Back to Journals » Clinical Ophthalmology » Volume 16
Impact of Inhaled and Intranasal Corticosteroids Exposure on the Risk of Ocular Hypertension and Glaucoma: A Systematic Review and Meta-Analysis
Authors Vinokurtseva A , Fung M, Ai Li E, Zhang R, Armstrong J , Hutnik CML
Received 12 January 2022
Accepted for publication 1 April 2022
Published 30 May 2022 Volume 2022:16 Pages 1675—1695
DOI https://doi.org/10.2147/OPTH.S358066
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Anastasiya Vinokurtseva,1 Matthew Fung,1 Erica Ai Li,2 Richard Zhang,1 James J Armstrong,1,2 Cindy ML Hutnik1– 3
1Department of Ophthalmology, Schulich School of Medicine and Dentistry, London, Ontario, Canada; 2Department of Pathology, Schulich School of Medicine and Dentistry, London, Ontario, Canada; 3Ivey Eye Institute, St Joseph’s Healthcare, London, Ontario, Canada
Correspondence: Anastasiya Vinokurtseva, Department of Ophthalmology, Schulich School of Medicine and Dentistry, 268 Grosvenor St., London, ON, N6A 4V2, Canada, Tel +1 519.646.6100 x.66272, Fax +1 519.646.6410, Email [email protected]
Purpose: Starting in 2019, the Global Initiative for Asthma recommended the use of inhaled corticosteroids (ICS) as part of reliever combination therapy in patients 12 years of age and older, thus dramatically increasing the population exposure to ICS. ICS and intranasal corticosteroids (INS) are commonly used for a variety of respiratory diseases. Chronic steroid use is a well-known risk factor for elevated intraocular pressure (IOP) and glaucoma regardless of route of administration. This study aimed to determine the reported risk of glaucoma, ocular hypertension (OHT) and IOP elevation associated with ICS and INS use.
Materials and Methods: Systematic literature search in MEDLINE, EMBASE, Cochrane, CINAHL, BIOSIS, and Web of Science databases from the date of inception identified studies that assess ocular outcomes related to glaucoma in ICS and INS users. Study selection, risk of bias assessment and data extraction were done independently in duplicate. Meta-analysis assessed glaucoma incidence, OHT incidence and IOP changes in patients using ICS and INS. Study adhered to PRISMA guidelines. Study protocol was registered with PROSPERO: CRD42020190241.
Results: Qualitative and quantitative analyses included 65 and 41 studies, respectively. Incidence of glaucoma was not significantly different in either ICS or INS users compared to control over 45,457 person-years of follow-up. Similarly, no significant difference in OHT incidence over 4431 person-years was detected. In studies reporting IOP, a significantly higher IOP was observed (0.69 mmHg) in 857 ICS or INS users compared to 615 controls. However, no significant increase in IOP was observed within ICS or INS users when compared to pre-treatment baseline.
Conclusion: Overall, use of ICS or INS does not significantly increase the incidence of glaucoma or OHT. However, ICS and INS patients had significantly higher IOPs compared to untreated patients. Awareness of these findings is significant in care of patients with additional risk factors for glaucoma.
Keywords: corticosteroids, asthma, glaucoma, intranasal, inhalation
Introduction
In 2019, the Global Initiative for Asthma (GINA) introduced what is described as the most radical change to the asthma treatment paradigm of the last 30 years.1 Previously, for patients with mild intermittent asthma, short-acting beta-agonists (SABA) alone were used as first-line therapy. The addition of inhaled corticosteroids (ICS) was reserved for more severely symptomatic cases.1 The new report from GINA recommended using SABA + ICS combination for both maintenance and relief therapy in patients of 12 years of age or older,2 affecting the patients with mild intermittent asthma who are not currently adherent to regular ICS treatment, thus increasing the population’s exposure to corticosteroids. These recommendations influence clinical decision-making and treatment guidelines throughout the world and these changes continue to be reflected in the 2020 and 2021 GINA reports.3,4
Asthma affects over 272 million people worldwide based on a 2017 report by Global Burden of Disease Study,5 which includes 3.8 million or 10.2% of Canadians.6,7 The new treatment paradigm exposes an entire new cohort of the patient population to ICS. In addition, many asthma patients are concurrently affected by rhinitis, conjunctivitis, rhinosinusitis and nasal polyps.3 Management of these conditions often warrants intranasal corticosteroid (INS) use, increasing patient exposure to corticosteroids even further.3 ICS are also extensively used for medical management of chronic obstructive pulmonary disease (COPD), another disease with significant health burden affecting over 251 million people worldwide,5 including 2.0 million Canadians.6
Corticosteroid use has been previously linked to increased intraocular pressure (IOP).8,9 When IOP rises beyond a certain threshold, typically defined as 21 mmHg,10 the condition is defined as ocular hypertension (OHT).8,10 Glaucoma is a disease defined by progressive damage of the optic nerve,9,11 and is the leading cause of irreversible blindness worldwide.12 OHT and glaucoma are closely related,9 and are often identified concurrently, as studies have shown that OHT is a major risk factor for development and progression of glaucoma.9,13,14 Anatomic proximity of the administration routes of ICS and INS, in addition to documented systemic effects,15,16 may increase exposure of ocular structures to corticosteroids.15,17
To date, multiple studies of various methodology, including randomized control trials (RCTs),18–20 prospective21,22 and retrospective23,24 studies, have looked at corticosteroid exposure via inhalation or intranasal routes, assessing systemic adverse effects, including ocular outcomes. As a result, some studies suggest increased incidence of ocular hypertension or glaucoma18,19 while others do not observe such an effect.25–28 However, systematic reviews on the topic tend to focus on ocular administration of corticosteroids, and there is a lack of recent syntheses appraising IOP elevation or glaucoma incidence in patients exposed to ICS/INS. The purpose of the current systematic review and meta-analysis is to analyze all available data on incidence of glaucoma, OHT and IOP elevation in ICS and INS users, as well as to summarize and quantify the effects ICS and INS have on IOP.
