Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Impact of Obesity on Microvascular Obstruction and Area at Risk in Patients After ST-Segment-Elevation Myocardial Infarction: A Magnetic Resonance Imaging Study
Authors Lan DH, Zhang Y, Hua B, Li JS, He Y, Chen H , Li WP, Li HW
Received 3 April 2022
Accepted for publication 20 July 2022
Published 28 July 2022 Volume 2022:15 Pages 2207—2216
DOI https://doi.org/10.2147/DMSO.S369222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Di-Hui Lan,1 Yue Zhang,1 Bing Hua,1 Jin-Shui Li,2 Yi He,2 Hui Chen,1 Wei-Ping Li,1,3 Hong-Wei Li1,3
1Department of Cardiology, Cardiovascular Center, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Radiology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Beijing Key Laboratory of Metabolic Disorder Related Cardiovascular Disease, Beijing, People’s Republic of China
Correspondence: Wei-Ping Li; Hong-Wei Li, Department of Cardiology, Cardiovascular Center, Beijing Friendship Hospital, Capital Medical University, 95 Yongan Road, Beijing, 100050, People’s Republic of China, Email [email protected]; [email protected]
Background: Better survival for overweight and obese patients after ST-segment elevation myocardial infarction (STEMI) has been demonstrated. The association between body mass index (BMI), microvascular obstruction (MVO), and area at risk (AAR) after STEMI was evaluated.
Methods: A prospective observational study was performed to enrolled patients undergoing primary percutaneous coronary intervention (pPCI) for STEMI and cardiac magnetic resonance was performed within 5– 7 days. Patients were classified as normal weight (18.5 ≤BMI < 24.0 kg/m2), overweight (24.0 ≤BMI < 28.0 kg/m2), or obese (BMI ≥ 28 kg/m2).
Results: Among 225 patients undergoing pPCI, 67 (30.00%) were normal weight, 113 (50.22%) were overweight, and 45 (20.00%) were obese. BMI ≥ 28 kg/m2 was significantly associated with less risk of MVO when compared with a normal BMI after multivariable adjustment (overweight: HR 0.29, 95% CI 0.13– 0.68, p = 0.004). Compared with normal weight patients, obese and overweight patients tend to have larger hearts (greater left ventricular end-diastolic volume [LVEDV] and left ventricular [LV] mass). In adjusted analysis, increased BMI was significantly associated with a smaller AAR. In addition, obese patients had a smaller AAR (β = − 0.252, 95% CI − 20.298- − 3.244, p = 0.007) and AAR, % LV mass (β = − 0.331, 95% CI − 0.211- − 0.062, p < 0.001) than normal weight patients.
Conclusion: Obesity (BMI ≥ 28 kg/m2) is independently associated with lower risks of MVO and a smaller AAR, % LV mass than normal weight patients among subjects undergoing pPCI for STEMI.
Keywords: ST-segment elevation myocardial infarction, STEMI, microvascular obstruction, MVO, cardiac magnetic resonance, CMR, body mass index, BMI, area at risk, AAR
Introduction
Obesity remains a major health problem, as it is associated with numerous diseases including an increased risk for acute myocardial infarction.1 Obesity also increases risk for developing other cardiovascular risk factors such as diabetes, dyslipidemia, and hypertension.2 Despite its established adverse impact on general and cardiovascular health, numerous studies have demonstrated a better prognosis for overweight or obese patients after an acute coronary syndrome compared with their leaner counterparts.3–5 The mechanisms underlying this “obesity paradox” remain unknown.
Relatively favorable outcomes among obese patients with ST-segment elevation myocardial infarction (STEMI) may be related to smaller infarcts in overweight patients. Several studies found that reduced myocardial infarct size among obese patients versus nonobese patients.6,7 While an obesity paradox is documented, several studies question its presence in STEMI patients.8,9 Thus, it remains unclear whether a true “obesity paradox” exists, which could be attributed to the inherent limitations of body mass index (BMI) as a marker of adiposity.
Cardiovascular magnetic resonance (CMR) has emerged as an important imaging modality for assessing microvascular obstruction (MVO) and relevant prognostic pathophysiological consequences of myocardial ischemia and reperfusion after an acute reperfused STEMI.10,11 Thus, CMR is uniquely positioned to be used to comprehensively evaluate the morphological, functional, and microvascular sequelae of the post-infarction patient.
