Back to Journals » Clinical Ophthalmology » Volume 15
Impact of Pseudoexfoliative Syndrome on Effective Lens Position, Anterior Chamber Depth Changes, and Visual Outcome After Cataract Surgery
Authors Müller M, Pawlowicz K, Böhm M, Hemkeppler E, Lwowski C, Hinzelmann L, Shajari M, Kohnen T
Received 10 March 2021
Accepted for publication 20 April 2021
Published 5 July 2021 Volume 2021:15 Pages 2867—2873
DOI https://doi.org/10.2147/OPTH.S307487
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Michael Müller, Katarzyna Pawlowicz, Myriam Böhm, Eva Hemkeppler, Christoph Lwowski, Lisa Hinzelmann, Mehdi Shajari, Thomas Kohnen
Department of Ophthalmology, Goethe-University, Frankfurt, Germany
Correspondence: Thomas Kohnen
Department of Ophthalmology, Goethe-University, Theodor-Stern-Kai 7, Frankfurt, 60590, Germany
Tel +49 69 6301 3945
Fax +49 69 6301 3893
Email [email protected]
Purpose: To compare the effective lens position (ELP), anterior chamber depth (ACD) changes, and visual outcomes in patients with and without pseudoexfoliation syndrome (PEX) after cataract surgery.
Design: Prospective, randomized, fellow-eye controlled clinical case series.
Methods: This prospective comparative case series enrolled 56 eyes of 56 consecutive patients with (n = 28) or without PEX (n = 28) and clinically significant cataract who underwent standard phacoemulsification and were implanted with single-piece acrylic posterior chamber intraocular lenses (IOLs). The primary outcome parameters were the ACD referring to the distance between the corneal anterior surface and the lens anterior surface, which is an indicator of the postoperative axial position of the IOL (the so-called ELP) and distance corrected visual acuity (DCVA).
Results: Before surgery, the ACD was 2.54 ± 0.42 mm in the PEX group and 2.53 ± 0.38 mm in the control group (p = 0.941). Postoperatively, the ACD was 4.29 ± 0.71 mm in the PEX group and 4.33 ± 0.72 mm in the normal group, respectively (p = 0.533). There was no significant difference in ACD changes between groups (PEX group: 1.75 ± 0.74 mm, control group: 1.81 ± 0.61 mm, p = 0.806) and DCVA pre- (p = 0.469) and postoperatively (PEX group: 0.11 ± 0.13 logMAR, control group: 0.09 ± 0.17 logMAR, p = 0.245) between groups.
Conclusion: Preoperative and postoperative ACD, as an indicator of ELP, between PEX eyes and healthy eyes after cataract surgery showed no significant difference. Phacoemulsification induced similar changes in eyes with PEX compared to healthy eyes.
Keywords: pseudoexfoliative syndrome, effective lens position, anterior chamber depth changes, cataract surgery
Introduction
Pseudoexfoliation (PEX) syndrome is described as an age-related disorder in which abnormal fibrillary extracellular material from the lens and iris pigment epithelium gradually accumulates on ocular tissues.1 Knowledge of the pseudoexfoliation syndrome has existed for decades, as Lindberg focused in detail on it in his doctoral thesis more than 100 years ago.2 Since then, there has been considerable advancement in understanding its pathogenesis and resulting clinical implications.2 For a time, it was thought to be peculiar to Scandinavia, but exfoliation syndrome has been recorded in almost every race and ethnic group around the world.3 It is well known to be associated with cataract and glaucoma, putting these patients at an increased risk to a broad spectrum of surgical ocular complications.4 Common complications associated with PEX are late-in-the-bag spontaneous IOL dislocations5 resulting from alterations of anterior segment tissues and unsatisfactory visual outcome after cataract surgery due to lens placement.6,7 Due to its world-wide presence and the great impact, it has on visual outcomes, PEX syndrome is a condition of international significance.
