Back to Journals » Nature and Science of Sleep » Volume 14
Impact of Persistent Poor Sleep Quality on Post-Stroke Anxiety and Depression: A National Prospective Clinical Registry Study
Authors Fan XW , Yang Y, Wang S, Zhang YJ, Wang AX, Liao XL, Ma GW , Zhang N, Wang CX, Wang YJ
Received 10 January 2022
Accepted for publication 23 May 2022
Published 13 June 2022 Volume 2022:14 Pages 1125—1135
DOI https://doi.org/10.2147/NSS.S357536
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Xiao-Wei Fan,1– 4,* Yang Yang,2– 5,* Shuo Wang,2– 5 Yi-Jun Zhang,1– 4 An-Xin Wang,1– 4 Xiao-Ling Liao,1– 4 Wei-Guo Ma,6 Ning Zhang,2– 5 Chun-Xue Wang,2– 5,7 Yong-Jun Wang1– 4
1Department of Neurology, Capital Medical University, Beijing, People’s Republic of China; 2Center of Stroke, Beijing Institute for Brain Disorders, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3China National Clinical Research Center for Neurological Diseases, Beijing, People’s Republic of China; 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 5Department of Neuropsychiatry and Behavioral Neurology and Clinical Psychology, Capital Medical University, Beijing, People’s Republic of China; 6Beijing Anzhen Hospital, Capital Medical University, Beijing, People’s Republic of China; 7Beijing Institute of Brain Disorders, Collaborative Innovation Center for Brain Disorders, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ning Zhang; Chun-Xue Wang, Beijing Tiantan Hospital, 119 South 4th Ring West Road, Beijing, 100070, People’s Republic of China, Tel +86-15801203052 ; +86-13701193710, Email [email protected]; [email protected]
Purpose: The impact of poor sleep quality after stroke, especially persistent poor sleep quality, on poststroke anxiety and depression is unclear. We seek to investigate the impact of baseline and persistent poor sleep quality on short-term poststroke anxiety and depression.
Patients and Methods: Data were analyzed for 1619 patients with acute ischemic stroke from the Impairment of Cognition and Sleep after Acute Ischemic Stroke or Transient Ischemic Attack in Chinese Patients study (ICONS). The sleep quality was assessed at 2 weeks and 3 months using the Pittsburgh Sleep Quality Index scale (PSQI). Poor sleep quality was defined as a PSQI score of > 5, and persistent poor sleep quality was defined as a PSQI score of > 5 at each time point. Patients were divided into three groups according to the quality of sleep: good sleep quality, baseline poor sleep quality and persistent poor sleep quality. Patient Health Questionnaire-9 (PHQ-9), General Anxiety Disorder-7 scale (GAD-7), and Modified Rankin Scale (mRS) at 3 months after stroke were taken as the study outcomes.
Results: Persistent poor sleep quality was present in 70.2% of patients after stroke. Compared to those with good sleep quality, patients with baseline poor sleep quality did not show significant differences in disability, anxiety and depression. However, patients with persistent poor sleep were at increased risk of depression (odds ratio, OR 3.04, 95% confidence interval, CI 1.66– 5.57, P < 0.01) and anxiety (OR 3.20, 95% CI 1.42– 7.19, P < 0.01) at 3 months after stroke. Persistent poor sleep quality was not identified as a risk factor for functional disability at 3 months.
Conclusion: Patients with persistent poor sleep quality are at added risks for depression and anxiety after stroke.
Keywords: sleep quality, depression, anxiety, ischemic stroke
Introduction
Stroke is the first leading cause of death in China, accounting for one third of the world’s stroke mortality.1 Most stroke survivors sustain disability, sleep disorders and emotional disorders.2,3 Among emotional disorders, post-stroke depression and anxiety are most common, with a prevalence of 24–31%.4,5 A meta-analysis showed a 1.59-fold increased risk of mortality in patients with post-stroke depression.6 In addition, post-stroke anxiety negatively affects the functional outcomes in both acute and chronic phases.7,8
Literature on risk factors for disability, anxiety, and depression after stroke is mixed and inconsistent, including lesion location, severity, previous history of anxiety and depression, cognitive function, and sleep disorders.9 Although sleep disorders are easier to manage and modify, they are associated with worse functional outcome and increased risk of depression and anxiety after stroke.10–15 They can manifest as subjective poor sleep quality, which may be missed by common sleep parameters16–18 that help in early detection of sleep problems. Up to date, the majority of multicenter studies on post-stroke sleep was conducted in western countries,12,19–21 most of which were focused on a certain sleep disease. There is a paucity of large multicenter series on sleep quality in patients with stroke, while available studies are limited to the effect of poor sleep quality at one particular time point on the outcomes of stroke, and few examined this issue over a period of time.
