Back to Journals » OncoTargets and Therapy » Volume 8
Impact of lymphovascular invasion on recurrence and progression rates in patients with pT1 urothelial carcinoma of bladder after transurethral resection
Authors Sha N, Xie L, Chen T, Xing C, Liu X, Zhang Y, Shen Z, Xu H, Wu Z, Hu H, Wu C, Tian D
Received 2 September 2015
Accepted for publication 14 October 2015
Published 18 November 2015 Volume 2015:8 Pages 3401—3406
DOI https://doi.org/10.2147/OTT.S95609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Nan Sha,* Linguo Xie,* Tao Chen,* Chen Xing, Xiaoteng Liu, Yu Zhang, Zhonghua Shen, Hao Xu, Zhouliang Wu, Hailong Hu, Changli Wu
Department of Urology, Tianjin Key Laboratory of Urology, Tianjin Institute of Urology, Second Hospital of Tianjin Medical University, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Objective: To evaluate the clinical significance of lymphovascular invasion (LVI) on recurrence and progression rates in patients with pT1 urothelial carcinoma of bladder after transurethral resection.
Methods: This retrospective study was performed with 155 patients with newly diagnosed pT1 urothelial carcinoma of bladder who were treated with transurethral resection of bladder tumor at our institution from January 2006 to January 2010. The presence or absence of LVI was examined by pathologists. Chi-square test was performed to identify the correlations between LVI and other clinical and pathological features. Kaplan–Meier method was used to estimate the recurrence-free survival (RFS) and progression-free survival curves and difference was determined by the log-rank test. Univariate and multivariate analyses were performed to determine the predictive factors through a Cox proportional hazards analysis model.
Results: LVI was detected in a total of 34 patients (21.9%). While LVI was associated with high-grade tumors (P<0.001) and intravesical therapy (P=0.009). Correlations with age (P=0.227), sex (P=0.376), tumor size (P=0.969), tumor multiplicity (P=0.196), carcinoma in situ (P=0.321), and smoking (P=0.438) were not statistically significant. There was a statistically significant tendency toward higher recurrence rate and shorter RFS time in LVI-positive patients. However, no statistically significant differences were observed in progression rate between the two groups. Moreover, multivariate Cox proportional hazards analysis revealed that LVI, tumor size, and smoking were independent prognostic predictors of recurrence. The hazard ratios (95% confidence interval) were 2.042 (1.113–3.746, P=0.021), 1.817 (1.014–3.256, P=0.045), and 2.079 (1.172–3.687, P=0.012), respectively.
Conclusion: The presence of LVI in transurethral resection of bladder tumor specimens is significantly associated with higher recurrence rate and shorter RFS time in patients with newly diagnosed T1 urothelial carcinoma of the bladder. It is an independent prognostic predictor for disease recurrence. Thus, patients with LVI should be followed up closely.
Keywords: bladder urothelial carcinoma, TURBT, lymphovascular invasion, recurrence, progression
Introduction
Bladder cancer is one of the most common malignant neoplasms in the world. It is the sixth leading cause of new cancer cases and ninth leading cause of cancer-related mortality worldwide.1 According to data from 2012 provided by American Cancer Society, the incidence of bladder cancer in men and women was 5.3/100,000 and 1.5/100,000, respectively, in less developed countries. In developed countries, the bladder cancer incidence in men (16.9/100,000) was four times as high as that in women (3.7/100,000).1 On average, 75% of patients with bladder cancer are diagnosed with nonmuscle invasive tumors limited to the mucosa or lamina propria. For nonmuscle invasive bladder tumors, the probability of recurrence and progression after transurethral resection of bladder tumor (TURBT) at 1 year is 15%–70% and 7%–40%, respectively.2,3 Lymphovascular invasion (LVI) is a pathological feature that has been receiving attention as the presence of tumor cells within the lumen of the blood and/or lymphatics; the process of which leads to circulating tumor cells. Several prior studies have evaluated that patients with muscle-invasive bladder tumors after radical cystectomy (RC) have an LVI incidence of 30%–50%.4–8 The prognostic value of LVI in these patients has been previously investigated by others and it showed that it was independently associated with overall and recurrence-free survival (RFS).4,6,7 However, the conclusions referred above are largely limited in muscle invasive tumors treated with RC. It is still debatable that whether LVI in patients with pT1 urothelial carcinoma of bladder after TURBT has a statistically significant impact on prognosis. A retrospective study conducted by Cho et al9 revealed that LVI, as an independent prognostic factor in pT1 bladder cancer, was significantly associated with disease recurrence. However, others contradicted this finding, they maintained that LVI in stage T1 tumors was unusual and frequently misdiagnosed on hematoxylin and eosin (H&E) stain; it did not necessarily portend a poor prognosis.10 Thus, the purpose of this study is to assess the independent prognostic role of LVI in patients with newly diagnosed pT1 urothelial carcinoma of the bladder.
