Back to Journals » OncoTargets and Therapy » Volume 12
Impact of liver kinase B1 on p53 and survivin and its correlation with prognosis in gastric cancer
Authors Li W, Luo S, Ma G, Wang L
Received 21 December 2018
Accepted for publication 1 February 2019
Published 22 February 2019 Volume 2019:12 Pages 1439—1445
DOI https://doi.org/10.2147/OTT.S199138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Weiwei Li,1 Shunxiang Luo,1 Guowei Ma,2 Lin Wang3
1Department of Oncology, The First People’s Hospital of Tianmen City, Hubei, China; 2Department of Gastrointestinal Surgery, The First People’s Hospital of Tianmen City, Hubei, China; 3Department of Pathology, The First People’s Hospital of Tianmen City, Hubei, China
Background: Liver kinase B1 (LKB1) is a newly discovered tumor suppressor gene that plays a role in apoptosis induction. However, the precise impact of LKB1 expression on gastric cancer (GC) progression and its correlation with survivin and p53 in GC have not yet been elucidated.
Purpose: The aim of this study was to explore the significance of LKB1 expression and its correlation with p53 and survivin in GC.
Patients and methods: In this study, LKB1 expression was detected in GC and adjacent paracancerous tissues from 150 patients through immunohistochemical (IHC) staining. The relationship between LKB1 expression and clinical pathological factors in GC was analyzed, alongside its correlation with p53 and survivin expression.
Results: LKB1 expression was reduced in GC tissues compared with adjacent paracancerous tissues (P=0.001). In patients with GC, lower LKB1 expression was associated with greater invasion depth (P=0.013), higher pTNM stage (P=0.009), and lymph node metastasis (P=0.029). Furthermore, LKB1 expression in GC was inversely associated with p53 (r=−0.181, P=0.027) and survivin expression (r=−0.198, P=0.015). Kaplan–Meier analysis indicated that the expression of LKB1, p53 and survivin, as well as tumor differentiation, invasion, and pTNM and lymph node metastasis were all associated with overall survival (OS) (all P<0.05). Finally, multivariate analysis showed that LKB1 expression [hazard ratio (HR): 0.605 (0.414–0.882), P=0.009], p53 expression [hazard ratio (HR): 1.840 (1.232–2.750), P=0.003], and survivin expression [hazard ratio (HR): 1.561 (1.039–2.345), P=0.032] were all independent prognostic factors for patients with GC.
Conclusion: Our study suggests that LKB1 expression is reduced in GC, negatively correlated with p53 and survivin expression, and plays an important role in predicting invasion and metastasis of GC.
Keywords: gastric cancer, liver kinase B1, p53, survivin, prognosis
Introduction
Gastric cancer (GC) is the fifth most common malignancy and the second major cause of cancer-related deaths worldwide; in China, it is one of the four most common malignancies.1,2 Surgery is the main therapeutic option for GC, and the 5-year survival rate for patients with early GC exceeds 90% following curative resection;3 however, most patients are diagnosed at an advanced stage, with a 5-year survival rate below 25%, due to local invasion or lymph node metastasis.4,5 Therefore, there is an urgent need for the discovery of new diagnostic and prognostic markers and therapeutic targets for GC.
Liver kinase B1 (LKB1), also called serine/threonine protein kinase 11 (STK 11), was first identified as the causative gene in inherited Peutz–Jeghers syndrome (PJS).6 Subsequent studies have reported that LKB1 gene mutations are found in sporadic cancers, including gastric, colorectal, lung, breast, and cervical cancers.7 LKB1 has been shown to function as a tumor suppressor gene, playing a role in inhibiting growth and migration of tumor cells, inducing cell cycle arrest, and promoting tumor cell apoptosis.8,9 It has been reported that LKB1’s regulatory role in apoptosis depends upon the tumor suppressor p53. Karuman et al10 demonstrated previously that LKB1 was physically associated with p53, regulating specific p53-dependent apoptosis pathways; Zeng and Berger11 also reported that LKB1 linked with p53 in promoting cell apoptosis. Survivin is an important inhibitor of apoptosis and is found to be selectively expressed in tumors, including gastric, colorectal, and breast cancers, and neuroblastoma.12 Pizem et al13 reported that the loss of p53 was a possible underlying mechanism in the upregulation of survivin in laryngeal squamous cell carcinoma. However, the precise impact of LKB1 expression on GC progression and its correlation with p53 and survivin in GC have not yet been elucidated.
Considering the above, we aimed to investigate LKB1 expression, alongside its correlation with clinicopathological features and prognoses of patients with GC. Furthermore, we explored the correlation between LKB1, p53, and survivin by analyzing their correlation and impact on overall survival (OS).