Hypothetically, patients with poor sleep quality after stroke would have worse outcomes. In this study, we seek to investigate the association of baseline and persistent poor sleep quality after stroke with the functional disability and emotional outcomes (depression and anxiety) at 3 months in patients from a Chinese national registry.
Materials and Methods
Study Design and Participants
This study uses data derived from the Impairment of Cognition and Sleep after Acute Ischemic Stroke or Transient Ischemic Attack in Chinese Patients (ICONS) study. ICONS is one of the research subgroups of China National Stroke Registry-III (CNSR-III),22 which was conducted in 40 hospitals representing populations all across China.23 Briefly, patients with acute ischemic stroke (AIS) or transient ischemic attack (TIA) within 7 days after onset were consecutively recruited from August 2015 to March 2018. AIS and TIA were diagnosed based on the WHO diagnostic criteria and confirmed by cerebral magnetic resonance imaging (MRI) or computerized tomography (CT).
Inclusion criteria for ICONS included all of the following conditions:
- Age ≥18 years;
- Diagnosis of AIS/TIA;
- Admission within 7 days after onset of AIS/TIA;
- Ability to understand, confirm, and sign the informed consent.
Patients with any of the following conditions were excluded from ICONS:
- Silent cerebral infarction diagnosed by MRI or CT without symptoms and signs;
- Illiterate patients;
- History of cognitive impairment, psychosis, or schizophrenia (as documented in medical records);
- Other conditions that interfere with cognitive or sleep evaluation.
The present study included patients from ICONS who were diagnosed with AIS and had completed Pittsburgh Sleep Quality Index scale (PSQI) at 2 weeks and 3 months after stroke. After inclusion, those with new episodes of poor sleep quality at 3 months after stroke were excluded from the final analysis.
The sleep quality was assessed at 2 weeks and 3 months using the Pittsburgh Sleep Quality Index scale (PSQI). Patients were divided into three groups according to the quality of sleep: good sleep quality, baseline poor sleep quality and persistent poor sleep quality. Patient Health Questionnaire-9 (PHQ-9), General Anxiety Disorder-7 scale (GAD-7), and Modified Rankin Scale (mRS) at 3 months after stroke were taken as the study outcomes.
This study was approved by the Ethics Committee of Beijing Tiantan Hospital (#KY2015-001-01) and conducted in accordance with the Declaration of Helsinki. All patients have signed written informed consent prior to participation in this study.
Data Collection
Patients were interviewed face-to-face upon admission into the hospital, at 2 weeks and 3 months by trained clinical research coordinators. Baseline data were collected on admissions, which included demographics (age, gender, marital status, education, employment, monthly income, smoking history, and alcohol use), clinical data (medical history) and scales (mRS; and National Institutes of Health Stroke Scale, NIHSS). Other scales, including the GAD-7, PHQ-9, and PSQI were collected at 2 weeks. All five scales were measured again at 3 months by trained examiners.
Definition and Classification of Sleep Quality
PSQI was used to evaluate sleep quality.21 The scores of all components are added to obtain a value of 0 to 21, and the higher the score, the poorer the sleep quality. A PSQI global score of >5 was defined as poor sleep quality, while a PSQI global score of 5 or lower was defined as good sleep quality.24
According to the total PSQI score at each time point, patients were divided into three groups: good sleep quality, baseline poor sleep quality and persistent poor sleep quality. Among them, good sleep quality was defined as no poor sleep quality during follow-up after stroke (PSQI score of ≤5 at 2 weeks and 3 months after stroke), baseline poor sleep quality was defined as PSQI score of >5 at 2 weeks and PSQI score of ≤5 at 3 months after stroke, persistent poor sleep quality was defined as PSQI score of >5 at 2 weeks and 3 months after stroke.