Patients and methods
Clinical data
With the approval from the institutional review board of the Second Hospital of Tianjin Medical University, a total of 155 patients newly diagnosed with pT1 bladder cancer who underwent TURBT at our institution from January 2006 to January 2010 were selected retrospectively for the analysis. Clinical and pathological information was retrospectively obtained from patient charts and electronic medical records, including age, sex, tumor size, tumor multiplicity, tumor grade, carcinoma in situ (CIS), and smoking. Tumor size was considered as the greater diameter on microscopic analysis of the surgical specimen.
Our inclusion criteria were 1) TURBT was performed as initial treatment for all patients; 2) evidence of urothelial carcinoma of the bladder; 3) an available pathology report for review; and 4) tumor was pathologically diagnosed as pT1. Individuals were excluded if they met all of the following inclusion criteria: 1) evidence of nonurothelial carcinoma histology; 2) presence of adenocarcinoma, squamous cell carcinoma, or other histological variants; 3) presence of upper tract carcinoma, or distant metastasis (including regional nodal metastasis) at diagnosis. Due to the inaccessible Bacillus Calmette–Guerin in the People’s Republic of China, patients received the anthracycline antibiotic chemotherapy drugs such as epirubicin or pirarubicin with an 8-week course. The study population comprised two groups, patients with LVI-positive and LVI-negative at TURBT. We compared those LVI patients with a group of patients who had no evidence of LVI.
Treatments
All patients underwent TURBT that was carried out by all surgeons according to a standard procedure in our study. All visible tumors or suspicious mucosal lesions were resected until the tumor base reached the deep muscle layer and showed perivesical fat transparently. T stage was determined according to the 2002 American Joint Committee on Cancer tumor, node, metastasis (TNM) staging system, and tumor grade was determined using the 2004 World Health Organization grading system. LVI status, defined as the presence of vascular and/or lymphatic invasion, was confirmed by reviewing the pathology reports. If the LVI was not depicted in the pathology report, then it would be reassigned by two independent uropathologists in our institute. LVI was considered present when tumor cells were unequivocally noted within or attached to the wall of a vascular or lymphatic space on H&E-stained sections. The presence of LVI was only assessed on the first TURBT.
Cystoscopy has been suggested to be given during the regular postoperative follow-up at 3-month interval for the first 2 years, biannually for the subsequent 3 years, and annually thereafter according to the US and European guidelines.11 The end point was the date of the last visit to our institute. Of course, patients were monitored with physical examinations, routine ultrasonic tests, and urine cytology at each visit. Outcomes of interest were RFS and progression-free survival (PFS). The RFS period was obtained from the date of first surgery to the date of first clinical recurrence (any histopathological staging and/or grading). The PFS period was estimated from the time of first surgery to the date of disease developed to higher histopathological staging and/or grading and/or to metastasis. Time of collecting data was the end point for patients without recurrence and progression.
Statistical analysis
Chi-square test was used to compare the association between LVI and other clinicopathological characteristics. We examined the impact of LVI on disease recurrence and progression. Univariable and multivariable Cox proportional hazards analysis models were used to analyze the prognostic significance of the clinical and pathological variables for RFS and PFS. Kaplan–Meier analysis and the log-rank test were done to calculate RFS and PFS curves and analyzed the significance of differences between survival curves. Statistical software SPSS (IBM Corporation, Armonk, NY, USA) version 20.0 was used for statistical analysis with two-sided P<0.05 considered significant.