For cataract and refractive surgeons, the most critical side effect of pseudoexfoliation is zonular instability.8 The accumulation of exfoliative deposits adjacent to the ciliary process and the anterior lens capsule have been associated with subsequent zonular rupture.9 Therefore, exfoliation is related to a higher risk of intraoperative and postoperative complications due to zonular instability, phacodonesis, impaired blood-aqueous barrier, melanin dispersion, posterior synechiae and keratopathy.10 This means that exfoliative deposits need to be considered prior to and during cataract surgery. Sastry and Singal suggested that intraoperative complications should be anticipated in patients with PEX even without glaucomatous changes due to poor pupillary dilation and zonular weakness.11
The combination of zonular instability and unstable capsular bag makes it difficult to predict the anterior chamber depth (ACD) deepening or shallowing and the stability of the IOL position might be compromised. The ACD measures the distance between the cornea anterior surface and the lens anterior surface, which is the primary indicator of the effective lens position (ELP), namely the axial position of the IOL postoperatively.
Prior studies have shown that cataract surgery induces more significant ACD changes associated with a hyperopic shift in patients with PEX compared to patients without PEX.12,13 However, these studies have shown no difference in mean absolute error calculated with different IOL formulas.12,13 The aim of this study is to investigate and compare the impact of PEX syndrome on cataract surgery by examining the ELP, the ACD changes, and the visual outcomes after cataract surgery in PEX patients and non-PEX patients. Furthermore, this research adds, while performing careful surgery, there is no difference between patients with PEX compared to patients without PEX to be expected.
Materials and Methods
Study Design
This prospective comparative study enrolled 56 eyes of 56 consecutive patients with (n = 28) or without PEX (n = 28) and clinically significant cataracts who underwent standard phacoemulsification and were implanted with single-piece acrylic posterior chamber intraocular lenses (IOLs). The study protocol was approved by the local Ethics Committee of the University Frankfurt, Germany, approval number 410/17 and followed the tenets of the Declaration of Helsinki. If patients met the inclusion criteria, they signed an informed consent form. The inclusion criteria were adults with a bilateral cataract surgery with the implantation of a monofocal IOL. Exclusion criteria were a history of previous intraocular surgery, ocular trauma and ocular pathologies that could possibly influence the operative complications and postoperative visual acuity (eg, age-related macular degeneration, severe glaucoma reducing visual acuity (VA). Surgeries were performed by one experienced surgeon (Michael Müller [MM]) at the Department of Ophthalmology, Goethe University, Frankfurt, Germany.
Patient Recruitment and Enrolment
Cataract patients with PEX and patients with otherwise healthy eyes for the control group were screened for enrolment. Each patient in the cataract consultation was examined and eligibility for the study was assessed. All participants were recruited between January 2019 and January 2020. The three-month follow-up was completed by April 2020. The first eye receiving surgery was evaluated from each participant, yielding a sample of 28 PEX eyes and 28 control eyes.
Preoperative and Postoperative Examination
Preoperative keratometry (K), axial length (AL), ACD, and white to white distance (WTW) were collected with a partial coherence interferometer (IOL Master 700, Carl Zeiss Meditec AG, Jena, Germany) and an anterior segment tomography Pentacam AXL (Oculus, Wetzlar, Germany). Preoperative and postoperative distance corrected visual acuity (DCVA) on the logMAR (Minimum angle of resolution) unit was obtained with undilated pupils with an auto kerato-refractometer (Topcon, model KR-800S). A slit lamp examination was also conducted. The posterior segment has been examined carefully with a 90D non-contact lens. Additionally, demographic information was recorded. The postoperative examination was performed three months after the IOL implantation. We have determined the post-op ACD uniformly with the anterior segment tomography Pentacam AXL. Both the pre-op as well as the post-op ACD was determined in a dilated state.