Study Outcomes
The mRS score at 3 months after stroke was used to evaluate the functional disability. The degree of functional disability was assessed at 6 levels ranging from 0 to 5, and an mRS score of >2 was defined as poor functional disability.25
The PHQ-9 was used to measure the severity of depression at 3 months after stroke. PHQ-9 is composed of nine items, each ranging from 0 (not at all) to 3 (nearly every day), and the total score is from 0 to 27. A PHQ-9 score of ≥5 was defined as depression, while patients with PHQ-9 score of <5 were considered as no depression.26
The severity of anxiety at 3 months after stroke was assessed by GAD-7. It consists of seven items, each of which ranges from 0 to 3, and the total score ranges from 0 to 21. A GAD-7 score of ≥ 5 was defined as anxiety, while a GAD-7 score of < 5 defined as no anxiety.26
Statistical Analysis
Variables are expressed as mean ± standard deviation, or median (interquartile range), or frequency (percentage). Data were compared using the Fisher’s exact test or Chi-square test for categorical variables, and Kruskal–Wallis test or Student’s t-test for continuous variables.
Logistic regression was used to evaluate the impact of baseline and persistent poor sleep quality on anxiety, depression and function disability at 3 months after stroke, respectively. Wald test was used to evaluate the fitness of logistic regression models. Patients with good sleep quality were taken as the control group.
Based on the literature and clinical relevance,2,19,27–30 the following covariates were considered in the regression models: age, gender, education (high school and above or not), marital status (married or not), employment status (retired, unemployed, employed), monthly income (≥ vs < RMB 2300), Body Mass Index (normal <24, overweight 24–27.9, obesity ≥28 kg/m2), smoking (current smoker or no), alcohol use (current drinker or no), hypertension, diabetes, hyperlipidemia, history of stroke, classification of stroke (artery atherosclerosis, cardiac embolism, small vessel occlusion, others), infarction location (thalamus, brain stem, cerebellum, occipital lobe, temporal lobe, corpus callosum), medication (antiplatelet agents, anticoagulant, lipid-lowering agents), anxiety at 2 weeks (GAD-7), depression at 2 weeks (PHQ-9), functional disability (mRS) and NIHSS score on admission. The above covariables are redefined on the basis of the raw data.
In the models, age, gender, marital status, education, employment status, hypertension, diabetes, tobacco and alcohol use, history of stroke, NIHSS score on admission and further plus anxiety, and depression at 2 weeks were adjusted to offset the impact of potential confounding factors.
To evaluate potential interactions, patients with persistent poor sleep quality were stratified by gender and age (with the cut-off set at 60 years according to literature).31 Logistic regression was used to compare the effect on outcomes in patients of different ages with persistent poor sleep quality, and in the male and female subgroups. The stratified factor was not adjusted as confounding variable in logistic regression analysis of the corresponding subgroups.
A two-sided P value of <0.05 was considered to be statistically significant. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Among the 1756 patients included from the ICONS, 137 patients with new episode of poor sleep quality at 3 months after stroke were excluded, and 1619 were included in the final analysis, including 264 (16.3%) with good sleep, 218 (13.4%) with baseline poor sleep quality and 1137 (70.2%) with persistent poor sleep (Figure 1). At 3 months, 17 patients were lost to follow-up because of failure to complete the PHQ-9 (n = 6), GAD-7 (n = 2), and mRS (n = 9) scales.
Mean age was 60.8 ± 10.7 years, and 446 were female (27.5%). At 2 weeks after stroke, depression occurred in 421 patients (26.1%) and anxiety in 280 patients (17.3%), and 1355 patients (83.6%) reported poor sleep quality (PSQI score >5). At 3 months, depression was diagnosed in 342 patients (21.2%), anxiety in 216 patients (13.3%), poor sleep quality in 1137 patients (70.2%), and 126 patients (7.8%) were functionally disabled (Figure 2).
Compared to those with good sleep quality, patients with baseline poor sleep quality and persistent poor sleep quality were more likely to have attended high school or above (25.7% vs 40.8% vs 30.1%, P < 0.01), be retired (17.8% vs 26.6% vs 29.2%, P < 0.01), and suffer from anxiety (4.6% vs 10.5% vs 21.4%, P < 0.01) and depression (5.0% vs 12.8% vs 33.1%, P < 0.01) at 2 weeks (Table 1).
 |
Table 1 Baseline Characteristics |
Impact of Poor Sleep Quality on Prognosis of Stroke
Multivariate analysis revealed no statistically significant association of baseline poor sleep quality with depression, anxiety and functional disability at 3 months after stroke, compared to patients with good sleep quality (Table 2).