Results
The clinicopathological demographics of patients who were newly diagnosed with pT1 urothelial carcinoma of bladder after TURBT with LVI (+) and those with LVI (-) are shown in Table 1. Among a total of 155 patients, LVI was histologically confirmed in 34 patients (21.9%). Of these tumors, 30 occurred in men and four in women. There were no significant differences between the two groups in terms of age, sex, tumor size, tumor multiplicity, CIS, and smoking. However, high-grade tumors were more common in LVI (+) than in LVI (-) (70.6% versus 30.6%, P<0.001).
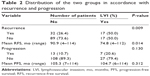
The mean (range) follow-up time for the group with LVI was 86.4 (6–114) months and was 78.4 (7–114) months for the controls. During the follow-up, disease recurrence and progression developed in 49 (31.6%) and 20 (12.9%) patients, respectively (Table 2). Patients with LVI (+) were significantly more likely to recur than those with LVI (-) (50.0% versus 26.4%, P=0.009). There was also a trend for patients with LVI (+) to be high in the rates of progression comparing with patients with LVI (-) (20.6% versus 10.7%), but the difference was not statistically significant (P=0.130).
We used Cox proportional hazard analysis for further analysis (Table 3). According to the results of univariate analysis, we found that tumor multiplicity (hazard ratio [HR] 1.790, 95% confidence interval [CI] 1.019–3.143, P=0.043), tumor size (HR 1.971, 95% CI 1.118–3.474, P=0.019), smoking (HR 2.282, 95% CI 1.297–4.013, P=0.004), and the presence of LVI (HR 2.060, 95% CI 1.142–3.714, P=0.016) significantly influenced disease recurrence time. However, in multivariate Cox proportional hazard analysis, only tumor size (HR 1.817, 95% CI 1.014–3.256, P=0.045), smoking (HR 2.079, 95% CI 1.172–3.687, P=0.012), and the presence of LVI (HR 2.042, 95% CI 1.113–3.746, P=0.021) were the independent prognostic factors associated with disease recurrence.
  | Table 3 Univariable and multivariable analyses according to recurrence |
The Kaplan–Meier analysis was used to estimate RFS and PFS stratified by LVI (+) versus LVI (-) in Figures 1 and 2. Patients with LVI (+) had shorter mean RFS duration than those with LVI (-) (74.8 versus 90.9 months, P=0.014). However, no significant difference was observed between the two groups in terms of PFS (104.7 versus 105.3 months, P=0.312).
Discussion
The value of LVI as a criterion to evaluate the severity of urothelial bladder tumors was reported for the first time by McDonald and Thompson.12 Evidence on the clinical significance of LVI in bladder cancer is increasing, and a great many recent cystectomy series have strengthened the significance of LVI for urothelial carcinoma of the bladder. Some series indicated that LVI was a significant and independent prognostic factor for disease-specific survival, as well as pathological staging and presence of lymph node metastasis in patients who underwent RC.13,14 Patients with LVI at RC were significantly more likely to have disease recurrence than those with no evidence of LVI.15 Kim et al16 conducted a systematic review and meta-analysis including a total of 12,527 patients to assess the association between LVI and prognosis of bladder cancer after RC. The result revealed that LVI was detected in 34.6% in RC specimens and it was a significant predictor for poor survival.
Nonetheless, there are only limited data on the clinical guiding significance of LVI in TURBT specimens in patients with nonmuscle invasive bladder cancer, especially pT1 urothelial carcinoma of bladder. Cho et al,9 who performed retrospective analyses of 118 patients reported that LVI, as an independent prognostic factor of progression and metastasis in pT1 bladder cancer, was significantly associated with disease recurrence. The result was consistent with the study conducted by Lopez and Angulo,17 in which multivariate analysis revealed that LVI proved to be an independent prognostic factor in TURBT surgical specimens of T1 bladder cancer. However, several previous reports held a contrary opinion, maintaining that LVI did not necessarily portend a poor prognosis in pT1 bladder cancer,10 and the presence of LVI in patients with node-negative was not an unfavorable factor of prognosis on multivariate analysis.18 They revealed that the pathologic stage was the only independent prognostic factor for survival. This study provided further evidence suggesting that LVI was a pathological variable that might play an important role as a prognostic indicator in patients with newly diagnosed pT1 bladder cancer. Our study showed that the presence of LVI was the independent prognostic factor associated with disease recurrence (HR 2.042, 95% CI 1.113–3.746, P=0.021). However, the association between LVI and progression was not statistically significant (P=0.130).