The Pentacam Nucleus Staging (PNS) of the Pentacam AXL was used, which is a quantitative method of measuring nuclear cataract that provides average and maximal lens density.14 It is measuring the optical density of the nucleus by blue light illumination.15 The densitometry software evaluates the lens’s optical densities by analysing backward scatter.16
Intraocular Lenses and Surgery
All eyes were implanted with a foldable single-piece acrylic posterior chamber monofocal biconvex intraocular lens (IOL) with an overall diameter of 13 mm, an optical zone of 6 mm, a zero degree haptic angulation and the same A constant of 118.4.17,18 In the PEX group, 23 and in the non-PEX group 22 SA60AT (Alcon Laboratories, Fort Worth, Texas, USA) were used. The remaining were implanted an AAB00 (Johnson & Johnson Surgical Vision, Santa Ana, California, USA). All cataract surgeries were performed by one surgeon (MM) under topical anaesthesia. Surgeons technique: clear cornea incision of 2.2 mm, two paracenteses, the use of cohesive and dispersive viscoelastic material (OcuCoat, Bausch+Lomb, USA and Provisc, Alcon, USA), capsulorhexis of the anterior lens capsule with forceps, hydrodissection and hydrodelineation, phacoemulsification in divide and conquer technique, irrigation/aspiration, insertion of the IOL in the capsular bag. No patients were excluded due to intraoperative problems. At most, 2 push pull hooks were used to dilate the pupil. There were no capsular tension rings nor capsular hooks used. Rhexis was performed at least 4.5 mm in diameter.
Outcome Measures
The primary outcome parameters were the ACD, an indicator of the ELP, and the DCVA at three months after surgery. The ACD measuring from the anterior corneal surface to the lens anterior surface, which is indicating the axial position of the IOL (the so-called ELP), was measured using anterior segment tomography with Pentacam AXL. DCVA was mentioned in logMAR.
Statistical Analysis
The sample-size calculation was based on the primary outcome parameter of the ACD. A study by Ermis et al19 evaluated the effects of postural variation on ACD in PEX eyes found a mean ACD of 2.71 mm. Considering an SD of 0.23 mm, a difference of 0.2 mm between PEX and healthy eyes was assumed to be clinically significant. Based on these assumptions, 28 patients in each group were required for a significance level (a) of 0.05 and a test power of 0.9 (BiAS for Windows, Version 11.01, Epsilon-Verlag).
Data were presented as mean ± standard deviation (SD). All compared parameters were tested on normal distribution by the Kolmogorov–Smirnov test. If the data in both groups were normally distributed, the unpaired t-test was applied. If one of the parameters was not normally distributed, the Mann–Whitney U-test was used. P values lower than 0.05 were considered statistically significant. A multiple regression was conducted to show if preoperative parameters like age, gender, preoperative DCVA and the axial length have an influence in the ACD difference. Statistical analyses were performed using Excel 2011 (Version 16.16.22; Microsoft, WA, USA) and SPSS (Version 26.0; IBM, NY, USA).
Results
The mean age of PEX patients was 75 ± 6.2 years, whereas the mean age of controls was 71 ± 8.0 years (p = 0.074; Table 1); thus, there was no statistical difference. In both, the PEX group and the control group, more females than males (64% and 57%) were included, respectively (p = 0.584). As expected, glaucoma was significantly more common in PEX eyes with 36% (10 of 28 eyes) compared to control eyes with 4% (1 of 28 eyes) (p = 0.002) (Table 1).
 |
Table 1 Baseline Demographics in the Two Study Groups, with and without Pseudoexfoliation Syndrome (PEX) |
The Pentacam nucleus staging (PNS) score was also comparable in both groups (p = 0.860), with a mean of 1.4 for the PEX group and the control group.
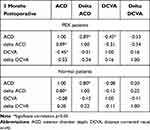
The preoperative DCVA showed no significant difference (p = 0.469) with 0.45 ± 0.30 LogMAR for the PEX group and 0.40 ± 0.29 LogMAR for the control group. The postoperative DCVA in the PEX group improved to 0.11 ± 0.13 logMAR and 0.09 ± 0.17 logMAR in the control group, respectively, with no significant difference between groups (p = 0.245) (Table 2).