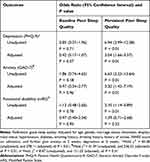 |
Table 2 Impact of Poor Sleep Quality on Outcomes at 3 Months After Stroke |
In unadjusted analysis, persistent poor sleep quality was predictive of depression (odds ratio, OR 6.94, 95% confidence interval, CI 3.99–12.08, P < 0.01), anxiety (OR 6.63, 95% CI 3.23–13.64, P < 0.01) and functional disability (OR 2.10, 95% CI 1.14–3.89, P = 0.01). After adjustment for age, gender, marital status, education, employment status, hypertension, diabetes, tobacco and alcohol use, history of stroke, NIHSS score on admission, and anxiety and depression at 2 weeks, persistent poor sleep quality was identified as a risk factor for depression (OR 3.04, 95% CI 1.66–5.57, P < 0.01) and anxiety (OR 3.20, 95% CI 1.42–7.19, P < 0.01) at 3 months after stroke; while no significant association was found between persistent poor sleep quality and functional disability (Table 2).
Subgroup Analysis
Compared to good sleep quality, the association between persistent poor sleep quality and depression at 3 months was significantly affected by gender (P for interaction = 0.01), which revealed that persistent poor sleep quality with male gender (P < 0.01) had a higher risk of depression at 3 months. However, age and gender did not interact with the association between persistent poor sleep quality and functional disability and anxiety at 3 months (Table 3).
 |
Table 3 Association of Persistent Poor Sleep Quality with Outcomes at 3 Months |
Relationship of Sleep Quality to Severity of Depression and Anxiety
Among 1355 patients with poor sleep quality, 1038 (64.1%) were categorized as mild, 271 (16.7%) as moderate and 46 (2.8%) as severe, respectively. After adjustment for age, gender, marital status, education, employment status, hypertension, diabetes, tobacco and alcohol use, history of stroke, NIHSS score on admission, and anxiety and depression at 2 weeks, the degree of baseline poor sleep quality was significantly correlated to the severity of depression (mild, OR 2.16, CI 1.21–3.85, P < 0.01; moderate, OR 3.18, CI 1.70 5.96, P < 0.01; severe, OR 2.79, CI 1.19–6.53, P < 0.01) and anxiety (mild, OR 2.38, CI 1.11–5.11, P < 0.01; moderate, OR 2.92, CI 1.28–6.63, P < 0.01; severe, OR 2.85, CI 0.99–8.17, P = 0.05) at 3 months after stroke (Table 4).
 |
Table 4 Relationship of Baseline Poor Sleep Quality to Severity of Anxiety and Depression at 3 Months |
Discussion
To the best of our knowledge, this study may represent the first multi-center, large sample, prospective study investigating the association of poor sleep quality after stroke with emotional and functional outcomes. Many previous studies were cross-sectional and focused on the prognosis of functional rehabilitation, which cannot elucidate causal relationship, ignoring the psychological prognosis. In contrast, more confounders were adjusted in the present study, which makes the findings more robust.
In this multicenter prospective registry study, we found that the incidence of poor sleep quality in stroke survivors decreased gradually over time but remained at a high level, which was 70% at 3 months and higher compared to previous studies (31.8–64.0%).14,27,32 This may be ascribed to the different definitions of poor sleep quality (eg sleep-related items in the Hamilton Depression Scale), variance in severity and types of stroke (eg TIA versus ischemic stroke), and markedly different follow-up durations.9,10 In consistence with the previous studies,13 we found that sleep quality tended to slightly improve over time within 3 months after stroke. Potential reasons included that disturbed sleep architecture in the acute phase of stroke returned to normal gradually,33,34 and, possibly, the use of sleep medication.35
An important finding in our study was that patients with persistent poor sleep quality after stroke were more likely to suffer from depression and anxiety. Sleep and stroke and mood interact with each other. Sleep disorders and anxiety and depression are not only considered as risk factors for stroke but also predict recurrence and mortality.2,36,37 Stroke patients are more likely to sustain sleep disorders, depression and anxiety.4,38,39 In addition, insomnia has been proven to increase the risk factor of depression and anxiety after stroke.19 In patients with poor sleep quality after a stroke, damage to the central nervous system leads to changes in brain activity and sleep structure.28,34 There may be biological responses to poor sleep quality, such as increased cortisol, hypothalamic–pituitary–adrenal (HPA) axis dysregulation, and increased inflammatory cytokines.40,41 These mechanisms may partly explain the correlation between poor sleep quality and depression and anxiety. Besides biological factors, psychosocial factors may be at play,42,43 such as personality traits, marriage, living alone, social activities, and so on. From a bio-psycho-social perspective, persistent poor sleep quality, while increasing the risk of depression and anxiety after stroke, together with psychosocial factors, will ultimately affect the functional prognosis and quality of life in patients with stroke. Therefore, the results of this study strongly highlight that stroke patients with persistent poor sleep quality should be given prioritized attention, and, if necessary, early intervention.
