Back to Journals » Research Reports in Clinical Cardiology » Volume 5
Impact of rivaroxaban on stent thrombosis and secondary prevention of cardiovascular events in acute coronary syndrome
Authors Krohn-Grimberghe M, Bode C, von zur Muhlen C
Received 4 January 2014
Accepted for publication 3 March 2014
Published 13 May 2014 Volume 2014:5 Pages 103—109
DOI https://doi.org/10.2147/RRCC.S38727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Marvin Krohn-Grimberghe, Christoph Bode, Constantin von zur Muhlen
Department of Cardiology and Angiology I, Heart Center, University of Freiburg, Germany
Abstract: Angioplasty and stent implantation have greatly improved the outcome of patients with acute coronary syndrome. However, stents come with the risk of stent thrombosis, which is associated with a high rate of revascularization, myocardial infarction, and death. The inhibition of factor Xa due to rivaroxaban leads to an interruption of the intrinsic as well as the extrinsic coagulation pathway, which reduces thrombus formation as a potential mechanism to diminish the rate of stent thrombosis. In this review, we evaluate the role of rivaroxaban in the prevention of stent thrombosis and its general role in patients with acute coronary syndrome.
Keywords: Rivaroxaban, stent thrombosis, acute coronary syndrome, secondary prevention
Introduction
Before the era of coronary artery stent implantation, the high rate of restenosis was one of the major problems after angioplasty. With the introduction of drug-eluting stents, the rate of restenosis was significantly reduced. However, drug-eluting stents come with the disadvantage of delayed endothelial healing, which can result in stent thrombosis. Since stent thrombosis is related to a high rate of repeated revascularization, myocardial infarction, and death,1–4 a lot of effort has been invested in reducing its rate.
During the early years, different definitions of stent thrombosis made the comparison between trials difficult, and led to conflicting results.5–8 In the meantime, the Academic Research Consortium (ARC) developed uniform definitions weighting and defining the likelihood of stent thrombosis (see Table 1).9 The ARC definitions added a lot of uniformity between the end points in clinical trials related to myocardial infarction and coronary revascularization, making interpretation and comparison easier, while still being imperfect.9
  | Table 1 The Academic Research Consortium (ARC) definition of stent thrombosis weights the likelihood of stent thrombosis. The probability is divided into definite, probable, and possible stent thrombosis |
There are many known factors for stent thrombosis. In a study with sirolimus-eluting stents, stent underexpansion and residual stenosis were significantly associated with stent thrombosis after successful stent placement.10 Late stent thrombosis seems to be related to incomplete healing and/or inadequate neointimal coverage of the stent.8 While it is still under debate whether the rate of stent thrombosis is higher in drug-eluting stents, it can definitely be found with bare-metal stents as well.5–7 Since the rate of stent thrombosis is higher in patients after acute coronary syndrome (ACS) compared to patients with stable coronary disease, regardless of the type of stent placed,11 the grade of the underlying vascular inflammation seems to play an important role in stent thrombosis. Due to the devastating outcome of stent thrombosis, much effort is invested into reducing its rate of occurrence.
Patients with an ACS have an increased level of thrombin, which persists for at least 6 months.12 Thrombin can activate platelets through the thrombin protease-activated receptor 1, leading to a positive-feedback loop. Rivaroxaban as a competitive inhibitor of factor Xa causes an interruption of the intrinsic as well as the extrinsic coagulation pathway.13 In a porcine model of stent thrombosis, rivaroxaban significantly reduced thrombus formation in addition to aspirin and clopidogrel.14 Therefore, the combination of rivaroxaban with dual-antiplatelet therapy consisting of aspirin in combination with a P2Y12-receptor inhibitor might offer new opportunities to reduce the rate of stent thrombosis.
Pharmacologic properties of rivaroxaban
Rivaroxaban is an oral, highly selective, direct, and competitive inhibitor of factor Xa. The inhibition of factor Xa by rivaroxaban causes an interruption of the intrinsic as well as the extrinsic coagulation pathway (see Figure 1).13 This prevents thrombin generation, one of the most potent activators of primary (platelet-mediated) and secondary (clotting factor-mediated) hemostasis, and subsequent thrombus formation. Rivaroxaban’s inhibition of both free and fibrin-bound factor Xa differentiates its action from low-molecular-weight heparin or fondaparinux.