Materials and Methods
Literature Search Strategy
Details of the protocol for this systematic review and meta-analysis were registered with PROSPERO (registration number CRD42020190241) and can be accessed at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42020190241.29 A predefined search strategy was used to obtain relevant literature from the following databases: MEDLINE, EMBASE, Cochrane, CINAHL, BIOSIS, and Web of Science. Databases were first searched on May 23, 2020 using key terms and subject headings related to 1) corticosteroids 2) inhalational and intranasal administration routes and 3) glaucoma and relevant clinical findings such as IOP or OHT. Additional records were identified through screening of relevant studies’ citation lists. References were managed in Mendeley (Mendeley Ltd, Version 1.19.8, London, England) and Zotero software (Version 5.0.96.2, Virginia, US), deduplicated and entered into Covidence software (Veritas Health Innovation, Melbourne, Australia). Database searches were rerun on August 31, 2021, to retrieve any further studies for inclusion before final analyses. This work adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA; Supplementary Material, Table 1).30
Study Selection
References imported into Covidence software first underwent level 1 screening based on title and abstract using pre-defined criteria. Studies advanced to level 2 screening if they 1) involved human patients; 2) were published in the English language; 3) involved administration of pre-defined corticosteroid drugs through inhalational or intranasal route; 4) measured an ocular outcome. Case reports, reviews, editorials, posters, abstracts, non-English language studies, and studies involving corticosteroids use through any other route than inhalation or intranasal were excluded. Full-texts were obtained. In level 2 screening, studies were selected for inclusion if they 1) reported IOP or any other clinical diagnostic features indicating glaucoma diagnosis or progression; 2) involved administration of corticosteroids through inhalational or intranasal routes; 3) possessed a sample size of at least 10 participants. All studies were screened by at least two independent reviewers and conflicts were resolved through consensus, and all studies passing level 2 screening were included in qualitative review.
Data Collection and Quality Assessment
The following information was extracted from included studies for input into quantitative and qualitative analyses: first author, publication year, study design, study location, funding sources, medication used, dose, route and indication, patient age (mean ± standard deviation (SD) when available, or derived from available data, and range as reported by the authors), follow-up duration, sample size at the study conclusion, and reported ocular outcomes such as IOP change, glaucoma or OHT diagnosis in ophthalmological assessment during study follow-up.
Methodological quality of the studies included after level 2 screening was assessed independently and in duplicate by two reviewers using Cochrane RoB-2 Risk of Bias31 tool for randomized trials and Cochrane ROBINS-I Risk Of Bias In Non-randomized Studies – of Interventions32 tool for non-randomized studies of interventions (NRSIs).33 Any discrepancies were resolved by consensus. Summary of the results of the risk of bias was presented using the robvis tool.34
Data Analysis and Synthesis
Meta-analysis was completed using Review Manager (RevMan, version 5.4; The Nordic Cochrane Center, Copenhagen). Among the studies that passed inclusion criteria for qualitative review, studies were included in the meta-analysis if they reported data on the outcomes of interest as described below. Studies were excluded from quantitative meta-analysis and remained in qualitative review only, if their results did not provide sufficient detail regarding ocular outcomes, lacked quantitative outcomes, or did not have a clear control group. The main outcomes of interest were incidence of glaucoma and OHT, as well as mean ± SD of IOP in controls and patients before and after ICS or INS use. Incidence of new glaucoma diagnoses, OHT and IOP increase was expressed as number of cases per person-years (PPY) based on study follow-up, in order to allow weighted measurement of the incidence rate, and was used to calculate risk differences. For incidence of OHT, the clinical threshold chosen by each study was accounted for in subgroup analysis. For IOP, reported mean ± SD of ICS users, INS users, and controls before and after medication use were pooled for meta-analysis. For all outcomes, subgroup analyses pooled studies based on study methodology, separating RCT and NRSI to account for the different strength of evidence each design provides in summary estimates, and drug administration route, to examine whether proximity to ocular tissues could influence ophthalmic outcomes. Heterogeneity was tested by computing I2 value and for low-heterogeneity studies (I2 value <50%), the fixed-effect model was used, while for high-heterogeneity studies (I2 value ≥50%), the random-effect model was used.35 Sensitivity analyses were performed for large studies (>1000 participants/person-years) and for studies with pediatric populations (<18 years of age) versus adult population.