Despite obesity being prevalent in patients with STEMI,12 its effects on infarct size are largely unexplored. Whether less extensive myocardial damage represents a potential mechanism for the more favorable clinical outcomes in overweight and obese patients with myocardial infarction remains therefore controversial. In this study, we aimed to evaluate the association between BMI and the CMR prognosis of patients with STEMI undergoing primary percutaneous coronary intervention (pPCI).
Methods
Study Population
In this prospective observational study, consecutive patients were included with first acute STEMI admitted to the coronary care unit (CCU) of Beijing Friendship Hospital. Patients were included if they were first STEMI defined in accordance with the redefined committee criteria,13 and were successfully treated by pPCI within 12 h from symptom onset. Exclusion criteria were previous myocardial infarction (MI) or revascularization, congestive heart failure with left ventricular ejection fraction (LVEF) <40%, atrial fibrillation, renal failure with glomerular filtration <30 mL/min, acute infections disease within 3 months, rheumatic disease, malignant tumors, claustrophobia, and other contraindications to CMR. According to the above inclusion and exclusion criteria, a total of 226 patients were enrolled from December 11, 2018 to November 19, 2021. The study complied with the Declaration of Helsinki. The study data collections were approved by the Institutional Review Board of Beijing Friendship Hospital affiliated to Capital Medical University, and written informed consent was obtained from all patients.
CMR Protocol
All patients were studied with a 3.0-T scanner (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) within 5–7 days after pPCI. Patients were scanned with electrocardiogram (ECG) triggering in the supine position using 32-channel surface phased array coils. The imaging protocol included whole LV coverage for T1- and T2-weighted, perfusion, cines, and Late gadolinium enhancement (LGE) images. After obtaining T1- and T2-weighted images, gadolinium was administered intravenously (0.2 mmol/kg, gadopentetate dimeglumine, Magnevist, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA) for perfusion, and then obtaining cine images. Ten minutes after contrast administration, a segmented IR cine bSSFP inversion time (TI) scouting sequence was performed to null the signal of the normal myocardium to insure the quality of LGE images. LVEDV, LVESV, LVEF and LV mass were calculated from the short axis cine images. Area at risk (AAR) was defined as a hyperintense area on T2-weighted images when the signal intensity was >2 SD above the mean intensity of normal myocardium and it was measured as absolute mass and as a percentage of entire LV mass. Infarct size and AAR are often used by studies to show which effect myocardial infarction has on the heart.14 MVO was defined as the hypo-enhanced region within the LGE area and was quantified by careful manual delineation of this hypo-enhanced region. Both LGE and MVO were finally measured as absolute mass and as percentage of entire LV mass. Infarct size was also shown as percentage of LV mass. Infarct size >19% was defined as large infarct size according to the prognostic data published.15 For all post-processing analyses, commercially available software was used CVI42 (Release 5.12.2, Circle Cardiovascular Imaging, Calgary, Canada). All CMR images were evaluated by experienced observers, blinded to clinical events and angiographic results.
Clinical Characteristics
Clinical history was recorded from each patient by 1 trained physician. The following variables were collected: demographic characteristics (age, sex, and body mass index [BMI]) and cardiovascular risk factors including hypertension; current or previous smoking; hyperlipidemia; diabetes mellitus; family history of CAD in first-degree relatives; medical therapy; vital parameters including blood pressure and heart rate, site of MI and Killip class.
Definition of BMI
BMI was defined as weight in kilograms divided by the square of height in meters. Two sets of analyses were conducted to assess the association among BMI and CMR outcomes. In the first analysis, patients were categorized into three different BMI groups: normal weight (18.5≤BMI<24.0 kg/m2), overweight (24.0≤BMI<28.0 kg/m2), and obesity (BMI ≥28.0 kg/m2) according to the classification of the Criteria of Weight for Adults released by the Ministry of Health of China.16 For this analysis, 1 patient who was underweight (defined as BMI <18.5 kg/m2) was excluded. In the second analysis, BMI was modeled as a continuous variable.
Statistical Analysis
Baseline characteristics were summarized for patients in each BMI category. All variables were expressed as mean ± SD for normally distributed continuous variables or median (25th to 75th percentile) for non-normally distributed continuous variables or numbers (percent) for categorical variables. Comparisons between groups were performed using one-way analysis of variance (ANOVA) or Mann–Whitney U-test for continuous variables. Categorical variables were compared by Pearson’s Chi square test.