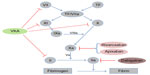  | Figure 1 Influence of vitamin K antagonists (VKAs) and the new oral anticoagulants rivaroxaban, apixaban, and dabigatran on the coagulation cascade. VKAs work by inhibiting the vitamin K epoxide reductase, leading to reduced carboxylation and subsequent synthesis of the clotting factors II, VII, IX, and X and the anticoagulation proteins C and S. Rivaroxaban and apixaban are selective, direct, and competitive inhibitors of the activated factor X (Xa), while dabigatran is a direct thrombin inhibitor. |
The pharmacokinetics of rivaroxaban are adequately described by an oral one-compartment model, and the bioavailability of rivaroxaban is dose-dependent.15,16 Age and renal function influence the clearance of rivaroxaban, while the body surface area affects the volume of distribution.15 However, the influence is small, and over a wide range of renal function no dose adjustment is needed, and no reactive metabolites of rivaroxaban are formed in humans.17 Therefore, it has highly predictable pharmacokinetics and pharmacodynamics, which may improve clinical outcomes and reduce unwanted bleeding events without a need for dose adjustments.16
The evidence for rivaroxaban
The efficacy and safety of rivaroxaban have been studied in several large clinical trials for different indications. These studies led to an approval of rivaroxaban for thromboprophylaxis in patients after total knee or hip arthroplasty,18–21 stroke prevention in nonvalvular atrial fibrillation,22 treatment of acute deep-vein thrombosis,23 and pulmonary embolism24 by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for these indications.
During the last few years, the role of different new oral anticoagulants as an antithrombotic option after ACS has been investigated. The first study of this kind was the ESTEEM (Efficacy and Safety of the oral direct Thrombin inhibitor ximElagatran in patients with rEcent Myocardial damage)25 trial, which used the oral direct thrombin inhibitor ximelagatran. In this study, a significant reduction in the composite end point of death, myocardial infarction, and stroke was shown for the combined treatment compared to aspirin monotherapy after 6 months. Ximelagatran was later withdrawn from the market, due to increased liver toxicity. In the RE-DEEM (RandomizEd Dabigatran Etexilate Dose Finding Study in Patients with Acute Coronary Syndromes) study26 (see Table 2), a Phase II study testing doses of 50–150 mg of the direct thrombin inhibitor dabigatran in addition to dual-antiplatelet therapy in patients with ACS, treatment with dabigatran was associated with a dose-related two- to fourfold increase in bleeding, while reducing surrogate parameters of coagulation activity. While the study was not adequately powered for this end point, no significant difference in death, myocardial infarction, or stroke was seen with the different doses of dabigatran compared to placebo. The APPRAISE (Apixaban for Prevention of Acute Ischemic Events)-2 study,27 a Phase III study using apixaban in high-risk ACS patients, was terminated prematurely, due to increased bleeding events without evidence for efficacy. In APPRAISE-2, the same dose of apixaban was used as for stroke prevention in atrial fibrillation. Therefore, the disproportionately high rate of bleeding might have been related to this high dose in the range of therapeutic anticoagulation.
