.272; there was no significant difference found statistically in the incidence of in-stent restenosis).
In a recent trial conducted by Frobert et al, who divided 93,697 deployed stents into five different pressure interval groups in a retrospective study, it was found that the incidence of stent restenosis was higher in the low- and very high-pressure deployment groups and when they redivided their stents into two groups (high and low pressure) for better interpretation of the effect of deployment pressure on the outcome, they also found that no statistical difference between the two groups as regards in-stent restenosis.44 Thus, the impact of stent deployment pressure on in-stent restenosis rate is markedly debated; we postulated that variable pressures applied during stent deployment should be related more to the lesion type and characteristics and that the variable in-stent restenosis rates in several trials could result from different stent and vascular wall interaction, and different lesion characteristics, and not only as a result of applying or modifying the stent deployment pressure.
Obviously, in the present work, there was a statistically significant lower incidence of angiographic restenosis at follow-up angiograms in the subgroup of patients who received poststenting dilatation (subgroup B1) compared to those who did not (subgroup B2) (26.7% vs 60%, P=0.018), yet there was a tendency toward lower incidence of MACE after 6 months in the postdilatation group but without any statistical significance (20% vs 26.7%, P=0.761). Similar to our results, in a retrospective analysis of 688 consecutive coronary stenoses, the group of patients in whom oversized balloon or high pressure inflations were done inside the stents showed less angiographic restenosis when compared to those in whom aggressive balloon stent dilatations were not performed.25
Contrary to our results, Frobert et al stated that in-stent restenosis was observed more frequently after poststenting dilatation and it was statistically significant.44 They explained that the higher restenosis rate after poststenting dilatation is due to the fact that postdilatation is traumatic to the vessel wall and usually operators use it in high-risk lesions (eg, clacific lesion, long lesion, and chronic total occlusion) that may have higher risk of restenosis irrespective of the poststenting dilatation.44 The difference between their and our results could result from the marked discrepancy in the number of studied population, also we included only BMS compared to both BMS and drug-eluting stents in their study, and importantly, in their study, postdilatation was more prevalent in high-pressure groups (more trauma to the vessel wall) while in our study, all postdilatations were done in the low-pressure stent deployment group.
Tahara et al stated that poststenting dilatation mandated accurate positioning of the NC balloon inside the stent, if this was not carefully done, it might result in geographic miss or edge dissection.45 Another possible complication of postdilation is the longitudinal stent deformation that may occur with the newer thin struts conformable stents.46
Thus, achieving optimal stent expansion during coronary interventions seems important to minimize in-stent restenosis. Although there are conflicting data, it seems reasonable to perform poststenting dilatation to achieve adequate stent expansion, especially in high-risk lesions where there is a high incidence of suboptimal stent deployment.
Limitations
Intravascular ultrasonogrpahy, which might provide accurate insights into optimal stent deployment, was not included in our study protocol.
Drug-eluting stents consistently reduces restenosis rates compared to BMS and should be the treatment of choice for patients who are at high risk of restenosis. This assumes that the patient will be able to afford, tolerate, and adhere to the prescribed dual-antiplatelet regimen, which in fact is difficult and costly in a developing country. In the current study, all the patients in the study groups were treated using BMS of the same type and material just for standardization and to minimize the confounding effect of the use of BMS instead of drug-eluting stent, thus yielding useful findings as regards solely the impact of deployment pressure on restenosis rate.
Our study was conducted on 120 patients only, being a self-funded research and concerning some statistically insignificant results observed in our study, it will be better to reevaluate again on a larger number of patients.
Conclusion
High-pressure stent deployment during elective PCI was associated with a statistically significant reduction in the in-stent restenosis rate compared to low-pressure stent deployment; however, there was no statistically significant difference in MACE after 6 months.
In the low-pressure stent deployment group, poststenting balloon dilatation was associated with a statistically significant lower incidence of in-stent restenosis.
Disclosure
The current study is a self-funded one. Only the abstract has been presented in the Society for Cardiovascular Angiography and Intervention, Las Vegas, Nevada and has been published in Catheterization and Cardiovascular Interventions, Supplement: The Society for Cardiovascular Angiography and Interventions in 2014. The author reports no conflicts of interest in this work.