The associations between BMI and LV parameters and AAR were assessed using multiple linear regression. Binary logistic regression was used to assess the association between baseline covariates and the BMI groups (results presented as odds ratio [OR] and 95% confidence interval [CI]). In the model, we adjusted for variables that were significant in the univariate analysis and variables with potential influence of presence of MVO. Also, intercorrelations among variables were taken into consideration in the multivariate analysis. All tests were 2-tailed, and a value of p < 0.05 was considered to be statistically significant. Statistical analysis was performed with SPSS version 25 (SPSS Inc., Chicago, Illinois) and R version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 225 patients with STEMI undergoing pPCI (83.1% men; mean age, 58.41 ± 11.53 years) were included in this analysis, with 67 (30.00%), 113 (50.22%) and 45 (20.00%) patients being categorized as normal weight, overweight, and obesity, respectively.
Characteristics of the Study Population
Baseline characteristics according to categories of BMI are detailed in Table 1. Compared with those of normal weight, overweight and obese patients were younger and have a higher prevalence of hypertension. They also had higher levels of SBP, DBP, waist circumference and triglyceride. The use of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEIs/ARB) was also more common among those patients. In contrast, normal weight patients were older, had a higher prevalence of dyslipidemia and family history of CAD, as well as higher level of peak CK-MB, total cholesterol, HDL-C and LDL-C.
 |
Table 1 Baseline Characteristic Comparisons According to General Obesity |
CMR Parameters
Overweight and obese patients showed a lower prevalence of MVO (40.0%, 50.4%3 vs 65.7%; p = 0.006) when compared with normal weight patients (Figure 1). Also, normal weight patients showed higher amounts of infarct size >19% compared with other patients (Figure 1), but the difference was not significant. Overall, BMI and most LV geometry and function parameters assessed early after STEMI were only weakly correlated. In agreement with previous reports, strongest correlations were seen for total LV mass, which were significantly greater in overweight and obese patients compared with normal weight patients (by 128.01 ± 25.17, 112.17 ± 23.31 and 105.24 ± 24.47, respectively, p < 0.001, Table 2). Overweight and obese patients also showed significant differences in diastolic parameters (higher left atrial volume) compared with normal weight patients (by 148.59 ± 36.84, 141.85 ± 32.76 and 130.58 ± 28.46, respectively, p = 0.011). Also, overweight and obese patients were found to have smaller AAR, % LV mass (Table 2).
 |
Table 2 CMR Baseline Characteristics |
 |
Figure 1 Percentage MVO and infarct size according to body mass index category. Abbreviations: BMI, body mass index; MVO, microvascular obstruction. |
BMI and imagine endpoints.
The results of the univariate and multivariate logistic regression analyses are shown in Table 3. On univariate analysis, increased waist circumference, Killip class, glycated hemoglobin and a higher BMI were significantly associated with MVO. Correlation analysis showed that waist circumference was significantly correlated with BMI (r = 0.690, p < 0.001). Therefore, waist circumference was not included in the multivariate model. After multivariable adjustment, when compared with a normal BMI, a higher BMI was associated with a lower risk of MVO (overweight: HR 0.44, 95% CI 0.23–0.86, p=0.017, and obesity: HR 0.29, 95% CI 0.13–0.68, p = 0.004) (Table 3). As a continuous variable, increased BMI was significantly associated with larger LVEDV and total LV mass in both unadjusted and adjusted analysis (Table 4). In contrast, increased BMI was significantly associated with smaller AAR, % LV mass (β = −0.151, 95% CI −0.017- −0.001, p = 0.023) (Table 4). When compared with normal weight patients, obese patients had a significantly decreased AAR (β = −0.252, 95% CI −20.298- −3.244, p = 0.007) and AAR, % LV mass (β = −0.331, 95% CI −0.211- −0.062, p < 0.001) (Table 5) (Figure 2).