Patients and methods
Patients
This was a retrospective study of 150 patients who underwent gastrectomy at the Tianmen First People’s Hospital (Tianmen, Hubei, China) between 2010 and 2017. GC and relevant adjacent (≥5 cm) non-tumor (paracancerous) specimens were obtained. None of the patients had received preoperative radiotherapy or chemotherapy. Clinicopathological features of each patient were obtained from the Department of Oncology at Tianmen First People’s Hospital (Tianmen, Hubei, China). TNM staging of GC was ascertained according to the eighth edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM system for differentiated gastric carcinoma.14 The study was approved by the scientific research ethics committee of Tianmen First People’s Hospital, and written informed consent for the use of tissues for ex vivo experimentation was obtained from each patient before surgery, which was conducted in accordance with the Declaration of Helsinki. OS was defined as the interval between GC diagnosis and patient’s death (any cause) or final visit.
Immunohistochemical (IHC) staining
A conventional IHC staining protocol was used for this study. Paraffin-embedded tumor tissue blocks were cut into 4 μm-thick sections, dried, deparaffinized, and dehydrated in a graded ethanol series. Tissue sections were treated with 1% hydrogen peroxide for 10 minutes to block endogenous tissue peroxidase activity, followed by treatment with bovine serum for 30 minutes to reduce nonspecific binding. Antigen retrieval was performed using citrate buffer (pH 6.0) and microwaving at high-heat microwave processing for 5 minutes followed by a low-heat microwave processing for 20 minutes. All slides were incubated overnight at 4°C with polyclonal rabbit antihuman LKB1 antibody (OM222158, 1:1,000; Omnimabs, Alhambra, CA, USA), monoclonal mouse antihuman p53 antibody (Ready-to-use, DO-7; Gene Tech. Co. Ltd., Shanghai, China), and polyclonal rabbit antihuman survivin antibody (Ready-to-use, RAB-0536; Maixin-Bio, Fuzhou, China), followed by a 30-minute incubation with an Ultra-Sensitive S-P Kit (Maixin-Bio). Slides were then rinsed with phosphate-buffered saline, color was developed using a 3,3′-diaminobenzidine substrate kit, and slides were counterstained with hematoxylin.
Slides were assessed by two senior pathologists who were blinded to clinicopathological data. Cytoplasmic LKB1, cytoplasmic and nucleic survivin, and nucleic p53 staining were defined as positive. IHC stainings for LKB1, survivin, and p53 proteins were all assessed for staining intensity and percentage of positive cells as follows: 0 (negative; ≤5% of the cells staining positive), 1+ (weak staining; 6%–25% of the cells staining positive), 2+ (moderate staining; 26%–50% of the cells staining positive), and 3+ (strong staining; >50% of the cells staining positive). The final score for each slide was calculated as the average score of three representative high-power fields (400× magnification). Scores <3 were defined as low expression and scores ≥3 were defined as high expression.
Statistical analysis
SPSS 21.0 software was used to perform statistical analysis. Wilcoxon signed-rank tests were used to analyze differences in LKB1 expression between gastric cancerous and paracancerous tissues. The correlations between LKB1 and p53, survivin, and clinicopathological features of patients with GC were assessed using chi-square tests and Mann–Whitney U tests. Correlations between OS and LKB1, p53, and survivin expression were analyzed using the Kaplan–Meier method and compared using the log-rank test. The Cox proportional hazards regression model was used to identify prognostic factors influencing survival. All two-sided P≤0.05 were deemed to be statistically significant.
Results
Expression of LKB1 in GC and paracancerous tissues
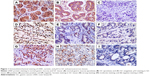
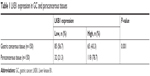
The HE staining of differentiated GC is shown in Figure S1. LKB1 expression was analyzed by IHC in GC tissue samples and corresponding paracancerous tissue samples from 150 patients. Positive LKB1 staining was mainly located in the cytoplasm of both tumor and paracancerous gastric cells (Figure 1A–C). Table 1 shows the results of LKB1 staining. LKB1 expression in paracancerous tissues was found to be significantly higher than in GC tissues (P=0.001).
  | Table 1 LKB1 expression in GC and paracancerous tissues |
Correlation between LKB1 expression and clinicopathological features in GC
Table 2 shows the correlation between LKB1 expression and clinicopathological features of patients with GC. It was found that lower LKB1 expression was associated in patients with GC with greater invasion depth (P=0.013), higher pTNM stage (P=0.009), and lymph node metastasis (P=0.029); however, LKB1 expression was not associated with patient gender (P=0.350), age (P=0.524), or differentiation (P=0.313).
