Back to Journals » Infection and Drug Resistance » Volume 14
Impact of Non-Alcoholic Simple Fatty Liver Disease on Antituberculosis Drug-Induced Liver Injury
Authors Liu YH, Guo Y, Xu H, Feng H, Chen DY
Received 24 June 2021
Accepted for publication 19 August 2021
Published 9 September 2021 Volume 2021:14 Pages 3667—3671
DOI https://doi.org/10.2147/IDR.S326386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yi-Hui Liu,1 Yan Guo,2 Hong Xu,1 Hui Feng,1 Dong-Ya Chen1
1Department of Digestive Hepatology, Hangzhou Red Cross Hospital, Hangzhou, Zhejiang, 310003, People’s Republic of China; 2Department of Gastroenterology, Hangzhou Third Hospital, Hangzhou, Zhejiang, 310009, People’s Republic of China
Correspondence: Yi-Hui Liu
Department of Digestive Hepatology, Hangzhou Red Cross Hospital, No. 208 of Huancheng East Road, Xiacheng District, Hangzhou, Zhejiang, 310003, People’s Republic of China
Tel +8613588227972
Email [email protected]
Objective: To observe the effect of non-alcoholic simple fatty liver disease on drug-induced liver injury caused by tuberculosis.
Methods: We retrospectively analyzed the incidence, characteristics, and risk factors of antituberculosis drug-induced liver injury in 104 patients with initial treatment of tuberculosis complicated with non-alcoholic simple fatty liver disease. The patients were divided into two groups according to whether there was liver injury or not. The differences in age, gender, body mass index (BMI), cholesterol, and triglycerides were studied between the two groups.
Results: Among the 104 patients with initial treatment of tuberculosis complicated with non-alcoholic fatty liver disease, 24 (23%) patients developed a drug-induced liver injury. The remaining 80 (77%) patients did not develop drug-induced liver injury (χ2 = 60.308, P < 0.05). In the liver injury group, there were 20 cases of mild liver injury, two cases of moderate liver injury, two cases of severe liver injury, 22 cases of hepatocellular injury, two cases of cholestasis, and no cases of mixed liver injury. The time of abnormal liver function in antituberculosis treatment was 16.42 ± 9.18 days from the beginning of the antituberculosis treatment. There were no significant differences in gender, age, BMI, or triglyceride between the liver injury group and the non-liver injury group (χ2 = 2.063, t = 0.179, t = 0.703, t = 1.12, P > 0.05 in all), but there were significant differences in cholesterol (t = 3.08, P < 0.05). By logistic regression analysis, cholesterol was a high-risk factor for liver injury.
Conclusion: Non-alcoholic simple fatty liver disease may increase the risk of antituberculosis drug-induced liver injury.
Keywords: non-alcoholic simple fatty liver disease, antituberculosis drugs, drug-induced liver injury
Introduction
Drug-induced liver injury (DILI) is one of the most common side effects of antituberculosis drugs. In addition to the effects of the drugs themselves, the risk factors for antituberculosis DILI are not completely clear. Theoretically, advanced age, female gender, poor nutritional status/ hypoproteinemia, HIV infection, diabetes, alcoholism, basic liver disease, Pregnancy/3 months post-partum, extrapulmonary tuberculosis (especially abdominal tuberculosis) etc., are risk factors of antituberculosis DILI. Previous studies have suggested that chronic hepatitis B and chronic hepatitis C can increase the incidence of antituberculosis DILI. The risk of DILI in patients with viral hepatitis is three to five times higher than that of non-viral hepatitis.1–5 With the effective control of hepatitis B and the prevalence of obesity, non-alcoholic fatty liver disease (NAFLD) has become the largest chronic liver disease in China. Although many patients with tuberculosis have NAFLD, there are few studies on the effect of NAFLD on antituberculosis DILI. Therefore, this study mainly studied the influence of non-alcoholic simple fatty liver disease on DILI caused by tuberculosis.