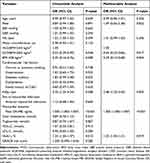 |
Table 3 Univariable and Multivariable Predictors of Presence of MVO |
 |
Table 4 Multivariable Adjusted Difference in LV Parameters and AAR per Unit Increase in Body Mass Index |
 |
Table 5 Multivariable Adjusted Difference in LV Parameters and AAR According to Body Mass Index Category |
Discussion
In the present study, overweight status was significantly associated with a lower risk of MVO presence and smaller AAR, % LV mass compared with normal BMI. BMI ≥28 kg/m2 was found to be independently and significantly associated with a smaller infarct size than normal BMI. Obese patients with greater BMI exhibit the most LV structural remodeling early after the infarction compared with normal and overweight patient groups. To the best of our knowledge, the present study is the first analysis examining the association between BMI and MVO measured by contrast-enhanced CMR in Chinese patients undergoing primary PCI for STEMI.
Recent studies raised the hypothesis that CMR-based parameters of irreversible myocardial ischemic damage, such as microvascular obstruction (MVO), are closely associated with adverse events among STEMI patients.11,17,18 Symons et al reported that MVO extent ≥2.6% of LV was a strong independent predictor of all deaths and HF hospitalizations in addition.11 In another pooled study which enrolled 1688 patients with STEMI after pPCI, a strong independent relationship between MVO measured within 7 days after reperfusion and the occurrence of mortality and heart failure hospitalization within 1 year was found.17 In addition, STEMI patients with MVO were associated with increased risks of major adverse cardiac events (MACEs). After 6 years of follow-up, the extent of MVO remained a strongest predictor for occurrence of MACEs.18 MVO is now firmly accepted to be a prognostic significance predictor of adverse left ventricular remodeling, major adverse cardiac events, and cardiovascular death.19 However, data on the association between MVO among STEMI patients and BMI was limited.
Obesity may be associated with a survival benefit once acute myocardial infarction has occurred.20 It is referred to as the “obesity paradox”. However, this pathophysiological mechanism behind this phenomenon remains controversial.21,22
In our study, compared with normal weight, the existence of MVO showed a graded reduction in obese and overweight patients when BMI was stratified according to Chinese classification. BMI ≥28 kg/m2 was associated with lower risks of MVO existence and smaller AAR, % LV mass. This is in accordance with existing studies that an obesity paradox is present in STEMI patients that patients with overweight might have a smaller infarct size as a possible explanation for better outcomes.6,7 However, these results were partially contradictory and derived from small numbers of included patients. Interestingly, in a pooled analysis performed from 6 randomized trials among 2238 patients undergoing pPCI, BMI was not associated with infarct size, MVO or LVEF.8 Another study also revealed no significant association between BMI and infarct size.9 Differences among these studies may be explained by differences in patient populations, therapies administered, and variables used for multivariable adjustment.
Several factors may contribute to this observed phenomenon. Age may partially explain the lower risk associated with overweight and obesity. Age was progressively lower in overweight and obese patients compared with normal weight subjects in most studies. In our study, we did observe a younger age in obese and overweight patients. However, the mean ages of subjects in three groups were not different and overweight was still an independent risk factor after multivariate analysis including age in our study. Therefore, the differences in baseline characteristics do not appear to be sufficient to explain the mechanism of the obesity paradox. Moreover, the association of BMI and adverse outcomes can also be modified by cardiorespiratory fitness (CRF). While obesity paradox has also been observed in patients with CHD, the prognostic role of BMI for adverse outcomes can be mitigated after adjusted for CRF.23 Studies have also shown that a higher level of CRF will substantially offset the adverse effects of obesity on morbidity and mortality.24 Besides, patients with overweight or obesity may get earlier and more aggressive intervention due to a higher prevalence of metabolic diseases such as hypertension or diabetes. In the present study, obese patients were more likely to have hypertension than nonobese patients, although there was no significant difference in baseline medications across BMI categories; however, data on whether obese patients were more aggressively treated than nonobese patients after admission were limited. In contrast, individuals with normal body weight have a lower pretest probability, and consequently present with more advanced disease, and thus a worse subsequent prognosis.