  | Table 2 Summary and comparison of the RE-DEEM, APPRAISE-2, and ATLAS ACS 2-TIMI 51 trials |
In the ATLAS ACS (A Randomized, Double-Blind, Placebo-Controlled, Event-Driven Multicenter Study to Evaluate the Efficacy and Safety of Rivaroxaban in Subjects With a Recent Acute Coronary Syndrome) 2–Thrombolysis in Myocardial Infarction (TIMI) 51 study, only a fraction of the dose of rivaroxaban as used for stroke prevention in atrial fibrillation was added to dual-antiplatelet therapy. To test the hypothesis of a further reduction of stent thrombosis in ACS patients, the ATLAS ACS 2-TIMI 51 study randomly assigned 15,526 patients in a 1:1:1 fashion to twice-daily administration of either 2.5 mg or 5 mg of rivaroxaban twice daily (bid) or placebo, with a maximum follow-up of 31 months (see Figure 2). This therapy was added to standard medical therapy at the enrolling physician’s discretion.28 Of the 15,342 patients included in the modified intention-to-treat analysis, 9,631 (63%) underwent percutaneous coronary intervention and had at least one stent placed either prior to randomization or during their index event.29 Apart from a slightly greater age among rivaroxaban patients (mean age 61.7 versus 61.1 years, P<0.001), no difference in baseline characteristics between stented patients randomly assigned to rivaroxaban versus placebo was noticed.29 When compared to placebo, the administration of 2.5 mg bid of rivaroxaban in addition to dual-antiplatelet therapy showed a significant reduction in stent thrombosis and mortality (hazard ratio 0.56, 95% confidence interval 0.35–0.89; P=0.014).29 Rivaroxaban 2.5 mg bid reduced the rates of death from cardiovascular causes (2.7% versus 4.1%, P=0.002) and death from any cause (2.9% versus 4.5%, P=0.002) (see Figure 3). This mortality benefit was not seen with 5 mg bid.30 In the ATLAS ACS 2-TIMI 51 trial, the discontinuation as well as the compliance rates were comparable between the 2.5 mg bid dose and the placebo groups.30
  | Figure 3 Cumulative incidence of the efficacy and primary safety end points for 2.5 mg bid rivaroxaban. The primary efficacy end point consisted of death from cardiovascular causes, myocardial infarction, or stroke. P-values for the modified intention-to-treat analyses are shown. |
Safety and tolerability
In general, rivaroxaban is well tolerated, and the rates of adverse events not related to bleeding including clinical and laboratory liver abnormalities were similar in the rivaroxaban and the placebo group.30 Rivaroxaban can be used in a broad range of patients, but it is not recommended in patients with a creatinine clearance of less than 15 mL/minute (<30 mL/minute for venous thromboembolism patients in the US). Special precaution should be taken in patients with liver disease and coagulopathies.31
In the ATLAS ACS 2-TIMI 51 trial, rivaroxaban significantly increased the major bleeding rates not related to coronary-artery bypass grafting (TIMI major bleeding, 2.1% versus 0.6% P<0.001), as well as the rate of intracranial hemorrhage (0.6% versus 0.2%, P=0.009), without a significant increase in fatal bleeding (0.3% versus 0.2%, P=0.66). The rates of TIMI minor bleeding (1.3% versus 0.5%, P=0.003) and TIMI bleeding requiring medical attention (14.5% versus 7.5%, P<0.001) increased significantly as well.
While the presence of rivaroxaban can be detected by prolonged prothrombin time as well as activated partial thromboplastin time, so far no routine specific coagulation assay for the monitoring of the therapeutic dosing of rivaroxaban is available. This is offset partially by the highly predictive pharmacokinetics and pharmacodynamics of rivaroxaban. However, in some clinical circumstances, such as active bleeding, a better way of monitoring rivaroxaban might be helpful. In addition, no specific antidote for the reversal of rivaroxaban is available, and due to its high plasma protein binding, it is not expected to be dialyzable. A study of a recombinant protein that was able to reverse the anticoagulative effect of rivaroxaban raises hopes that this might change in the near future.32 So far, in the case of severe bleeding under active treatment with rivaroxaban, reversal with prothrombin complex concentrate, activated prothrombin complex concentrate, or recombinant factor VIIa is recommended. These recommendations are not based on clinical trials, but on physiologic considerations and expert opinions.33 Therefore, the problem of severe bleeding under active treatment and how to manage it is still waiting for a solution.
Limitations and outlook
While the role of rivaroxaban has been established in such indications as treatment of patients with atrial fibrillation and venous thromboembolism, its role in patients with ACS is still developing. In the ATLAS ACS 2-TIMI 51 trial, rivaroxaban significantly improved ischemic outcomes in ACS patients on dual-antiplatelet therapy. However, the FDA declined approval of rivaroxaban for the treatment of patients with recent ACS three times. One of the main reasons for the first decline was the high rate of missing data. Therefore, the distributor submitted vital status on 843 of the 1,338 subjects in ATLAS for whom vital status was unknown at the end of the trial. However, approval was declined again, since the strength of evidence for the primary end point was judged to be inadequate by the Division of Cardiovascular and Renal Products (DCaRP) of the FDA.