References
Sarembock IJ, LaVeau PJ, Sigal SL, Timms I, Sussman J, Haudenschild C, Ezekowitz MD. Influence of inflation pressure and balloon size on the development of intimal hyperplasia after balloon angioplasty. a study in the atherosclerotic rabbit. Circulation. 1989;80(4):1029–1040. | ||
De Jaegere P, Mudra H, Figulla H, et al. Intravascular ultrasound-guided optimized stent deployment. immediate and 6 months clinical and angiographic results from the multicenter ultrasound stenting in coronaries study (MUSIC Study). Eur Heart J. 1998;19(8):1214–1223. | ||
Goldberg SL1, Colombo A, Nakamura S, Almagor Y, Maiello L, Tobis JM. Benefit of intracoronary ultrasound in the deployment of Palmaz-Schatz stents. J Am Coll Cardiol. 1994;24(4):996–1003. | ||
Blackman DJ, Porto I, Shirodaria C, Channon KM, Banning AP. Usefulness of high-pressure post-dilatation to optimize deployment of drug eluting stents for the treatment of diffuse in-stent coronary restenosis. Am J Cardiol. 2004;94(7):922–925. | ||
Muraoka Y, Sonoda S, Tsuda Y, Tanaka S, Okazaki M, Otsuji Y. Effect of intravascular ultrasound-guided adjuvant high-pressure non-compliant balloon post-dilation after drug-eluting stent implantation. Heart Vessels. 2011;26(6):565–571. | ||
Kim JS, Moon JY, Ko YG, et al. Intravascular ultrasound evaluation of optimal drug-eluting stent expansion after poststent balloon dilation using a noncompliant balloon versus a semicompliant balloon (from the Poststent Optimal Stent Expansion Trial [POET]). Am J Cardiol. 2008;102(3):304–310. | ||
Colombo A, Hall P, Nakamura S, et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation. 1995;91(6):1676–1688. | ||
Nakamura S, Hall P, Gaglione A, et al. High pressure assisted coronary stent implantation accomplished without intravascular ultrasound guidance and subsequent anticoagulation. J Am Coll Cardiol. 1997;29(1):21–27. | ||
Holmes DR Jr, Hirshfeld J Jr, Faxon D, Vlietstra R, Jacobs A, King SB 3rd. ACC expert consensus document on coronary artery stents. document of the American College of Cardiology. J Am Coll Cardiol. 1998;32(5):1471–1482. | ||
Neumann FJ, Gawaz M, Ott I, May A, Mössmer G, Schömig A. Prospective evaluation of hemostatic predictors of subacute stent thrombosis after coronary Palmaz-Schatz stenting. J Am Coll Cardiol. 1996;27(1):15–21. | ||
The EPISTENT Investigators. Randomized placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet. 1998;352(9122):87–92. | ||
Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary artery stents. N Engl J Med. 1996;334(17):1084–1089. | ||
Martin BL, Donald SB, Jeffrey JP, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med. 1998;339(23):1665–1671. | ||
Kuntz RE, Gibson CM, Nobuyoshi M, Baim DS. Generalized model of restenosis after conventional balloon angioplasty, stenting and directional atherectomy. J Am Coll Cardiol. 1993;21(1):15–25. | ||
Liu MW, Roubin GS, King SB 3rd. Restenosis after coronary angioplasty: potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79(6):1374–1387. | ||
Kastrati A, Schomig A, Elezi S, et al. Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol. 1997;30(6):1428–1436. | ||
Mercado N, Boersma E, Wijns W, et al. Clinical and quantitative coronary angiographic predictors of coronary restenosis: a comparative analysis from the balloon-to-stent era. J Am Coll Cardiol. 2001;38(3):645–652. | ||
Abizaid A, Kornowski R, Mintz GS, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998;32(3):584–589. | ||
Elezi S, Kastrati A, Neumann FJ, Hadamitzky M, Dirschinger J, Schomig A. Vessel size and long-term outcome after coronary stent placement. Circulation. 1998;98(18):1875–1880. | ||
Kuntz RE, Safian RD, Carozza JP, Fishman RF, Mansour M, Baim DS. The importance of acute luminal diameter in determining restenosis after coronary atherectomy or stenting. Circulation. 1992;86(6):1827–1835. | ||
Hoffmann R, Mintz GS, Dussailant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996;94(6):1247–1254. | ||
Goldberg SL, Loussararian A, De Gregorio J, Di Mario C, Albiero R, Colombo A. Predictors of diffuse and aggressive intra-stent restenosis. J Am Coll Cardiol. 2001;37(4):1019–1025. | ||
Hoffmann R, Mintz GS, Mehran R, Kint KM, Pichard AD, Satler LF, Leon MB. Tissue proliferation within and surrounding Palmaz-Schatz stents is dependent on the aggressiveness of stent implantation technique. Am J Cardiol. 1999;83(8):1170–1174. | ||
Finci L, Ferraro M, Kobayashi Y, et al. Coronary stent implantation throughout technical evolution: immediate and follow-up results. Int J Cardiovasc Interventions. 1998;1(1):29–39. | ||