Results
Search Results
The study selection process is outlined in the PRISMA flow diagram (Figure 1). The chosen search strategy yielded 2381 references, including 1764 from EMBASE, 205 from MEDLINE, 59 from CINAHL, 102 from Cochrane Library, 210 from Web of Science, and 41 from BIOSIS databases. An additional 7 studies were identified through a hand search of reference lists. After deduplication, 1805 references remained. The agreement between the reviewers was substantial36 – 0.79 for level 1 and 0.84 for level 2. After level 1 screening, 132 references remained. During level 2 screening, 67 references were excluded, while the remaining 65 studies met our inclusion criteria and thus were included in qualitative synthesis. Out of these 65, 24 studies were included only in qualitative synthesis because their results did not provide sufficient detail regarding ocular outcomes, lacked quantitative outcomes, or did not have a clear control group. The remaining 41 studies were included in quantitative meta-analysis.
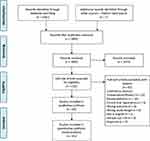 |
Figure 1 PRISMA flow diagram. Notes: PRISMA figure adapted from Moher D, Liberati A, Altman D, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health-care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.30. |
Study Characteristics
Characteristics of the included studies are summarized in Table 1. A total of 536,412 patients participated in the included studies, aged 0 to 97 years of age. Follow-up period varied between 1 week and 2 years. Number of patients enrolled per study varied between 10 and 459,795. Main indications for inhaled corticosteroids were asthma and COPD, while intranasal corticosteroids were mainly indicated for allergic rhinitis, and rhinosinusitis with or without nasal polyps. For both administration routes, a wide range of corticosteroids doses has been used, with a comparable spread. Methodological quality of the included studies was good overall, with RCTs having a lower risk of bias compared to NRSIs (Supplementary Material, Figure 1).34
 |  |  |  |  |  |
Table 1 Characteristics of Included Studies, (A) ICS, (B) INS |
Publication Bias Funnel Plots
Funnel plots did not display asymmetry for glaucoma incidence, OHT incidence, differences in IOP between ICS/INS users versus non-users, nor IOP elevation after corticosteroid use compared to pre-treatment baseline (Figure 2). Figure 2 Continued.
Main Outcomes
Main outcomes of this study were glaucoma and ocular hypertension incidence, as well as differences in IOP between ICS/INS users and controls, and change in IOP in corticosteroid users compared to pre-treatment baseline.
Glaucoma Incidence
There was no statistically significant risk difference in glaucoma incidence identified between corticosteroid users and non-users (Figure 3A). Subgroup analyses of both RCT and NRSI revealed no significant increase in glaucoma incidence for ICS or INS users. Sensitivity analysis (Supplementary Material, Figure 2) did not reveal significant influence of large studies by Sheffer26 or Chang37 on subgroup or overall risk difference, or heterogeneity. No significant differences were identified between adult and pediatric populations (Supplementary Material, Figure 3). Figure 3 Continued. Figure 3 Continued.

OHT Incidence
Studies reported different thresholds for classification of OHT, beginning at IOP above 20 mmHg, 21 mmHg, or 22 mmHg. Many authors reported only occurrence of IOP elevation, without specifying the magnitude. Overall, there was no statistically significant risk difference in OHT incidence or incidence of IOP elevation between corticosteroid users and non-users (Figure 3B). Subgroup analysis accounting for different reporting styles was overall consistent with the summary analysis, revealing two subgroups slightly favoring control. Both of these subgroups included RCT studies that used >22 mmHg as an IOP threshold for OHT, with one of these reporting a 27% increased risk with ICS use in a sample of 30 patients over 30 person-years,18 and the other reporting a 2% risk increase with INS use in a sample of 806 patients over 806 person years.19 In the latter subgroup, a 2% risk increase persisted despite a study that included 66 patients over 16 person years reporting 0% increased risk. Sensitivity analysis (Supplementary Material, Figure 2) did not reveal significant influence of the large study by LaForce38 on the analysis. Pediatric and adult populations did not display significantly different trends (Supplementary Material, Figure 3).