Correlation between LKB1 expression and expression of survivin and p53
Expression of p53 and survivin was also analyzed by IHC in GC tissue from 150 patients. Positive staining for p53 was mainly located in cell nuclei in GC tissue (Figure 1D–F), and positive staining for survivin was mainly located in both cytoplasm and cell nuclei (Figure 1G–I). Correlation of LKB1 expression with p53 and survivin expression was analyzed. LKB1 expression was inversely correlated with both p53 (r=−0.181, P=0.027) and survivin expression (r=−0.198, P=0.015; Table 3).
  | Table 3 Correlation between LKB1 expression and survivin and p53 expression in GC |
OS analysis of GC
The median OS time for patients was 14.5 months (range: 1–53 months). Kaplan–Meier and Cox proportional hazards regression methods were used to evaluate the risk factors considering OS for patients with GC. Kaplan–Meier analysis revealed that patients with high LKB1 expression (log-rank test, P<0.001), negative p53 (log-rank test, P<0.001), and low survivin expression (log-rank test, P=0.001) had longer OS compared to patients with low LKB1 expression, positive p53, and high survivin expression (Figure 2). OS was also significantly correlated with tumor differentiation (log-rank test, P=0.044), invasion depth (log-rank test, P=0.001), pTNM stage (log-rank test, P=0.001), and lymph node metastasis (log-rank test, P=0.006; Table 4).
Factors affecting OS in patients with GC were analyzed further using the Cox proportional hazards regression method. Among variables, LKB1 expression (HR: 0.605 [0.414–0.882]; P=0.009), p53 expression (HR: 1.840 [1.232–2.750]; P=0.032), and survivin expression (HR: 1.561 [1.039–2.345]; P=0.032) were all identified as independent factors for OS.
Discussion
LKB1 is a newly discovered tumor suppressor gene that is known to play a role in suppressing tumor progression in both lung and breast cancers.15,16 An early study on LKB1 by Park et al17 could not identify any common mutations associated with sporadic GC tumorigenesis. More recently, however, Jiang et al18 found that LKB1 expression was reduced in GC tissues, but that reduced LKB1 expression was not associated with clinicopathological features of patients. Meanwhile, Sun et al19 demonstrated that reduced LKB1 mRNA and protein levels were significantly inversely correlated with tumor progression, being associated with a poor prognosis and lower survival rate in GC. Therefore, it remains to be elucidated whether LKB1 expression is decreased in GC, and, if so, the mechanism underlying this reduced expression needs to be understood.
In this study, IHC staining revealed that LKB1 expression was reduced in GC tissues compared with adjacent paracancerous tissues, indicating that loss of LKB1 results in GC tumorigenesis. Evaluation of the correlation between LKB1 expression and clinicopathological features of patients with GC revealed that LKB1 expression was inversely correlated with invasion, pTNM stage, and lymph node metastasis. These results are consistent with the earlier findings of Sun et al,19 indicating that LKB1 plays a role in GC tumorigenesis and progression.
Several studies have demonstrated that the development of most types of human cancers, including GC, involves alterations to the p53 gene.20 There are two forms of p53. Wild-type p53 is an important tumor suppressor, which plays an important role in apoptosis but is rarely detected in tissues; conversely, mutated p53 inhibits apoptosis and can be detected using IHC. Previous studies have indicated that the LKB1 gene also plays a role in cell apoptosis and is mutated in several tumors.8,9 Furthermore, these studies also suggest that the regulation of apoptosis by LKB1 is dependent on tumor suppressor p53. Karuman et al10 demonstrated that LKB1 physically associates with p53 and regulates a specific p53-dependent apoptosis pathway. Furthermore, Zeng and Berger11 reported that LKB1 links with p53 to promote cell apoptosis, and Jimenez et al21 revealed that LKB1 regulates apoptosis of A549 lung adenocarcinoma cells, possibly mediated by the p53 pathway.
Survivin is a member of the inhibitor of apoptosis protein family, which plays important roles in inhibiting apoptosis. Wakana et al22 demonstrated previously that survivin is upregulated in GC, wherein it inhibits GC cell apoptosis. Pizem et al13 reported that loss of p53 is a possible underlying mechanism for the upregulation of survivin in laryngeal squamous cell carcinoma. However, the correlation between LKB1 expression and p53 and survivin expression in GC has not been elucidated yet. In the present study, we revealed that LKB1 expression was inversely associated with both survivin and p53 expression in GC. Furthermore, Kaplan–Meier analysis indicated that LKB1, survivin, and p53 expression were all associated with OS, acting as independent prognostic factors for patients with GC. These results were the same as those found by Hu et al,23 suggesting that LKB1 expression is reduced and negatively correlated with survivin and p53 expression in GC, also playing an important role in predicting the prognosis of GC.