Materials and Methods
I. The data of 104 patients with non-alcoholic simple fatty liver complicated with an initial treatment of tuberculosis in our hospital were collected from January 2019 to March 2021. This study was conducted in accordance with the Declaration of Helsinki, and approved by the ethics committee of Hangzhou Red Cross Hospital, and all patients had signed the informed consent.
II. The following information was recorded: age, gender, occupation, serum liver function (TBIL, ALT, AST, ALP, GGT), triglyceride, cholesterol, weight, and height. The observation time was eight weeks, and the time of abnormal liver function was recorded.
II. Inclusion criteria: (1) According to the Guidelines for Diagnosis and Treatment of Tuberculosis,6 all patients with initial treatment for tuberculosis received 2HRZE/4HR antituberculosis treatment. (2) The diagnostic criteria of DILI refer to the Guidelines for the Diagnosis and Treatment of Anti-tuberculous Drug-induced Liver Injury in 2019.7 (3) The diagnostic criteria of non-alcoholic simple fatty liver disease refer to the Guidelines for the Diagnosis and Treatment of Non-alcoholic Fatty Liver Disease in 2006.8 (4) NAFLD and normal liver function were diagnosed prior to antituberculosis treatment. (5) No prophylactic liver protective drugs were used. (6) Clinical data is complete. Exclusion criteria: (1) Patients who had tuberculosis combined with viral hepatitis A, B, C, D, E, autoimmune liver disease, genetic metabolic liver disease, etc. (2) Patients with a long history of alcohol consumption. (3) Patients whose basic liver function was abnormal.
IV. The type of liver injury was judged according to the Guidelines for Diagnosis and Treatment of Drug-induced Liver Injury in 2015.9 (1) Hepatocyte injury type: ALT ≥ 3 × ULN, and R ≥ 5. (2) Cholestasis type: ALP ≥ 2 × ULN, and R ≤ 2; (3) Mixed type: ALT ≥ 3 × ULN, ALP ≥ 2 × ULN, and 2<R<5. R = (ALT/ULN)/(ALP/ULN).
V. The degree of liver injury was classified as follows. Grade 1 (mild liver injury): a recoverable increase of serum ALT, ALP, or both, TBIL < 2.5 ULN. Grade 2 (moderate liver injury): elevated serum ALT, ALP, or both, TBIL ≥ 2.5 ULN. Grade 3 (severe liver injury): elevated serum ALT, ALP, or both, TBIL ≥ 5 ULN.
Statistical Analyses
Normally distributed variables were expressed as mean ± SD and were compared using Student’s t-test. Categorical variables were expressed in percentages and compared using the χ2 test. Logistic regression was performed to test the association between indicators and liver injury. All P-values were two-tailed, and values less than 0.05 were considered statistically significant. Analyses were carried out using SPSS software (version 26.0).
Results
Basic Characteristics
1. A total of 104 patients with pulmonary tuberculosis complicated with NAFLD participated in the study, including 56 males and 48 females. The mean age was 56.41 ± 14.75, with 64 farmers, 6 workers, 14 employees, and 20 other professions (retired/unemployed/not working). The mean BMI was 23.53 ± 3.57 kg/m2.
Characteristics of Liver Injury in Pulmonary Tuberculosis Complicated with NAFLD
There were 24 cases of pulmonary tuberculosis complicated with non-alcoholic simple fatty liver who suffered a liver injury, including 16 males and eight females. Of these cases, 20 had a mild liver injury, 2 had a moderate liver injury, and 2 had a severe liver injury. There were 22 cases of hepatocyte injury type, two cases of cholestasis type, and no cases of mixed type. The mean age was 57.08 ± 14.33. The abnormal liver function was found in (16.42 ± 9.18) days during antituberculosis treatment (Table 1).
 |
Table 1 Comparison of Liver Injury Group and Non-Liver Injury Group of Pulmonary Tuberculosis Complicated with Non-Alcoholic Simple Fatty Liver Disease (Mean ± SD) |
Comparison of Liver Injury Group and Non-Liver Injury Group of Pulmonary Tuberculosis Complicated with Non-Alcoholic Simple Fatty Liver Disease
There were 24 (23%) cases with liver injury and 80 (77%) cases without liver injury in pulmonary tuberculosis complicated with non-alcoholic simple fatty liver disease. There were no significant differences in gender, age, BMI, triglyceride between the liver injury group and the non-liver injury group (χ2 = 2.063, t = 0.179, t = 0.703, t = 1.12, P > 0.05 in all). The difference in cholesterol was statistically significant (t = 3.08, P < 0.05) (Table 2).