The association between subjective sleep quality and prognosis of stroke disability has been controversial in previous studies.14,44 The functional disability of stroke patients is not only affected by sleep quality but also by mood, stroke severity and psychosocial factors. In this study, 75.5% of patients sustained mild stroke, which may be reason why we did not find a direct relationship of persistent poor sleep quality to functional disability.
Although depression is more common in women generally,45 the relationship between different types of sleep problems and the risk of depression may differ between men and women. While Morita and associates reported that women with delayed sleep-wake time and men with difficulty falling asleep and daytime sleepiness are more likely to suffer from depression,46 the present study found a higher risk of depression in men than women in stroke patients with persistent poor sleep quality. This finding may be ascribed to the relatively low proportion of women in this study (27.5%) and the fact that ischemic stroke is more common in men.47
Upon further analysis of the relationship between the degree of baseline poor sleep quality and the severity of anxiety and depression at 3 months, we have found the worse sleep quality, the higher the risk of severe anxiety and depression. Previous studies have confirmed insomnia to be a risk factor for anxiety and depression after stroke,19 and the shorter sleep duration, the higher the risk of anxiety and depression.48,49 The severity of depression and anxiety was associated with functional impairment, lower quality of life and mortality after stroke.4,8 Therefore, sleep quality is crucial to the mood status, functional prognosis and survival of patients with stroke, and, in particular, more attention should be paid to those with poorer sleep quality in clinical practice.
Study Limitations
First, this study excluded patients who had not completed the scale evaluation, which may lead to an underestimation of the association between poorer sleep quality and worse stroke outcomes. Second, more objective measurements, such as polysomnograms or activity charts, were not used to assess the sleep quality, given the difficulty in performing polysomnography monitoring in all centers. Finally, due to the lack of uniformity in the definition of poor sleep quality after stroke, the results of this study may not be directly generalizable and should be interpreted with extra caution.
Conclusions
This study shows that poor sleep quality is common in stroke survivors, and persistent poor sleep quality is associated with a significantly increased risk for poststroke anxiety and depression. The results of this study suggest the importance of sleep quality screening after stroke and imply that early diagnosis and treatment of poor sleep quality may help improve the emotional outcomes in patients with stroke.
Acknowledgments
We gratefully acknowledge the clinicians and patients from the 40 centers involved in this project for their help and support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2020YFC2005300), Talents Project of Beijing (2018000021469G235), Dongcheng District Talents Project of Beijing (DCQYYRC-789-01-DR), Youth Research Funding, Beijing Tiantan Hospital, Capital Medical University (2018-YQN-18) and Beijing Key Clinical Specialty Program.
Disclosure
The authors declare no conflicts of interest related to this study.