Moreover, some studies showed a 10%–25% LVI rate in pT1 bladder cancer cases.17,19–21 In accordance with previous studies, we found that LVI was detected pathologically in 21.9% of cases in our series. Also, disease recurrence and progression developed in 17 (50.0%) and seven (20.6%) patients within a mean follow-up of 86.4 (6–114) months after TURBT, respectively.
Treatment for nonmuscle invasive bladder cancer with LVI is also under debate. Cho et al considered systemic chemotherapy a reasonable option based on the assumption that undetectable micrometastasis might be present in patients with LVI. While there were no statistically significant differences between patients who did and did not undergo adjuvant systemic chemotherapy in terms of time to disease recurrence, progression, or metastasis.9 Its presence in the TURBT specimen had special value, because if LVI was found before RC, then neoadjuvant chemotherapy or early RC might be considered.22 In addition to being used to select adjuvant therapy as predictive tools, LVI should be considered for inclusion in the TNM staging system. Despite a growing number of published studies have proved the important role of LVI on the prognosis of bladder cancer, it is not a part of the TNM staging system or treatment guidelines for bladder cancer. In other malignancies such as hepatic23 and testicular cancer,24 LVI has been added to the TNM staging system in case of underestimating disease staging, so that we can improve the decision-making of disease treatment. Upstaging tumors on the basis of LVI might improve the accuracy of prognosis in bladder cancer, therefore, it is a worthy consideration. Although it is mostly accepted that LVI is an indicator of bladder cancer aggressiveness, on account of difficult identification and questionable reproducibility of LVI in a limited TURBT surgical specimen, controversy on the impact of LVI on staging and therapeutic decision-making still remains. So further studies are still needed to evaluate potential treatment options for nonmuscle invasive bladder cancer with LVI.
Clearly, several limitations of this study need to be considered. First and foremost were its retrospective characteristic and the limited study sample, although all the data were extracted from our medical institution, the possibility of selection bias could not be excluded. In addition, the patients in our study underwent TURBT by multiple surgeons, and specimens were evaluated by different pathologists. In addition, we determined most of LVI cases by H&E staining, not based on immunohistochemical staining such as CD31 or CD34 staining, which might help in identification of LVI.25 Due to the misdiagnosis of LVI in H&E-stained specimens, it may have contributed to LVI overestimation. Moreover, we did not control for repeat TURBT on patients with a suspicion of residual tumor in initial TURBT specimens, which could have affected outcomes.26
Conclusion
This study provides further evidence that the presence of LVI in TURBT specimens is significantly associated with an increased risk of disease recurrence and a shorter RFS time in patients with newly diagnosed T1 urothelial carcinoma of the bladder. As an independent prognostic predictor for disease recurrence, patients with LVI should be followed up closely in case of recurrence.