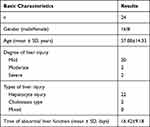 |
Table 2 Characteristics of Liver Injury in Pulmonary Tuberculosis Complicated with NAFLD |
Analysis of Risk Factors of Liver Injury in Pulmonary Tuberculosis Complicated with Non-Alcoholic Simple Fatty Liver Disease
Logistic regression analysis found that among factors such as gender, age, BMI, triglycerides, and cholesterol, only cholesterol was a statistically significant high-risk factor (Table 3).
 |
Table 3 Analysis of Risk Factors of Liver Injury in Pulmonary Tuberculosis Complicated with Non-Alcoholic Simple Fatty Liver Disease |
Discussion
China is a country with a large population of patients who have pulmonary tuberculosis, and many patients need antituberculosis treatment. Drug-induced liver injury is one of the most common side effects of antituberculosis drugs, with the greatest harm. This harm manifests as transient transaminase elevation in light cases and liver failure in serious cases, even endangering life. Therefore, some patients need to stop antituberculosis treatment, thus affecting the therapeutic effect of tuberculosis medication. In addition to the hepatotoxicity of the drugs themselves, advanced age, alcoholism, and basic liver disease are high-risk factors for liver injury. There are many studies on the impact of chronic viral hepatitis on antituberculosis DILI. Meta-analysis shows that both chronic hepatitis B and chronic hepatitis C may increase the risk of antituberculosis DILI.3,10,11 Patients with chronic hepatitis B co-infection receiving antituberculosis treatment are more prone to liver failure and progress to poor prognosis during antituberculosis treatment.5
Non-alcoholic fatty liver disease has become the most significant liver disease in China. However, there are relatively few studies on the influence of NAFLD on the incidence of antituberculosis DILI. Whether NAFLD will affect the incidence of antituberculosis DILI has not been confirmed. Mak et al used animal models to show that NAFLD does not increase the risk of liver injury induced by idiosyncratic drugs.12 When evaluating many drugs, a pre-existing liver disease did not seem to be associated with an increased risk of idiosyncratic hepatotoxicity. However, an increasing number of studies have suggested that certain drugs can induce liver injury more frequently and more severely when exposed to pre-existing fatty liver disease. These drugs can also exacerbate pre-existing NAFLD and accelerate the transformation from steatosis to steatohepatitis.13,14 Compared with chronic hepatitis C, patients with NAFLD have a 4-fold higher risk of developing DILI.15 Cao et al showed that the incidence of DILI in patients with pulmonary tuberculosis complicated with NAFLD was 24.56%, which was significantly higher than that in patients with NAFLD.16 Fang et al reported that the incidence of antituberculosis DILI in patients with pulmonary tuberculosis complicated with fatty liver was 22%, significantly higher than 14.3% in patients without fatty liver.17 Xu et al believed that the incidence of antituberculosis DILI in patients with pulmonary tuberculosis complicated with fatty liver was 32.1%, and fatty liver is a high-risk factor for liver injury.18
In clinical practice, NAFLD can be divided into non-alcoholic simple fatty liver disease, non-alcoholic steatohepatitis, and steatohepatitis-associated cirrhosis. We chose non-alcoholic simple fatty liver as the study object to better accord with clinical practice and avoid the influence of basic liver function and liver protection drugs on the study. Our study found that the incidence of liver injury in the initial treatment of tuberculosis complicated with non-alcoholic simple fatty liver disease was 23%. This is significantly higher than 9.5–10.6% of antituberculosis DILI in China,19 but consistent with other domestic studies.16–18 This suggests that basic liver disease increases the risk of antituberculosis DILI. In the present study, the degree of liver injury was mostly mild liver injury, and the type of liver injury was more common in hepatocyte types. The detection time of abnormal liver function in antituberculosis treatment was about two weeks. This was consistent with the relevant guidelines,7,9 