 |
Table 2 Anterior Chamber Depth (ACD) and Distance Corrected Visual Acuity (DCVA) Three Months After Cataract Surgery in Patients with and without Pseudoexfoliation Syndrome (PEX) |
Before surgery, the ACD was 2.54 ± 0.42 mm in the PEX group and 2.53 ± 0.38 mm in the control group. Postoperatively, the ACD was 4.29 ± 0.71 mm in the PEX group and 4.33 ± 0.72 mm in the control group. There were no significant differences in ACD preoperatively and 3 months after surgery between groups (p > 0.05) (Figures 1 and 2). A multiple regression including age (p = 0.175), gender (p = 0.404), a preoperative DCVA (p= 0.064) and the axial length (p = 0.226) shows no influence in ACD difference. Moreover, there were no significant differences in ACD changes and DCVA changes from preoperative to 3 months postoperatively between groups (p > 0.05) (Table 2). Figures 2 and 3 show that there is no relation between ACD change from preoperative to postoperative in relation to the axial length and c-factor (ACD plus 0.4 x pre-op LT (lens thickness).20 Furthermore, there is a significant correlation between the postoperative ACD and delta ACD, as expected. All other variables are not correlating with each other even in the PEX or normal group (Table 3). We did not encounter any intraoperative complications nor a post-op capsular phimosis. We have not excluded any patients before surgery due to the severity of PEX, eg, lentodonesis, nor after surgery because of complications or a difficult surgery. We had though 8 patients who have signed a consent, underwent the phacoemulsification, but unfortunately have not been willing to come back for the follow up, mainly because of the distance from home.
 |
Figure 1 Comparison of the preoperative and postoperative anterior chamber depth (ACD) in patients with and without pseudoexfoliation syndrome (PEX). |
 |
Figure 2 Comparison of the preoperative and postoperative anterior chamber depth (ACD) in patients with and without pseudoexfoliation syndrome (PEX) in relation to the axial length. |
 |
Figure 3 Comparison of the anterior chamber depth (ACD) difference in patients with and without pseudoexfoliation syndrome (PEX) in relation to C Factor. |
Discussion
This prospective study compared the ACD or ELP and the visual outcome changes in patients after cataract surgery with PEX vs non-PEX healthy eyes.
The study results show that there was no significant difference in ACD prior to surgery and no difference in ACD at three months postoperatively between the groups. Furthermore, no significant difference in the ACD changes, ie, the delta between pre- and postoperative ACD, was found to indicate the ELP. The DCVA and DCVA changes of both groups also showed no significant difference between those two groups.
A prior study12 showed similar results in terms of ACD change in PEX patient from preoperative to 1 month and to 6 months postoperatively, however missing a control group of non-PEX eyes. The authors find a significant ACD deepening from 2.63 ± 0.43 mm to 3.97 ± 0.93 mm at one month and 4.06 ± 0.36 mm at 6 months after cataract surgery (P < 0.001), thus backward movement of the IOL in the first six months, which was associated with a concurrent small hyperopic shift. This overall ACD change is in line with the results presented in this study (2.54 ± 0.42 mm to 4.29 ± 0.71 mm 3 months postoperatively).
Another study13 also compared PEX eyes with non-PEX healthy eyes after phacoemulsification with respect to ELP. In contrast to the current study, the authors find that phacoemulsification induces more significant ACD change in patients with PEX compared to normal patients. They show that the postoperative ACD values were significantly higher than the preoperative ACD in both groups (P < 0.0001). However, the ACD changes were found to be higher in the PEX group with 0.46 ± 0.3 mm compared to normal eyes group (0.12 ± 0.1 mm) (P = 0.04), which is smaller than our findings. It is notable that in the above-mentioned study the postoperative ACD changes in both groups are also different to other studies: Ning et al have found in normal eyes a postoperative ACD-increase of in the mean 1.33 mm, which is comparable to Kim 201121 after cataract surgery in glaucoma eyes. Kim described an ACD change after surgery of in the mean 1.31 mm in eyes with open-angle glaucoma (OAG) and 1.92 mm in eyes with angle closure glaucoma (ACG). The data of Ning and Kim are in line with our findings: In the current study, no significant difference in ACD change, as an indicator of ELP, between groups with a delta of 1.75 ± 0.77 mm in the PEX group and 1.81 ± 0.61mm in the control group was demonstrated.