The third time, the distributor was seeking approval for rivaroxaban for a limited duration of treatment – 90 days. The DCaRP questioned the superiority of the 2.5 mg bid dose to the 5 mg bid dose for the primary efficacy end point, because it would lack biological plausibility, and the only independent information from the much-smaller Phase II dose-ranging study ATLAS ACS TIMI 46 did not suggest such superiority. Furthermore, they disputed the exclusion of the three Indian study sites for misconduct, inclusion of which would modestly increase the P-value for the primary efficacy analysis from 0.024 to 0.032. Since ATLAS was not designed to provide information about the best duration of therapy and the P-value was deemed not sufficiently low for the approval of rivaroxaban based on a single trial, the DCaRP decided against approval of rivaroxaban for a limited duration of treatment on January 16, 2014.
The benefit of an additional treatment with rivaroxaban in ATLAS was especially pronounced during the first 30–90 days of treatment. The strong appeal of such shorter duration of treatment over only 1–3 months was appreciated by the DCaRP; nevertheless, the data from the ATLAS trial were deemed not sufficient to proof this theory, since the trial was not designed to discriminate between different treatment durations.34,35 In March 2013, the EMA Committee for Medicinal Products for Human Use granted an indication for rivaroxaban 2.5 mg bid in patients with ACS.36
Besides these issues, rivaroxaban might have a much more important role in the treatment of ACS without the use of the new P2Y12-receptor antagonists ticagrelor37 and prasugrel.38 Both have become new options for patients with ACS at high risk for coronary events or stent thrombosis. Since rivaroxaban has only been tested in addition to a combined treatment with aspirin and clopidogrel or aspirin and ticlopidine, but not with one of the new P2Y12-receptor antagonists, its role in combination with one of the new P2Y12-receptor antagonists remains unclear. Due to a potential further increase in adverse bleeding events, such a combination cannot be recommended at present.
One potential treatment area for rivaroxaban in ACS might be in patients at very high risk for another cardiovascular event, for a limited duration of time of about 30 days. Treatment with rivaroxaban for a limited period of time after an ACS has an intuitive appeal, since the absolute rate of cardiovascular events is highest immediately after an episode of ACS and declines to a rather steady level thereafter.39 The hazard ratio for efficacy was best at 30 days in ATLAS, and a good net clinical benefit was reached.34 However, as mentioned earlier, ATLAS was not designed for answering questions about treatment duration, and even at the 30-day time point the confidence intervals are broad. Therefore, another trial would be needed to definitively prove the superiority of rivaroxaban for a limited duration of treatment.
Another potential area of improved outcomes with rivaroxaban might be in patients with an indication for a therapeutic anticoagulation (eg, atrial fibrillation) and an ACS with a newly placed stent. Here, the combination of rivaroxaban with a P2Y12-receptor antagonist with or without aspirin might show improved outcomes when compared to warfarin. Further clinical trials are needed to address these questions.
Conclusion
Rivaroxaban has become a preferred treatment in many indications. In patients with ACS, the ATLAS ACS 2-TIMI 51 study showed that rivaroxaban reduces the rate of stent thrombosis, with the downside of increased bleeding complications. However, it remains an interesting and promising option in selected patients. Especially for patients at high risk of a secondary cardiovascular event, a short duration of treatment with low-dose rivaroxaban for about 30 days might be beneficial. In ACS patients with an additional indication for a therapeutic anticoagulation, such as atrial fibrillation, rivaroxaban might be a promising therapeutic strategy as well. Further studies will be needed to answer these questions.
Disclosure
MK-G reports no conflicts of interest in this work. CB received consulting fees/honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi-Aventis. CvzM received speaker honoraria from Merck, BerlinChemie, Bayer, AstraZeneca, Pfizer, Bristol-Myers Squibb, GlaxoSmithKline, Abbott Vascular, Edwards, and Medtronic.