indicating that most liver injuries could be controlled. Due to the limited observation time, it was not clear whether the liver injury would affect the antituberculosis efficacy. Our study suggested that age, gender, BMI, and triglyceride were not high-risk factors for antituberculosis DILI, but cholesterol was a high-risk factor for liver injury. Xu et al believed that dyslipidemia and being female were significantly correlated with DILI.20 The increased risk of DILI in dyslipidemia may be explained by two mechanisms. First, malnutrition could slow drug clearance and subsequently lead to delayed drug elimination and higher drug plasma levels.21 Second, host factors, such as overnutrition and alcohol, may increase the pre-existing cellular oxidants of the host, modifying the drug-induced oxidative liver damage, resulting in steatosis, lipid peroxidation, and mitochondrial degeneration.22 Omaima et al believed that age, gender, alcohol consumption, and smoking status had no significant correlation with antituberculosis DILI.23 Muzamil et al found that women were associated with an increased risk of antituberculosis DILI, while age and BMI did not significantly increase the risk of liver injury.24,25 Abbara et al believed that the risk factors for antituberculosis DILI included low body weight, HIV-combined infection, and alcohol consumption.26 Therefore, the effect of influencing factors on the antituberculosis DILI was not determined from various studies and still excite controversy.
So far, the mechanism by which NAFLD increases liver injury with anti-tuberculosis drugs is unclear. It may be due to hepatic blood perfusion disorders, decreased hepatic blood flow, and decreased activity of liver metabolic enzymes, leading to slower drug clearance, prolonged biological half-life, and free drug concentration. Increased, thereby increasing the toxicity of the drug.
In summary, our study found that non-alcoholic simple fatty liver disease may increase the risk of antituberculosis DILI. However, there are some limitations. The sample size was small, and it was not possible to thoroughly observe whether antituberculosis treatment and prognosis were affected. There was no definite conclusion about the risk factors of antituberculosis DILI. Whether the non-alcoholic simple fatty liver disease impacts liver injury caused by antituberculosis drugs and other drugs has not yet been determined, and further studies are needed to better prevent and treat DILI.
Funding
This study was supported by Project of Administration of Traditional Chinese medicine in Zhejiang Province of China (2020ZB189).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lomtadze N, Kupreishvili L, Salakaia A, et al. Hepatitis C virus co-infection increases the risk of anti-tuberculosis drug-induced hepatotoxicity among patients with pulmonary tuberculosis. PLoS One. 2013;8(12):e83892. doi:10.1371/journal.pone.0083892
2. Kim WS, Lee SS, Lee CM, et al. Hepatitis C and not Hepatitis B virus is a risk factor for anti-tuberculosis drug induced liver injury. BMC Infect Dis. 2016;16:50. doi:10.1186/s12879-016-1344-2
3. Chang TE, Huang YS, Chang CH, Perng CL, Huang YH, Hou MC. The susceptibility of anti-tuberculosis drug-induced liver injury and chronic hepatitis C infection: a systematic review and meta-analysis. J Chin Med Assoc. 2018;81(2):111–118. doi:10.1016/j.jcma.2017.10.002
4. Chien JY, Huang RM, Wang JY, et al. Hepatitis C virus infection increases hepatitis risk during anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2010;14(5):616–621.
5. Chen L, Bao D, Gu L, et al. Co-infection with hepatitis B virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment. BMC Infect Dis. 2018;18(1):295. doi:10.1186/s12879-018-3192-8
6. Tuberculosis Branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of tuberculosis. Chin J Tubercul Respir Dis. 2001;24(2):70–74.
7. Tuberculosis Branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of antituberculous drug-induced liver injury. Chin J Tubercul Respir Dis. 2019;42(5):343–356.
8. Fatty Liver and Alcoholic Liver Disease Group, Hepatology Society, Chinese Medical Association. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver disease. Chin J Hepatol. 2006;14(3):161–163.