Goldberg SL, Di Mario C, Hall P, Colombo A. Comparison of aggressive versus non aggressive balloon dilatation for stent deployment on late loss and restenosis in native coronary arteries. Am J Cardiol. 1998;81(6):708–712. | ||
Yang P, Gyongyosi M, Hassan A, et al. Short-and long-term outcomes of Wiktor stent implantation at low versus high pressures. Austrian Wiktor Stent Study Group. Am J Cardiol. 1999;84(6):644–649. | ||
Uretsky BF, Rosanio S, Lerakis S, et al. A prospective evaluation of angiography-guided coronary stent implantation with high versus very high balloon inflation pressure. Am Heart J. 2000;140(5):804–812. | ||
Bermejo J, Botas J, García E, et al. Mechanisms of residual lumen stenosis after high-pressure stent implantation: a quantitative coronary angiography and intravascular ultrasound study. Circulation. 1998;98(2):112–118. | ||
Park SW, Hong MK, Lee CW, et al. Immediate and late clinical and angio graphic outcomes after GFX coronary stenting: is high-pressure balloon dilatation necessary? Clin Cardiol. 2000;23(8):595–599. | ||
Dirschinger J, Kastrati A, Neumann FJ, et al. Influence of balloon pressure during stent placement in native coronary arteries on early and late angiographic and clinical outcome: a randomized evaluation of high-pressure inflation. Circulation. 1999;100(9):918–923. | ||
Caixeta AM, Brito FS, Rati M, et al. High versus low-pressure balloon inflation during multilink stent implantation: acute and long-term angiographic results. Cathet Cardiovasc Intervent. 2000;50(4):398–401. | ||
Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–1058. | ||
Ozaki Y, Okumura M, Ismail TF, et al. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur Heart J. 2010;31(12):1470–1476. | ||
Van der Hoeven BL, Liem SS, Dijkstra J, et al. Stent malapposition after sirolimus-eluting and bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: acute and 9-month intravascular ultrasound results of the MISSION! intervention study. JACC Cardiovasc Interv. 2008;1(2):192–201. | ||
Cheneau E, Leborgne L, Mintz GS, et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003;108(1):43–47. | ||
Chan AW, Moliterno DJ. Restenosis: the clinical issues. In: Topol EJ, editor. Text Book of Interventional Cardiology. 4th ed. Saunders publishing, Philadelphia, Pennsylvania; 2003:415–453. | ||
Mehilli J, Schühlen H, Pache J, et al. Influence of balloon pressure during stent placement in native coronary arteries on early and late angiographic and clinical outcome: a randomized evaluation of high-pressure inflation. Circulation. 1999;100(9):918–923. | ||
vom Dahl J, Hanrath P, Mintz GS, et al. The impact of high pressure vs low pressure stent implantation on intimal hyperplasia and follow-up lumen dimensions; results of a randomized trial. Eur Heart J. 2001;22(21):2015–2024. | ||
deQuadros AS, Sarmento-Leite R, Gottschall CA, Silva GV, Perin EC. Hyperexpansion of coronary stents and clinical outcomes. Tex Heart Inst J. 2006;33(4):437–444. | ||
Mattos LA, Sousa AG, Chaves A, et al. Influence of balloon pressure inflation in patients undergoing primary coronary stent implantation during acute myocardial infarction. a quantitative coronary angiography analysis. Arq Bras Cardiol. 2003;80(3):260–268. | ||
Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR. Restenosis and the proportional neointimal response to coronary artery injury results in a porcine model. J Am Coll Cardiol. 1992;19(2):267–274. | ||
Frab A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999;99(1):44–52. | ||
Hoffmann R, Haager P, Mintz GS, et al. The impact of high pressure vs low pressure stent implantation on intimal hyperplasia and follow up lumen dimensions. results of a randomized trial. Eur Heart J. 2001;22(21):2015–2024. | ||
Frobert O, Samo G, James SK, Saleh N, Lagerqvist B. Effect of stent inflation pressure and post dilatation on the outcome of coronary artery intervention A report of more than 90000 stent implantations. PLoS One. 2013;8(2):e 56348. | ||
Tahara S, Bezerra HG, Kyono H, Carrigan T, Mehanna E, Wang W, Costa MA. Impact of acute gain on clinical outcomes of patients treated with sirolimus eluting stent A subanalysis study from the STLLR Trial. Circ J. 2011;75(9):2133–2119. | ||
Williams P, Mamas M, Morgan K, et al. Longitudinal stent deformation: A retrospective analysis of frequency and mechanisms. EuroIntervention. 2011;8(2):267–274. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.