Differences in IOP Between Corticosteroid Users and Controls
Among 857 ICS/INS users and 615 non-user controls (Figure 3C), corticosteroid users had significantly higher IOP with a mean difference of +0.69 mmHg (95% CI: 0.15, 1.23; p<0.01) compared to controls. In a subgroup analysis of RCTs, ICS users were statistically indistinguishable from controls with a mean difference in IOP of –0.10 mmHg (95% CI: −2.89, 2.69; p=0.94). In NRSIs, ICS users had a higher mean IOP compared to controls by 0.70 mmHg (95% CI: −0.21, 1.61; p=0.13). In a subgroup analysis of RCTs, INS users had a higher mean IOP compared to controls (+0.06 mmHg; 95% CI: −0.85, 0.98; p=0.89). In NRSIs, INS users had significantly higher mean IOP than controls by 1.21 mmHg (95% CI: 0.57, 1.85; p<0.0002). There was significant heterogeneity between the studies (I2 = 75%), however no large studies were identified to explain the large heterogeneity. Largest effect was noted by the study by Shroff39 (Supplementary Material, Figure 2), however overall result remained consistent even with this study excluded. Both adult and pediatric populations have displayed these statistically significant trends (Supplementary Material, Figure 3). However adult studies showed a higher mean IOP by 0.90 mmHg (95% CI: 0.08, 1.73, p<0.02) in ICS/INS users compared to controls, while in pediatric population, this difference was smaller at 0.45 mmHg (95% CI: 0.33, 0.57, p<0.00001).
Change in IOP from Pre-Treatment Baseline
Collectively, 1360 patients had IOP measurements reported prior to initiating ICS/INS use, as well as at the end of follow-up. These data are summarized in Figure 3D. Overall, there was a 0.25 mmHg (95% CI: −0.85, 0.34, p=0.41) nonsignificant increase in IOP following corticosteroid use. Subgroup analysis of RCTs showed a significant increase in mean IOP after ICS use of 1.09 mmHg (95% CI: 0.18, 1.99; p<0.05). In NRSIs, IOP increased after ICS use by 0.04 mmHg (95% CI: −0.65, 0.74; p=0.90). Within RCTs, IOP increased after INS use by 0.18 mmHg (95% CI:-0.20, 0.56; p=0.36). In NRSIs, IOP after INS use was lower by 0.2 mmHg (95% CI: −0.55, 0.95; p=0.60). There was significant heterogeneity between the studies (I2=79%), and sensitivity analysis (Supplementary Material, Figure 2) identified significant influence of large study by Chylack;40 however, overall conclusions remained the same with the study excluded. Similarly, separate analyses of adult and pediatric populations displayed similar trends (Supplementary Material, Figure 3).
Discussion
The new GINA treatment guidelines recommend increased exposure to ICS, notably daily use starting in patients as young as 12 years of age. Given the known effect of corticosteroids on IOP elevation and risk of glaucoma, widespread use of ICS and INS may raise concerns regarding OHT and glaucoma risks as even low absolute risks when applied to hundreds of millions of patients can be of significant impact.41–43 Even a 0.1 mmHg increase in IOP over a population can increase the risk of glaucoma or its progression.44
Our search identified 65 primary studies discussing ocular outcomes in patients using ICS and INS, compared to control. Qualitative assessment revealed that most studies report ICS and INS as well tolerated, and do not raise ocular safety concerns.37,45–50 Quantitative assessment of 41 primary studies including 54,080 patients assessed difference in glaucoma incidence, OHT incidence, IOP between users and non-users, as well IOP changes following ICS or INS use.
Overall, there was no statistically significant difference in glaucoma incidence in ICS and INS users compared to controls in overall and subgroup analyses over 45,457 person-years51 of follow-up.
With respect to OHT incidence, there was a lack of consistency in IOP threshold between studies, ranging between 20 and 22 mmHg. Regardless of these differences, tests for overall effect and most subgroup analyses did not identify significant trends in risks for OHT incidence in corticosteroid users compared to control over 4431 person-years of follow-up. While single subgroup analysis of OHT incidence using a 22 mmHg IOP threshold demonstrated significant risk differences between ICS users and non-users, this subgroup includes only a single study with 30 person-years of follow-up,18 thus this finding is potentially of limited clinical significance.
Interestingly, overall analysis identified a statistically significant increase of 0.69 mmHg IOP in ICS or INS users compared to non-users. Subgroup analysis supported this finding only in NRSI INS studies, with a mean difference between groups of 1.21 mmHg.
There was no significant increase in IOP following ICS or INS use compared to patients’ baseline. However, subgroup analysis identified a significant increase in IOP of 1.09 mmHg following ICS use in RCTs. Potentially, these differences are related to longer duration of use and follow-up (between 6 months and up to 4 years) compared to other groups (between 6 weeks and 1 year). Within this subgroup, the study by Chylack 200940 possessed the greatest weight, resulting in a significant mean increase in IOP for the overall subgroup. Although this study was double-blinded, it lacked a placebo control group. Furthermore, there is well-documented evidence demonstrating that IOP measurements can fluctuate due to patient factors, timing, and evaluator variability.52,53 Therefore, caution is warranted when determining whether these results are truly clinically significant.