References
Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. | |
Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103(15):1967–1971. | |
Doyle B, Rihal CS, O’Sullivan CJ, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007;116(21):2391–2398. | |
Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369(9562):667–678. | |
Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356(10):1020–1029. | |
Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998–1008. | |
Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):989–997. | |
Holmes DR Jr, Kereiakes DJ, Garg S, et al. Stent thrombosis. J Am Coll Cardiol. 2010;56(17):1357–1365. | |
Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. | |
Fujii K, Carlier SG, Mintz GS, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45(7):995–998. | |
Kukreja N, Onuma Y, Garcia-Garcia HM, Daemen J, van Domburg R, Serruys PW. The risk of stent thrombosis in patients with acute coronary syndromes treated with bare-metal and drug-eluting stents. JACC Cardiovasc Interv. 2009;2(6):534–541. | |
Skeppholm M, Kallner A, Malmqvist K, Blombäck M, Wallén H. Is fibrin formation and thrombin generation increased during and after an acute coronary syndrome? Thromb Res. 2011;128(5):483–489. | |
Gulseth MP, Michaud J, Nutescu EA. Rivaroxaban: an oral direct inhibitor of factor Xa. Am J Health Syst Pharm. 2008;65(16):1520–1529. | |
Becker EM, Perzborn E, Klipp A, et al. Effects of rivaroxaban, acetylsalicylic acid and clopidogrel as monotherapy and in combination in a porcine model of stent thrombosis. J Thromb Haemost. 2012;10(12):2470–2480. | |
Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban – an oral, direct factor Xa inhibitor – in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47(3):203–216. | |
Xu XS, Moore K, Burton P, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with acute coronary syndromes. Br J Clin Pharmacol. 2012;74(1):86–97. | |
Lang D, Freudenberger C, Weinz C. In vitro metabolism of rivaroxaban, an oral, direct factor Xa inhibitor, in liver microsomes and hepatocytes of rats, dogs, and humans. Drug Metab Dispos. 2009;37(5):1046–1055. | |
Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–2775. | |
Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. July 5, 2008;372(9632):31–39. | |
Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776–2786. | |
Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373(9676):1673–1680. | |
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. | |
Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. | |
Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. | |
Wallentin L, Wilcox RG, Weaver WD, et al. Oral ximelagatran for secondary prophylaxis after myocardial infarction: the ESTEEM randomised controlled trial. Lancet. 2003;362(9386):789–797. | |
Oldgren J, Budaj A, Granger CB, et al. Dabigatran vs placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011;32(22):2781–2789. | |
Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365(8):699–708. | |
Gibson CM, Mega JL, Burton P, et al. Rationale and design of the Anti-Xa therapy to lower cardiovascular events in addition to standard therapy in subjects with acute coronary syndrome – thrombolysis in myocardial infarction 51 (ATLAS-ACS 2 TIMI 51) trial: a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of rivaroxaban in subjects with acute coronary syndrome. Am Heart J. 2011;161(5):815–821. e6. | |
Gibson CM, Chakrabarti AK, Mega J, et al. Reduction of stent thrombosis in patients with acute coronary syndromes treated with rivaroxaban in ATLAS-ACS 2 TIMI 51. J Am Coll Cardiol. 2013;62(4):286–290. | |
Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9–19. | |
Alquwaizani M, Buckley L, Adams C, Fanikos J. Anticoagulants: a review of the pharmacology, dosing, and complications. Curr Emerg Hosp Med Rep. 2013;1(2):83–97. | |
Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446–451. | |
Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother. 2013;47(6):841–855. | |
US Food and Drug Administration. 2014 meeting materials, Cardiovascular and Renal Drugs Advisory Committee. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/ucm378911.htm. Accessed March 4, 2014. | |
Krantz MJ, Kaul S. The ATLAS ACS 2-TIMI 51 trial and the burden of missing data: (anti-Xa therapy to lower cardiovascular events in addition to standard therapy in subjects with acute coronary syndrome ACS 2-thrombolysis in myocardial infarction 51). J Am Coll Cardiol. 2013;62(9):777–781. | |
Wood S. Rivaroxaban gets ACS indication recommendation from European regulators. Available from: http://www.medscape.com/viewarticle/791540?t=1. Accessed March 4, 2013. | |
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. | |
Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. | |
Mehta SR, Yusuf S. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. 2000;21(24):2033–2041. | |
Paikin JS, Eikelboom JW, Cairns JA, Hirsh J. New antithrombotic agents – insights from clinical trials. Nat Rev Cardiol. 2010;7(9):498–509. | |
Costopoulos C, Niespialowska-Steuden M, Kukreja N, Gorog DA. Novel oral anticoagulants in acute coronary syndrome. Int J Cardiol. 2013;167(6):2449–2455. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