Multiple large registry studies investigating the effect of BMI on clinical outcomes after an acute coronary syndrome have either not described LV function according to BMI or have limited their analysis to LVEF.5 Obesity has been consistently associated with adverse, frequently subclinical, cardiac structural, and functional changes, leading to the development of established LV dysfunction and eventually heart failure.25 BMI was positively associated with increased LV mass and LV volume without change in ejection fraction among patients free of clinically apparent cardiovascular disease.25 Recognition of the independent structural and functional consequences of obesity itself on the myocardium has grown. Several studies have reported that there were significant positive correlations between severity of obesity and measures of LV mass, but not all studies assessing LV diastolic dimension or volume have reported a significant positive correlation between obesity and LV diastolic chamber size.26 Multiple factors have been identified that may increase LV mass in obese patients including hypertension and duration of obesity.27 Our findings are consistent with previous studies demonstrating LV mass was consistently significantly greater in obese than in normal weight subjects.25,28 We also find significant differences in LV end-diastolic volume across BMI groups in the present study. Obese patients had a significantly further impaired level of LV diastolic function compared with normal weight patients. It has been postulated that perivascular and interstitial fibrosis may contribute to LV diastolic dysfunction in obesity based on the presence of markers of collagen turnover and the high prevalence of diabetes mellitus in obese subjects.29 These results should be taken into consideration for future “obesity paradox” studies, particularly when evaluating optimized treatment strategies for this group of patients suffering acute STEMI.
Limitations
Our study had several limitations. First, our study was single-center study and the sample size was small, leading to a cautious generation of the results. Second, cardiorespiratory fitness data which may affect the obesity paradox was not assessed in this cohort. Third, updated information during follow-up was not included, which BMI may play an important role on prognosis of STEMI patients. Although BMI is the most commonly used measure of obesity, it failed directly distinguish between adipose and lean tissue or central and peripheral adiposity. Waist circumference or direct body fat measuring modalities, are likely to more accurately reflect true obesity burden. In addition, the present study did not take into account recent weight loss and shifts in body weight, which may be associated with significant increases in risk. Last, the classification of BMI in our study is from Criteria of Weight for Adults released by the Ministry of Health of China, this may not be applicable to other countries.
Conclusion
In conclusion, the current study demonstrated that BMI was associated with MVO and AAR, % LV mass among subjects undergoing pPCI for STEMI. BMI ≥28 kg/m2 in this population is independent of MVO and smaller AAR, % LV mass suggests focusing on alternative mechanisms by which higher BMI might confer better prognosis in the contemporary STEMI era.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study data collections were approved by the Institutional Review Board of Beijing Friendship Hospital affiliated to Capital Medical University, and written informed consent was obtained from all patients.
Consent for Publication
All authors have participated in the work and have read and approved the content, and agree to submit for consideration for publication in the journal.
Acknowledgments
We sincerely thank all staffs who have contributed to this subject.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Key R&D Program of China (2021ZD0111004), National Natural Science Foundation of China (Grant No. 82070357), Beijing Municipal Administration of Hospitals Incubating Program (Grant No. PX2018002) and Beijing Key Clinical Subject Program.
Disclosure
The authors declare that they have no competing interests.
References
1. Yusuf S, Hawken S, Ounpuu S, Bautista L, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi:10.1016/S0140-6736(05)67663-5
2. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi:10.1016/j.jacc.2008.12.068
3. Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yen RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta-analysis. Heart. 2015;101:1631–1638. doi:10.1136/heartjnl-2014-307119
4. Coutinho T, Goel K, Sá D, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57:1877–1886. doi:10.1016/j.jacc.2010.11.058
5. Das SR, Alexander KP, Chen AY, et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58:2642–2650. doi:10.1016/j.jacc.2011.09.030
6. Cepeda-Valery B, Slipczuk L, Figueredo VM, et al. Association between obesity and infarct size: insight into the obesity paradox. Int J Cardiol. 2012;167:604–606. doi:10.1016/j.ijcard.2012.09.230
7. Sohn GH, Kim EK, Hahn JY, et al. Impact of overweight on myocardial infarct size in patients undergoing primary percutaneous coronary intervention: a magnetic resonance imaging study. Atherosclerosis. 2014;235:570–575. doi:10.1016/j.atherosclerosis.2014.05.961
8. Shahim B, Redfors B, Chen S, Thiele H, Stone GW. BMI, infarct size, and clinical outcomes following primary PCI. JACC Cardiovasc Interv. 2020;13:965–972. doi:10.1016/j.jcin.2020.02.004
9. Reinstadler SJ, Reindl M, Tiller C, Holzknecht M, Klug G, Metzler B. Obesity paradox in ST-elevation myocardial infarction: is it all about infarct size? Eur Heart J Qual Care Clin Outcomes. 2018;5:180–182.