Kristianslund et al22 focused on the corneal endothelial cell loss following cataract surgery in patients with PEX and also evaluated the DCVA. Preoperatively, the groups were comparable regarding the DCVA with 0.34 ± 0.25 logMAR in the PEX eyes and 0.25 ± 0.18 logMAR for the control group (p= 0.03). At the 6 months follow-up the groups also improved similarly to 0.01 ± 0.12 logMAR and −0.02 ± 0.14 logMAR, respectively. Worse initial DCVA with 0.47 ± 0.35 logMAR for the PEX group and 0.43 ± 0.35 logMAR for the control group was apparent in the current study. The DCVA after three months showed comparable results as Kristianslund et al after 6 months with 0.11 ± 0.13 logMAR and 0.11 ± 0.20 logMAR for the control group. Fallah Tafti et al 201712 also found a significant improvement in DCVA in their PEX eyes collective from 0.83 ± 0.19 logMAR, which improved to 0.06 ± 0.05 logMAR at one month after cataract surgery (p < 0.001).
This prospective study found no statistical difference in ACD or DCVA between PEX eyes and healthy eyes after cataract surgery. The strength of the present study is its prospective nature and the age-gender-matched control group design. The study has some limitations. First, two different intraocular lenses were implanted in both groups; however, the (non-existent) haptic angulation and the A-constant of IOLs are the same and a subgroup analysis within IOL groups revealed similar results. Further research with a longer follow up with a larger sample size would be recommendable.
In summary, if the finding of no significant difference in ACD or ELP between PEX and non-PEX eyes after cataract surgery holds, the implication for practice is that for PEX patients operated by an experienced surgeon no special precautions with respect to ELP are required compared to healthy eyes and that the expected VA in PEX and non-PEX eyes is similar.
Conclusion
This prospective study reveals no significant difference – neither before nor after cataract surgery – in ACD, as an indicator of ELP, between PEX eyes and non-PEX eyes. Moreover, cataract surgery induces similar ACD changes in eyes with PEX compared to normal eyes. In line with these findings, there was no significant difference in DCVA between groups postoperatively.
Disclosure
All authors declare that they were not guided by economic interests in the preparation of the contribution. Michael Müller reports lecture activity for Alcon/Novartis, Allergan, Thea. Prof Kohnen is a consultant and researcher for Abbott/J&J, Alcon/Novartis, Avedro, Oculentis, Oculus, Presbia, Schwind, Zeiss; consultant for Allergan, Bausch & Lomb, Dompé, Geuder, Med Update, Merck, Rayner, Santen, Staar, Tear Lab, Théa, Thieme, Ziemer; researcher for Hoya. Christoph Lwowski reports lecture fees from Johnson & Johnson. All other authors report no conflicts of interest in this work.
References
1. Erkayhan GE, Dogan S. Cataract surgery and possible complications in patients with pseudoexfoliation syndrome. Eurasian J Med. 2017;49(1):22–25. doi:10.5152/eurasianjmed.2016.0060
2. Lindberg JG. Clinical investigations on depigmentation of the pupillary border and translucency of the iris in cases of senile cataract and in normal eyes in elderly persons. Acta Ophthalmol Suppl. 1989;190:1–96.