Overall lack of significant increases in glaucoma or OHT incidence suggests that the use of INS and ICS at the doses prescribed and over the periods studied are alone not a major risk factor. This conclusion, of course is reliant upon the definitions for glaucoma and OHT used in the various reported studies. On a per patient level, a 1 mmHg rise in IOP is unlikely clinically significant. However, at a population level, this number would be significant for a risk of development and progression of glaucoma,44 especially when patients already have a number of other risk factors for the disease.
Steroid-induced glaucoma is a well-known secondary cause of open-angle glaucoma. A third of the population is designated as steroid responders, as they are more likely to develop IOP elevation, OHT and glaucoma in response to topical ocular steroid administration.8,9,54 Up to 95% of these patients have a family history of glaucoma.8,9 Interestingly, many authors excluded patients with family history of glaucoma from enrollment in their studies, which may have resulted in an underestimation of risk by excluding potential steroid responders.55–57 As such, the current literature may be underestimating the risk of IOP increase, OHT and glaucoma incidence, which may limit applicability of the findings and of risk assessment to general population.
Glaucoma diagnosis and management depend upon an accurate development of a risk factor profile for each patient. The results of this study do support the need for awareness among both practitioners and patients of the potential risk of long-term use of ICS and INS. Upon prescription, it may be prudent to recommend that patients actively seek regular examinations with an eye care professional and report the use of these medications.
Limitations of the current study are consistent with those previously identified by other authors working on the subject. Lack of inclusion of patients with family history of glaucoma is a potential confounder, leading to possible exclusion of potential steroid responders.58 Large variability in diagnostic methods, such as tonometry measurements, and reporting criteria for ocular outcomes, such as OHT threshold and glaucoma diagnosis, is also widespread.58,59 Similarly, there remains a relative lack of IOP data reporting in the field, making meta-analysis challenging and precluding dose-response analysis.58,60 Multiple studies48–50,61 were found to have industry sponsorship – on one hand, this may raise concerns of a risk of bias; however, these partnerships fuel research that leads to discovery of vision-saving treatments and is thus an important source of research support. Results suggest future studies may be warranted that are more representative of the at-risk population, capture more detailed risk factors, include tighter definitions of endpoints,62,63 and incorporate comparisons of corticosteroid potencies, dosing regimens, and lengths of exposure.
Conclusion
In conclusion, a small but significant IOP elevation of 0.69 mmHg was identified in patients using ICS and INS. Increases of this magnitude are known to increase the risk of glaucoma at a population level. The relatively recent GINA recommendations would cause a significant increase in the number of patients exposed to chronic daily corticosteroid use. Awareness of the potential risk of glaucoma may be an important consideration for patient-informed consent and recommendations for patient management.
Acknowledgments
We would like to thank Brad Dishan for assisting in obtaining article full-texts. We would also like to thank the Ivey Eye Institute Department of Ophthalmology at Schulich School of Medicine and Dentistry, the Summer Research Training Program of Schulich School of Medicine and Dentistry, and the Lawson Health Research Institute for their generous financial support of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Beauchesne M-F, Lemiere C. Global Initiative for Asthma report: how will new recommendations affect practice in Canada? [Internet]. Canadian Medical Association Journal. 2020;192(17):E456–8. doi:10.1503/cmaj.191445
2. Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents [Internet]. Eur Respir J. 2014;53:2548. doi:10.1183/13993003.01046-2019
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention: 2020 Update. Global Initiative for Asthma; 2020.
4. Reddel HK, Bacharier LB, Bateman ED, et al. Global Strategy for Asthma Management and Prevention (2021 update). 2021. p. 1–217.
5. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2017;392(10159):1789–1858.
6. Hamm NC, Pelletier L. Report from the Canadian chronic disease surveillance system: asthma And Chronic Obstructive Pulmonary Disease (COPD) In Canada. Chronic Dis Can. 2018;31:548.
7. Population estimates, quarterly; 2021. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901&cubeTimeFrame.startMonth=01&cubeTimeFrame.startYear=2018&cubeTimeFrame.endMonth=01&cubeTimeFrame.endYear=2020&referencePeriods=20180101%2C20200101.
8. Jones R, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17(2):163–167.
9. Phulke S, Kaushik S, Kaur S, Pandav SS. Steroid-induced Glaucoma: an avoidable irreversible blindness. J Curr Glaucoma Pract. 2014;11(2):67–72.
10. Burr J, Botello-Pinzon P, Takwoingi Y, et al. Surveillance for ocular hypertension: an evidence synthesis and economic evaluation. Health Technol Assess. 2014;16(29):95.