10. Marra MP, Lima J, Iliceto S. MRI in acute myocardial infarction. Eur Heart J. 2011;32:284–293. doi:10.1093/eurheartj/ehq409
11. Symons R, Pontone G, Schwitter J, et al. Long-term incremental prognostic value of cardiovascular magnetic resonance after ST-segment elevation myocardial infarction: a study of the collaborative registry on CMR in STEMI. Jacc Cardiovasc Imaging. 2017;11:813–825. doi:10.1016/j.jcmg.2017.05.023
12. Zhang M, Zuo HJ, Yang HX, Nan N, Song XT. Trends in conventional cardiovascular risk factors and myocardial infarction subtypes among young Chinese men with a first acute myocardial infarction. Clin Cardiol. 2021;45:129–135.
13. Jaffe AS. Third universal definition of myocardial infarction. Clin Biochem. 2013;46:1–4. doi:10.1016/j.clinbiochem.2012.10.036
14. Khoshnood A, Carlsson M, Akbarzadeh M, et al. Effect of oxygen therapy on myocardial salvage in ST elevation myocardial infarction: the randomized SOCCER trial. Eur J Emerg Med. 2018;25:78–84. doi:10.1097/MEJ.0000000000000431
15. Eitel I, Waha SD, WöHrle J, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1217–1226. doi:10.1016/j.jacc.2014.06.1194
16. Chinese Standard-National Health and Family Planning Commission of PRC. Criteria of weight for adults. Available from: https://www.chinesestandard.net/PDF/English.aspx/WST428-2013. Accessed July 25, 2022.
17. Waha SD, Patel MR, Granger CB, et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J. 2017;38:3502–3510.
18. Regenfus M, Schlundt C, Krähner R, et al.Six-year prognostic value of microvascular obstruction after reperfused ST-elevation myocardial infarction as assessed by contrast-enhanced cardiovascular magnetic resonance. Am J Cardiol. 2015;116(7):1022–1027.
19. Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:940–952. doi:10.1016/j.jcmg.2014.06.012
20. Wang L, Liu W, He X, et al. Association of overweight and obesity with patient mortality after acute myocardial infarction: a meta-analysis of prospective studies. Int J Obes. 2015;40:220–228. doi:10.1038/ijo.2015.176
21. Younge JO, Damen NL, Van Domburg RT, Pedersen SS. Obesity, health status, and 7-year mortality in percutaneous coronary intervention: in search of an explanation for the obesity paradox. Int J Cardiol. 2013;167:1154–1158. doi:10.1016/j.ijcard.2012.03.105
22. Neeland IJ, Das SR, Simon DN, et al. The obesity paradox, extreme obesity, and long-term outcomes in older adults with ST-segment elevation myocardial infarction: results from the NCDR. Eur Heart J Qual Care Clin Outcomes. 2017;3:183–191. doi:10.1093/ehjqcco/qcx010
23. Mcauley PA, Kokkinos PF, Oliveira RB, Emerson BT, Myers JN. Obesity paradox and cardiorespiratory fitness in 12,417 male veterans aged 40 to 70 years. Mayo Clin Proc. 2010;85:115–121. doi:10.4065/mcp.2009.0562
24. Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Prog Cardiovasc Dis. 2014;56:382–390. doi:10.1016/j.pcad.2013.09.002
25. Turkbey EB, Mcclelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (Mesa). JACC Cardiovasc Imaging. 2010;3:266–274. doi:10.1016/j.jcmg.2009.10.012
26. Aurigemma GP, De Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging. 2013;6:142–152. doi:10.1161/CIRCIMAGING.111.964627
27. Yan Y, Li S, Guo Y, et al. Life-course cumulative burden of body mass index and blood pressure on progression of left ventricular mass and geometry in midlife: the Bogalusa heart study. Circ Res. 2020;126:633–643. doi:10.1161/CIRCRESAHA.119.316045
28. Joyce E, Hoogslag GE, Kamperidis V, et al. Relationship between myocardial function, body mass index, and outcome after ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2017;10. doi:10.1161/CIRCIMAGING.116.005670
29. Ren J, Wu NN, Wang S, Sowers JR, Zhang Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev. 2021;101:1745–1807. doi:10.1152/physrev.00030.2020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.