References
1. Li Z, Jiang Y, Li H, Xian Y, Wang Y. China’s response to the rising stroke burden. BMJ. 2019;364:l879. doi:10.1136/bmj.l879
2. Khot SP, Morgenstern LB. Sleep and stroke. Stroke. 2019;50(6):1612–1617. doi:10.1161/STROKEAHA.118.023553
3. Hackett ML, Köhler S, O’Brien JT, Mead GE. Neuropsychiatric outcomes of stroke. Lancet Neurol. 2014;13(5):525–534. doi:10.1016/S1474-4422(14)70016-X
4. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry. 2016;173(3):221–231. doi:10.1176/appi.ajp.2015.15030363
5. Kim JS. Post-stroke mood and emotional disturbances: pharmacological therapy based on mechanisms. J Stroke. 2016;18(3):244–255. doi:10.5853/jos.2016.01144
6. Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102–109. doi:10.1016/j.arr.2019.01.013
7. Maaijwee NA, Tendolkar I, Rutten-Jacobs LC, et al. Long-term depressive symptoms and anxiety after transient ischaemic attack or ischaemic stroke in young adults. Eur J Neurol. 2016;23(8):1262–1268. doi:10.1111/ene.13009
8. Li W, Xiao WM, Chen YK, et al. Anxiety in patients with acute ischemic stroke: risk factors and effects on functional status. Front Psychiatry. 2019;10:257. doi:10.3389/fpsyt.2019.00257
9. Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke. 2014;9(8):1026–1036. doi:10.1111/ijs.12356
10. Li LJ, Yang Y, Guan BY, et al. Insomnia is associated with increased mortality in patients with first-ever stroke: a 6-year follow-up in a Chinese cohort study. Stroke Vasc Neurol. 2018;3(4):197–202. doi:10.1136/svn-2017-000136
11. Leppävuori A, Pohjasvaara T, Vataja R, Kaste M, Erkinjuntti T. Insomnia in ischemic stroke patients. Cerebrovasc Dis. 2002;14(2):90–97. doi:10.1159/000064737
12. Lisabeth LD, Sanchez BN, Lim D, et al. Sleep-disordered breathing and poststroke outcomes. Ann Neurol. 2019;86(2):241–250. doi:10.1002/ana.25515
13. Harris AL, Elder J, Schiff ND, Victor JD, Goldfine AM. Post-stroke apathy and hypersomnia lead to worse outcomes from acute rehabilitation. Transl Stroke Res. 2014;5(2):292–300. doi:10.1007/s12975-013-0293-y
14. Sonmez I, Karasel S. Poor sleep quality I related to impaired functional status following stroke. J Stroke Cerebrovasc Dis. 2019;28(11):104349. doi:10.1016/j.jstrokecerebrovasdis.2019.104349
15. Davis JC, Falck RS, Best JR, Chan P, Doherty S, Liu-Ambrose T. Examining the inter-relations of depression, physical function, and cognition with subjective sleep parameters among stroke survivors: a cross-sectional analysis. J Stroke Cerebrovasc Dis. 2019;28(8):2115–2123. doi:10.1016/j.jstrokecerebrovasdis.2019.04.010
16. Frankel BL, Coursey RD, Buchbinder R, Snyder F. Recorded and reported sleep in chronic primary insomnia. Arch Gen Psychiatry. 1976;33(5):615–623. doi:10.1001/archpsyc.1976.01770050067011
17. Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133(12):1382–1388.
18. Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9:S10–S17. doi:10.1016/S1389-9457(08)70011-X
19. Glozier N, Moullaali TJ, Sivertsen B, et al. The course and impact of poststroke insomnia in stroke survivors aged 18 to 65 years: results from the Psychosocial Outcomes in Stroke (POISE) Study. Cerebrovasc Dis Extra. 2017;7(1):9–20. doi:10.1159/000455751
20. Iddagoda MT, Inderjeeth CA, Chan K, Raymond WD. Post-stroke sleep disturbances and rehabilitation outcomes: a prospective cohort study. Intern Med J. 2020;50(2):208–213. doi:10.1111/imj.14372
21. Bassetti CLA, Randerath W, Vignatelli L, et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur J Neurol. 2020;27(7):1117–1136. doi:10.1111/ene.14201
22. Wang Y, Jing J, Meng X, et al. The Third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol. 2019;4(3):158–164. doi:10.1136/svn-2019-000242
23. Liao XL, Zuo LJ, Zhang N, et al. The occurrence and longitudinal changes of cognitive impairment after acute ischemic stroke. Neuropsychiatr Dis Treat. 2020;16:807–814. doi:10.2147/NDT.S234544
24. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
25. Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi:10.1161/01.STR.0000258355.23810.c6
26. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