Acknowledgments
This work is funded by grants from the Natural Science Foundation of Tianjin (No 14JCYBJC26300) and National Key Specialty Construction of Clinical Projects, the Natural Science Foundation of Tianjin (No 15JCYBJC24600), and Tianjin Major Scientific and Technological Special Project (No 12ZCDZSY16900). We thank all the study participants, urologists, and study coordinators for participating in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Kurth KH, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer. 1995;31A(11):1840–1846. | ||
Allard P, Bernard P, Fradet Y, Tetu B. The early clinical course of primary Ta and T1 bladder cancer: a proposed prognostic index. Br J Urol. 1998;81(5):692–698. | ||
Hong SK, Kwak C, Jeon HG, Lee E, Lee SE. Do vascular, lymphatic, and perineural invasion have prognostic implications for bladder cancer after radical cystectomy? Urology. 2005;65(4):697–702. | ||
Bassi P, Ferrante GD, Piazza N, et al. Prognostic factors of outcome after radical cystectomy for bladder cancer: a retrospective study of a homogeneous patient cohort. J Urol. 1999;161(5):1494–1497. | ||
Quek ML, Stein JP, Nichols PW, et al. Prognostic significance of lymphovascular invasion of bladder cancer treated with radical cystectomy. J Urol. 2005;174(1):103–106. | ||
Leissner J, Koeppen C, Wolf HK. Prognostic significance of vascular and perineural invasion in urothelial bladder cancer treated with radical cystectomy. J Urol. 2003;169(3):955–960. | ||
Harada K, Sakai I, Hara I, Eto H, Miyake H. Prognostic significance of vascular invasion in patients with bladder cancer who underwent radical cystectomy. Int J Urol. 2005;12(3):250–255. | ||
Cho KS, Seo HK, Joung JY, et al. Lymphovascular invasion in transurethral resection specimens as predictor of progression and metastasis in patients with newly diagnosed T1 bladder urothelial cancer. J Urol. 2009;182(6):2625–2630. | ||
Larsen MP, Steinberg GD, Brendler CB, Epstein JI. Use of Ulex europaeus agglutinin I (UEAI) to distinguish vascular and “pseudovascular” invasion in transitional cell carcinoma of bladder with lamina propria invasion. Mod Pathol. 1990;3(1):83–88. | ||
Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639–653. | ||
McDonald JR, Thompson GJ. Carcinoma of the urinary bladder: a pathologic study with special reference to invasiveness and vascular invasion. J Urol. 1948;60(3):435–445. | ||
Palmieri F, Brunocilla E, Bertaccini A, et al. Prognostic value of lymphovascular invasion in bladder cancer in patients treated with radical cystectomy. Anticancer Res. 2010;30(7):2973–2976. | ||
Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. | ||
Streeper NM, Simons CM, Konety BR, et al. The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. BJU Int. 2009;103(4):475–479. | ||
Kim H, Kim M, Kwak C, Kim HH, Ku JH. Prognostic significance of lymphovascular invasion in radical cystectomy on patients with bladder cancer: a systematic review and meta-analysis. PLoS One. 2014;9(2): e89259. | ||
Lopez JI, Angulo JC. The prognostic significance of vascular invasion in stage T1 bladder cancer. Histopathology. 1995;27(1):27–33. | ||
Manoharan M, Katkoori D, Kishore TA, et al. Lymphovascular invasion in radical cystectomy specimen: is it an independent prognostic factor in patients without lymph node metastases? World J Urol. 2010;28(2):233–237. | ||
Kakizoe T, Tobisu K, Mizutani T, et al. Analysis by step sectioning of early invasive bladder cancer with special reference to G3.pT1 disease. Jpn J Cancer Res. 1992;83(12):1354–1358. | ||
Gohji K, Nomi M, Okamoto M, et al. Conservative therapy for stage T1b, grade 3 transitional cell carcinoma of the bladder. Urology. 1999;53(2):308–313. | ||
Andius P, Johansson SL, Holmang S. Prognostic factors in stage T1 bladder cancer: tumor pattern (solid or papillary) and vascular invasion more important than depth of invasion. Urology. 2007;70(4):758–762. | ||
Tilki D, Shariat SF, Lotan Y, et al. Lymphovascular invasion is independently associated with bladder cancer recurrence and survival in patients with final stage T1 disease and negative lymph nodes after radical cystectomy. BJU Int. 2013;111(8):1215–1221. | ||
Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20(6):1527–1536. | ||
Albers P, Siener R, Kliesch S, et al. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol. 2003;21(8):1505–1512. | ||
Lapham RL, Grignon D, Ro JY. Pathologic prognostic parameters in bladder urothelial biopsy, transurethral resection, and cystectomy specimens. Semin Diagn Pathol. 1997;14(2):109–122. | ||
Cookson MS, Herr HW, Zhang ZF, et al. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997; 158(1):62–67. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.