To the best of our knowledge, this is the first report to explore the correlation between LKB1, survivin, and p53 in GC and also the first report to demonstrate the prognostic value of LKB1 in patients with GC, associated with apoptosis. However, the mechanism by which LKB1 influences or inhibits GC progression requires further investigation. Furthermore, due to the limited patient numbers in this study, a larger study is required, including prolonged follow-up to allow analysis of 5-year OS rates.
Conclusion
This study reveals that LKB1 expression is reduced in patients with GC and that reduced LKB1 expression results in deeper invasion, higher pTNM stage, and lymph node metastasis, as well as shorter OS, which might result from increased expression of survivin and mutated p53. Therefore, LKB1 can be considered a promising candidate target for tumor therapy in GC. However, further studies are required to elucidate fully the mechanism through which LKB1 participates in the inhibition or progression of GC.
Disclosure
The authors report no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Elimova E, Shiozaki H, Wadhwa R, et al. Medical management of gastric cancer: a 2014 update. World J Gastroenterol. 2014;20(38):13637–13647. | ||
Lochhead P, El-Omar EM. Gastric cancer. Br Med Bull. 2008;85:87–100. | ||
Chen R, He Q, Cui J, Bian S, Chen L. Lymph node metastasis in early gastric cancer. Chin Med J. 2014;127(3):560–567. | ||
Momcilovic M, Shackelford DB. Targeting LKB1 in cancer – exposing and exploiting vulnerabilities. Br J Cancer. 2015;113(4):574–584. | ||
Avizienyte E, Loukola A, Roth S, et al. LKB1 somatic mutations in sporadic tumors. Am J Pathol. 1999;154(3):677–681. | ||
Zhao RX, Xu ZX. Targeting the LKB1 tumor suppressor. Curr Drug Targets. 2014;15(1):32–52. | ||
Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27(55):6908–6919. | ||
Karuman P, Gozani O, Odze RD, et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001;7(6):1307–1319. | ||
Zeng PY, Berger SL. LKB1 is recruited to the p21/WAF1 promoter by p53 to mediate transcriptional activation. Cancer Res. 2006;66(22):10701–10708. | ||
Li F. Survivin study: what is the next wave? J Cell Physiol. 2003;197(1):8–29. | ||
Pizem J, Cör A, Gale N. Survivin expression is a negative prognostic marker in laryngeal squamous cell carcinoma and is associated with p53 accumulation. Histopathology. 2004;45(2):180–186. | ||
Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2016:203–220. | ||
Liang X, Li ZL, Jiang LL, et al. Suppression of lung cancer cell invasion by LKB1 is due to the downregulation of tissue factor and vascular endothelial growth factor, partly dependent on Sp1. Int J Oncol. 2014;44(6):1989–1997. | ||
Zhuang ZG, Di GH, Shen ZZ, Ding J, Shao ZM. Enhanced expression of LKB1 in breast cancer cells attenuates angiogenesis, invasion, and metastatic potential. Mol Cancer Res. 2006;4(11):843–849. | ||
Park WS, Moon YW, Yang YM, et al. Mutations of the STK11 gene in sporadic gastric carcinoma. Int J Oncol. 1998;13(3):601–604. | ||
Jiang S, Chen R, Yu J, et al. Clinical significance and role of LKB1 in gastric cancer. Mol Med Rep. 2016;13(1):249–256. | ||
Sun J, Ling B, Xu X, et al. Decreased expression of tumor-suppressor gene LKB1 correlates with poor prognosis in human gastric cancer. Anticancer Res. 2016;36(3):869–875. | ||
Kubota E, Williamson CT, Ye R, et al. Low ATM protein expression and depletion of p53 correlates with olaparib sensitivity in gastric cancer cell lines. Cell Cycle. 2014;13(13):2129–2137. | ||
Jimenez AI, Fernandez P, Dominguez O, Dopazo A, Sanchez-Cespedes M. Growth and molecular profile of lung cancer cells expressing ectopic LKB1: down-regulation of the phosphatidylinositol 3′-phosphate kinase/PTEN pathway. Cancer Res. 2003;63(6):1382–1388. | ||
Wakana Y, Kasuya K, Katayanagi S, et al. Effect of survivin on cell proliferation and apoptosis in gastric cancer. Oncol Rep. 2002;9(6):1213–1218. | ||
Hu M, Zhao T, Liu J, et al. Decreased expression of LKB1 is associated with epithelial-mesenchymal transition and led to an unfavorable prognosis in gastric cancer. Hum Pathol. 2019;83:133–139. |
Supplementary material
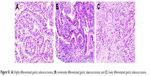  | Figure S1 (A) Highly differentiated gastric adenocarcinoma; (B) moderately differentiated gastric adenocarcinoma; and (C) lowly differentiated gastric adenocarcinoma. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.