11. Sihota R, Konkal VL, Dada T, Agarwal HC, Singh R. Prospective, long-term evaluation of steroid-induced glaucoma. Eye. 2008;22(1):26–30.
12. Rouland J-F, Berdeaux G, Lafuma A. The Economic Burden of Glaucoma and Ocular Hypertension Implications for Patient Management: a Review. Durgs Agins. 2005;22(4):315–321.
13. Bonomi L, Marchini G, Marraffa M, Morbio R. The relationship between intraocular pressure and glaucoma in a defined population: data from the Egna-Neumarkt glaucoma study. Ophthalmologica. 2015;215(1):34–38.
14. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2014;120(10):1268–1279.
15. Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–1317.
16. Patel R, Naqvi SA, Griffiths C, Bloom CI. Systemic adverse effects from inhaled corticosteroid use in asthma: a systematic review. BMJ Open Respir Res. 2020;7:1.
17. Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992;10(3):505–512.
18. Novak-Lauš K, Kukulj S, Iveković R, Tedeschi-Reiner E, Koršić J, Matanić D. Inhaled corticosteroids and the risk of glaucoma and intraocular hypertension. Acta Clin Croat. 2003;42(1):41–45.
19. Rosenblut A, Bardin PG, Muller B, et al. Long-term safety of fluticasone furoate nasal spray in adults and adolescents with perennial allergic rhinitis. Allergy Eur J Allergy Clin Immunol. 2007;62(9):1071–1077.
20. Rotenberg BW, Zhang I, Arra I, Payton KB. Postoperative care for Samter’s triad patients undergoing endoscopic sinus surgery: a double-blinded, randomized controlled trial. Laryngoscope. 2011;121(12):2702–2705.
21. Öztürk F, Yücetürk AV, Kurt E, Ünlü HH, Ilker SS. Evaluation of intraocular pressure and cataract formation following the long-term use of nasal corticosteroids. Ear Nose Throat J. 1998;77(10):846–851.
22. Behbehani A, Owayed A, Hijazi Z, Eslah E, Al-Jazzaf A. Cataract and Ocular Hypertension in Children on Inhaled Corticosteroid Therapy. J Pediatr Ophthalmol Strabismus. 2005;42:23–27.
23. Dereci S, Pirgon O, Akcam M, Turkyilmaz K, Dundar B. Effect of inhaled fluticasone propionate on retinal nerve fiber layer thickness in asthmatic children. Eur J Ophthalmol. 2015;25(6):535–538.
24. Soudry E, Wang J, Vaezeafshar R, Katznelson L, Hwang PH. Safety analysis of long-term budesonide nasal irrigations in patients with chronic rhinosinusitis post endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016;6(6):568–572.
25. Kerwin EM, Ferguson GT, Mo M, Deangelis K, Dorinsky P. Bone and ocular safety of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a 52-week randomized study. Respir Res. 2019;20(1):1–14.
26. Sheffer AL, Silverman M, Woolcock AJ, Díaz PV, Lindberg B, Lindmark B. Long-term safety of once-daily budesonide in patients with early-onset mild persistent asthma: results of the Inhaled Steroid Treatment as Regular Therapy in Early Asthma (START) study. Ann Allergy Asthma Immunol. 2005;94(1):48–54. doi:10.1016/S1081-1206(10)61285-9
27. Weinstein SF, Andrews CP, Shah SR, et al. Long-term efficacy and safety of once-daily treatment with beclomethasone dipropionate nasal aerosol. Allergy Asthma Proc. 2014;35(4):323–331.
28. Moss EB, Buys YM, Low SA, et al. A Randomized Controlled Trial to Determine the Effect of Inhaled Corticosteroid on Intraocular Pressure in Open-Angle Glaucoma and Ocular Hypertension: the ICOUGH Study. J Glaucoma. 2017;26(2):182–186.
29. Vinokurtseva A, Fung M, Li E, Zhang R, Armstrong JJ, Hutnik CML. Impact of inhaled and intranasal corticosteroids exposure on the risk of ocular hypertension and glaucoma: systematic review and meta-analysis. Prospero. 2020;1:CRD42020190241.
30. Moher D, Liberati A, Tetzlaff J, Altman DGD, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097.
31. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;1:366.
32. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;1:355.
33. Farrah K, Young K, Tunis MC, Zhao L. Risk of bias tools in systematic reviews of health interventions: an analysis of PROSPERO-registered protocols. Syst Rev. 2019;8(1):1–9.
34. Mcguinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020;26:584. doi:10.1002/jrsm.1411
35. Armstrong JJ, Wasiuta T, Kiatos E, Malvankar-Mehta M, Hutnik CML. The Effects of Phacoemulsification on Intraocular Pressure and Topical Medication Use in Patients with Glaucoma: a Systematic Review and Meta-analysis of 3-Year Data. J Glaucoma. 2017;26(6):511–522.