3. Ringvold A. Epidemiology of the pseudo-exfoliation syndrome, a review. Acta Ophthalmol Scand. 1999;77(4):371–375. doi:10.1034/j.1600-0420.1999.770401.x
4. Naumann GO, Schlötzer-Schrehardt U, Küchle M. Pseudoexfoliation syndrome for the comprehensive ophthalmologist. Intraocular and systemic manifestations. Ophthalmology. 1998;105(6):951–968. doi:10.1016/S0161-6420(98)96020-1
5. Subasi S, Yuksel N, Karabas VL, Yilmaz Tugan B. Late in-the-bag spontaneous IOL dislocation: risk factors and surgical outcomes. Int J Ophthalmol. 2019;12(6):954–960. doi:10.18240/ijo.2019.06.12
6. Scharfenberg E, Schlötzer-Schrehardt U. [PEX syndrome. Clinical diagnosis and systemic manifestations]. Ophthalmologe. 2012;109(10):952–961. (German). doi:10.1007/s00347-012-2534-y
7. Nobl M, Mackert M. [Pseudoexfoliation syndrome and glaucoma]. Klin Monbl Augenheilkd. 2019;236(9):1139–1155. (German). doi:10.1055/a-0972-4548
8. Schlötzer-Schrehardt U, Naumann GOH. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141(5):921–937. doi:10.1016/j.ajo.2006.01.047
9. Grzybowski A, Kanclerz P, Ritch R. The history of exfoliation syndrome. Asia Pac J Ophthalmol (Phila). 2019;8(1):55–61. doi:10.22608/APO.2018226
10. Tekin K, Inanc M, Elgin U. Monitoring and management of the patient with pseudoexfoliation syndrome: current perspectives. Clin Ophthalmol. 2019;13:453–464. doi:10.2147/OPTH.S181444
11. Sastry PV, Singal AK. Cataract surgery outcome in patients with non-glaucomatous pseudoexfoliation. Rom J Ophthalmol. 2017;61(3):196–201. doi:10.22336/rjo.2017.36
12. Fallah Tafti MR, Abdollah Beiki H, Mohammadi SF, Latifi G, Ashrafi E, Fallah Tafti Z. Anterior chamber depth change following cataract surgery in pseudoexfoliation syndrome; a Preliminary Study. J Ophthalmic Vis Res. 2017;12(2):165–169. doi:10.4103/jovr.jovr_81_15
13. Gür Güngör S, Akman A, Asena L, Aksoy M, Sarıgül Sezenöz A. Changes in anterior chamber depth after phacoemulsification in pseudoexfoliative eyes and their effect on accuracy of intraocular lens power calculation. Turk J Ophthalmol. 2016;46(6):255–258. doi:10.4274/tjo.56659
14. Lim DH, Kim TH, Chung E-S, Chung T-Y. Measurement of lens density using Scheimpflug imaging system as a screening test in the field of health examination for age-related cataract. Br J Ophthalmol. 2015;99(2):184–191. doi:10.1136/bjophthalmol-2014-305632
15. Nixon DR. Preoperative cataract grading by Scheimpflug imaging and effect on operative fluidics and phacoemulsification energy. J Cataract Refract Surg. 2010;36(2):242–246. doi:10.1016/j.jcrs.2009.08.032
16. Mayer WJ, Klaproth OK, Hengerer FH, Kohnen T. Impact of crystalline lens opacification on effective phacoemulsification time in femtosecond laser-assisted cataract surgery. Am J Ophthalmol. 2014;157(2):426–432.e1. doi:10.1016/j.ajo.2013.09.017
17. Technical product information. Alcon.com. Available from: https://www.alcon.com/eye-care-products.
18. Sensar 1 piece - AAB00.pdf. Available from: https://www.dc-ophthalmology.com.
19. Ermis SS. Effects of postural variation on anterior chamber depth in pseudoexfoliative eyes with normal intraocular pressure. Curr Eye Res. 2010;35(10):888–891. doi:10.3109/02713683.2010.494242
20. Ning X, Yang Y, Yan H, Zhang J. Anterior chamber depth - a predictor of refractive outcomes after age-related cataract surgery. BMC Ophthalmol. 2019;19(1):134. doi:10.1186/s12886-019-1144-8
21. Kim M, Park KH, Kim T-W, Kim DM. Changes in anterior chamber configuration after cataract surgery as measured by anterior segment optical coherence tomography. Korean J Ophthalmol. 2011;25(2):77–83. doi:10.3341/kjo.2011.25.2.77
22. Kristianslund O, Pathak M, Østern AE, Drolsum L. Corneal endothelial cell loss following cataract surgery in patients with pseudoexfoliation syndrome: a 2-year prospective comparative study. Acta Ophthalmol. 2020;98(4):337–342. doi:10.1111/aos.14314
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.