27. Karaca B. Factors affecting poststroke sleep disorders. J Stroke Cerebrovasc Dis. 2016;25(3):727–732. doi:10.1016/j.jstrokecerebrovasdis.2015.11.015
28. Ferre A, Ribó M, Rodríguez-Luna D, et al. Strokes and their relationship with sleep and sleep disorders. Neurologia. 2013;28(2):103–118. doi:10.1016/j.nrl.2010.09.016
29. Xiaolin GMM. Risk factors of sleep disorder after stroke: a meta-analysis. Top Stroke Rehabil. 2017;24(1):34–40. doi:10.1080/10749357.2016.1188474
30. Choi-Kwon S, Han K, Choi S, et al. Poststroke depression and emotional incontinence: factors related to acute and subacute stages. Neurology. 2012;78(15):1130–1137. doi:10.1212/WNL.0b013e31824f8090
31. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi:10.1016/S1474-4422(18)30500-3
32. Kim KT, Moon HJ, Yang JG, Sohn SI, Hong JH, Cho YW. The prevalence and clinical significance of sleep disorders in acute ischemic stroke patients-a questionnaire study. Sleep Breath. 2017;21(3):759–765. doi:10.1007/s11325-016-1454-5
33. Gottselig JM, Bassetti CL, Achermann P. Power and coherence of sleep spindle frequency activity following hemispheric stroke. Brain. 2002;125(Pt 2):373–383. doi:10.1093/brain/awf021
34. Bassetti CL, Aldrich MS. Sleep electroencephalogram changes in acute hemispheric stroke. Sleep Med. 2001;2(3):185–194. doi:10.1016/S1389-9457(00)00071-X
35. Wang J, Wang Z, Wang X, et al. Combination of alprazolam and bailemian capsule improves the sleep quality in patients with post-stroke insomnia: a retrospective study. Front Psychiatry. 2019;10:411. doi:10.3389/fpsyt.2019.00411
36. Williams LS. Depression and stroke: cause or consequence? Semin Neurol. 2005;25(4):396–409. doi:10.1055/s-2005-923534
37. Lambiase MJ, Kubzansky LD, Thurston RC. Prospective study of anxiety and incident stroke. Stroke. 2014;45(2):438–443. doi:10.1161/STROKEAHA.113.003741
38. Baylan S, Griffiths S, Grant N, Broomfield NM, Evans JJ, Gardani M. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2020;49:101222. doi:10.1016/j.smrv.2019.101222
39. Cumming TB, Blomstrand C, Skoog I, Linden T. The high prevalence of anxiety disorders after stroke. Am J Geriatr Psychiatry. 2016;24(2):154–160. doi:10.1016/j.jagp.2015.06.003
40. Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9–15. doi:10.1016/S0022-3956(02)00052-3
41. Blake MJ, Trinder JA, Allen NB. Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: implications for behavioral sleep interventions. Clin Psychol Rev. 2018;63:25–40. doi:10.1016/j.cpr.2018.05.006
42. van Mierlo ML, van Heugten CM, Post MW, de Kort PL, Visser-Meily JM. Psychological factors determine depressive symptomatology after stroke. Arch Phys Med Rehabil. 2015;96(6):1064–1070. doi:10.1016/j.apmr.2015.01.022
43. De Ryck A, Fransen E, Brouns R, et al. Psychosocial problems associated with depression at 18 months poststroke. Int J Geriatr Psychiatry. 2014;29(2):144–152. doi:10.1002/gps.3974
44. Kim WH, Yoo YH, Lim JY, et al. Objective and subjective sleep problems and quality of life of rehabilitation in patients with mild to moderate stroke. Top Stroke Rehabil. 2020;27(3):199–207. doi:10.1080/10749357.2019.1673591
45. Hysing M, Pallesen S, Stormark KM, Lundervold AJ, Sivertsen B. Sleep patterns and insomnia among adolescents: a population-based study. J Sleep Res. 2013;22(5):549–556.
46. Morita Y, Sasai-Sakuma T, Asaoka S, Inoue Y. The impact of a delayed sleep-wake schedule on depression is greater in women–A web-based cross-sectional study in Japanese young adults. Chronobiol Int. 2015;32(7):952–958. doi:10.3109/07420528.2015.1055756
47. Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3):259–268. doi:10.1001/jama.2014.7692
48. Dong L, Brown DL, Chervin RD, Case E, Morgenstern LB, Lisabeth LD. Pre-stroke sleep duration and post-stroke depression. Sleep Med. 2021;77:325–329. doi:10.1016/j.sleep.2020.04.025
49. Liu F, Yang Y, Wang S, et al. Impact of sleep duration on depression and anxiety after acute ischemic stroke. Front Neurol. 2021;12:630638. doi:10.3389/fneur.2021.630638
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