36. McHugh ML. Interrater reliability: the kappa statistic. Biochem Medica. 2012;22(3):276.
37. Chang LS, Lee HC, Tsai YC, et al. Decreased incidence of glaucoma in children with asthma using inhaled corticosteroid: a cohort study. Oncotarget. 2017;8(62):105463–105471.
38. LaForce C, Journeay GE, Miller SD, et al. Ocular safety of fluticasone furoate nasal spray in patients with perennial allergic rhinitis: a 2-year study. Ann Allergy Asthma Immunol. 2013;111(1):45–50. doi:10.1016/j.anai.2013.04.013
39. Shroff S, Thomas RK, D’Souza G, Nithyanandan S. The effect of inhaled steroids on the intraocular pressure. Digit J Ophthalmol DJO. 2018;24(3):6–9.
40. Chylack LT, Gross GN, Pedinoff A, et al. A randomized, controlled trial to investigate the effect of ciclesonide and beclomethasone dipropionate on eye lens opacity. J Asthma. 2008;45(10):893–902.
41. Becker B. Intraocular Pressure Response To Topical Corticosteroids. Invest Ophthalmol. 1965;4:198–205.
42. Becker B, Mills DW. Corticosteroids and Intraocular Pressure. Arch Ophthalmol. 1963;70(4):500–507.
43. Armaly MF. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics: II. The Effect of Dexamethasone in the Glaucomatous Eye. Arch Ophthalmol. 2015;70(4):492–499.
44. Parrish RK, Palmberg P, Sheu WP. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135(5):688–703.
45. Howland WC, Hampel FC, Martin BG, Ratner PH, Van Bavel JH, Field EA. The efficacy of fluticasone propionate aqueous nasal spray for allergic rhinitis and its relationship to topical effects. Clin Ther. 1996;18(6):1106–1117.
46. Máspero JF, Rosenblut A, Finn A, Lim J, Wu W, Philpot E. Safety and efficacy of fluticasone furoate in pediatric patients with perennial allergic rhinitis. Otolaryngol. 2008;138(1):30–37.
47. Covelli H, Pek B, Schenkenberger I, Scott-Wilson C, Emmett A, Crim C. Efficacy and safety of fluticasone furoate/vilanterol or tiotropium in subjects with COPD at cardiovascular risk. Int J COPD. 2015;11:1–12.
48. Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once-daily ciclesonide in children: efficacy and safety in asthma. J Pediatr. 2006;148(3):377–383.
49. Duh MS, Walker AM, Lindmark B, Laties AM. Association between intraocular pressure and budesonide inhalation therapy in asthmatic patients. Ann Allergy Asthma Immunol. 2000;85(5):356–361. doi:10.1016/S1081-1206(10)62545-8
50. Ferguson GT, Tashkin DP, Skärby T, et al. Effect of budesonide/formoterol pressurized metered-dose inhaler on exacerbations versus formoterol in chronic obstructive pulmonary disease: the 6-month, randomized RISE (Revealing the Impact of Symbicort in reducing Exacerbations in COPD) study. Respir Med. 2017;132:31–41.
51. Tenny S, Boktor SW Incidence. StatPearls 2021 Available from: https://www-ncbi-nlm-nih-gov.proxy1.lib.uwo.ca/books/NBK430746/.
52. Bhorade AM, Gordon MO, Wilson B, Kass MA Variability of Intraocular Pressure Measurements in Observation Participants in the Ocular Hypertension Treatment Study. Available from: https://vrcc.wustl.edu/mop/ohtsmop.pdf.
53. Kim JH, Caprioli J. Intraocular Pressure Fluctuation: is It Important? J Ophthalmic Vis Res. 2018;13(2):170.
54. Feroze KB, Khazaeni L Steroid Induced Glaucoma. StatPearls; 2020 Available from: https://www-ncbi-nlm-nih-gov.proxy1.lib.uwo.ca/books/NBK430903/.
55. Man LX, Farhood Z, Luong A, et al. The effect of intranasal fluticasone propionate irrigations on salivary cortisol, intraocular pressure, and posterior subcapsular cataracts in postsurgical chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2013;3(12):953–957.
56. Alsaadi M, Osuagwu U, Almubrad T. Effects of inhaled fluticasone on intraocular pressure and central corneal thickness in asthmatic children without a family history of glaucoma. Middle East Afr J Ophthalmol. 2012;19(3):314–319.
57. Gunay M, Dogru M, Celik G, Gunay BO. Swept-source optical coherence tomography analysis in asthmatic children under inhaled corticosteroid therapy. Cutan Ocul Toxicol. 2019;38(2):131–135. doi:10.1080/15569527.2018.1539009
58. Donaldson AM, Choby G, Kim DH, Marks LA, Lal D. Intranasal Corticosteroid Therapy: systematic Review and Meta-analysis of Reported Safety and Adverse Effects in Adults. Otolaryngol Head Neck Surg. 2020;163(6):1097–1108.