9. Pharmaceutical Liver Disease Group, Liver Disease Branch, Chinese Medical Association. Guidelines for the diagnosis and treatment of drug-induced liver injury. Chin J Hepatol. 2015;23(11):810–820.
10. Wang NT, Huang YS, Lin MH, Huang B, Perng CL, Lin HC. Chronic hepatitis B infection and risk of antituberculosis drug-induced liver injury: systematic review and meta-analysis. J Chin Med Assoc. 2016;79(7):368–374. doi:10.1016/j.jcma.2015.12.006
11. Zheng J, Guo MH, Peng HW, Cai XL, Wu YL, Peng XE. The role of hepatitis B infection in anti-tuberculosis drug-induced liver injury: a meta-analysis of cohort studies. Epidemiol Infect. 2020;148:e290. doi:10.1017/S0950268820002861
12. Mak A, Cho T, Uetrecht J. Use of an animal model to test whether non-alcoholic fatty liver disease increases the risk of idiosyncratic drug-induced liver injury. J Immunotoxicol. 2018;15(1):90–95. doi:10.1080/1547691X.2018.1467982
13. Ortega-Alonso A, Stephens C, Lucena MI, Andrade RJ. Case characterization, clinical features and risk factors in drug-induced liver injury. Int J Mol Sci. 2016;17(5):714. doi:10.3390/ijms17050714
14. Massart J, Begriche K, Moreau C, Fromenty B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res. 2017;3(Suppl 1):212–232.
15. Lammert C, Imler T, Teal E, Chalasani N. Patients with chronic liver disease suggestive of nonalcoholic fatty liver disease may be at higher risk for drug-induced liver injury. Clin Gastroenterol Hepatol. 2019;17(13):2814–2815. doi:10.1016/j.cgh.2018.12.013
16. Cao SP, Yang L, Gao LC, Fu MJ. Liver injury of 182 primary pulmonary tuberculosis patients induced by antituberculosis drugs. Cent South Pharm. 2015;13(9):1003–1006.
17. Wang F, Yang YF. Analysis of risk factors of drug induced liver injury caused by antituberculosis treatment. Pract Pharm Clin Remedies. 2014;17(10):1326–1328.
18. Chi X, Qi Y, Chen J, Cai HF, Li WF, Song SB. Logistic regression analysis of clinical characteristics and risk factors of drug-induced liver injury induced by primary antituberculous therapy. Shaanxi Med J. 2019;48(1):67–70.
19. Zhang T, Du J, Yin X, et al. Adverse events in treating smear-positive tuberculosis patients in China. Int J Environ Res Public Health. 2015;13(1):86. doi:10.3390/ijerph13010086
20. Li X, Wang L, Li D, Niu J, Gao P. Dyslipidemia is a risk factor for the incidence and severity of Drug-Induced Liver Injury (DILI): a retrospective population-based study in China. Med Sci Monit. 2019;25:3344–3353. doi:10.12659/MSM.916687
21. Chen M, Suzuki A, Borlak J, Andrade Raúl J, Isabel LM. Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol. 2015;63:503–514. doi:10.1016/j.jhep.2015.04.016
22. Fromenty B. Drug-induced liver injury in obesity. J Hepatol. 2013;58:824–826. doi:10.1016/j.jhep.2012.12.018
23. Bouazzi OE, Hammi S, Bourkadi JE, et al. First line anti-tuberculosis induced hepatotoxicity: incidence and risk factors. Pan Afr Med J. 2016;25:167. doi:10.11604/pamj.2016.25.167.10060
24. Latief M, Dar WR, Sofi N, et al. Novel risk factors and early detection of anti tubercular treatment induced liver injury-looking beyond American Thoracic Society Guidelines. Indian J Tuberc. 2017;64(1):26–32. doi:10.1016/j.ijtb.2016.11.002
25. Zhao H, Wang Y, Zhang T, Wang Q, Xie W. Drug-induced liver injury from anti-tuberculosis treatment: a retrospective cohort study. Med Sci Monit. 2020;26:e920350.
26. Abbara A, Chitty S, Roe JK, et al. Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK. BMC Infect Dis. 2017;17(1):231. doi:10.1186/s12879-017-2330-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