59. Valenzuela CV, Liu JC, Vila PM, Simon L, Doering M, Lieu JEC. Intranasal Corticosteroids Do Not Lead to Ocular Changes: a Systematic Review and Meta-analysis. Laryngoscope. 2019;129:6–12.
60. Ishii M, Horita N, Takeuchi M, et al. Inhaled corticosteroid and secondary glaucoma: a meta-analysis of 18 studies. Allergy Asthma Immunol Res. 2021;13(3):435–449.
61. Kemp JP, Osur S, Shrewsbury SB, et al. Potential Effects of Fluticasone Propionate on Bone Mineral Density in Patients with Asthma: a 2-Year Randomized, Double-Blind, Placebo-Controlled Trial. Mayo Clin Proc. 2004;79(4):458–466.
62. Schlenker M, Kansal V. How Mean Intraocular Pressures Are Failing Patients. Ophthalmol Glaucoma. 2021;1:1–5. 10.1016/j.ogla.2021.04.008.
63. Shaarawy TM, Sherwood MB, Grehn F. WGA Guidelines on Design and Reporting of Glaucoma Surgical Trials. J Med. 2009;2:93.
64. Emin O, Fatih M, Mustafa O, Nedim S, Osman C. Evaluation impact of long-term usage of inhaled Fluticasone propionate on ocular functions in children with asthma. Steroids. 2011;76(6):548–552.
65. Mitchell P, Cumming RG, Mackey DA. Inhaled corticosteroids, family history, and risk of glaucoma. Ophthalmology. 1999;106(12):2301–2306.
66. Marcus MW, Müskens RPHM, Ramdas WD, et al. Corticosteroids and open-angle glaucoma in the elderly: a population-based cohort study. Drugs Aging. 2012;29(12):963–970.
67. Nath T, Roy SS, Kumar H, Agrawal R, Kumar S, Satsangi SK. Prevalence of steroid-induced cataract and glaucoma in chronic obstructive pulmonary disease patients attending a tertiary care center in India. Asia-Pacific J Ophthalmol. 2017;6(1):28–32.
68. Boulet LP, Cartier A, Ernst P, Larivée P, Laviolette M. Safety and efficacy of HFA-134a beclomethasone dipropionate extra-fine aerosol over six months. Can Respir J. 2004;11(2):123–130.
69. Busse WW, O’Byrne PM, Bleecker ER, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the β2 agonist vilanterol administered once daily for 52 weeks in patients ≥12 years old with asthma: a randomised trial. Thorax. 2013;68(6):513–520.
70. Li JTC, Ford LB, Chervinsky P, et al. Fluticasone propionate powder and lack of clinically significant effects on hypothalamic-pituitary-adrenal axis and bone mineral density over 2 years in adults with mild asthma. J Allergy Clin Immunol. 1999;103(6):1062–1068.
71. Maspero JF, Nolte H, Chérrez-Ojeda I. Long-term safety of mometasone furoate/formoterol combination for treatment of patients with persistent asthma. J Asthma. 2010;47(10):1106–1115.
72. Ratner PH, Wingertzahn MA, Van Bavel JH, et al. Effectiveness of ciclesonide nasal spray in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97(5):657–663. doi:10.1016/S1081-1206(10)61097-6
73. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild-to-moderate asthma. J Allergy Clin Immunol. 1998;101(1):14–23.
74. Tinkelman DG, Reed CE, Nelson HS, Offord KP. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92(1):64–77.
75. Garbe E, Lelorier J, Boivin J, Suissa S. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucoma. Am J Rhinol. 1997;11(4):331–332.
76. Gonzalez AV, Li G, Suissa S, Ernst P. Risk of glaucoma in elderly patients treated with inhaled corticosteroids for chronic airflow obstruction. Pulm Pharmacol Ther. 2010;23(2):65–70. doi:10.1016/j.pupt.2009.10.014
77. Miller DP, Watkins SE, Sampson T, Davis KJ. Long-term use of fluticasone propionate/ salmeterol fixed-dose combination and incidence of cataracts and glaucoma among chronic obstructive pulmonary disease patients in the UK general practice research database. Int J COPD. 2011;6(1):467–476.
78. Baan EJ, de Smet VA, Hoeve CE, et al. Exploratory Study of Signals for Asthma Drugs in Children, Using the EudraVigilance Database of Spontaneous Reports. Drug Saf. 2020;43(1):7–16. doi:10.1007/s40264-019-00870-x
79. Johnson LN, Soni CR, Johnson MAJ, Madsen RW. Short-term use of inhaled and intranasal corticosteroids is not associated with glaucoma progression on optical coherence tomography. Eur J Ophthalmol. 2012;22(5):695–700.
80. Manji J, Singh G, Okpaleke C, et al. Safety of long-term intranasal budesonide delivered via the mucosal atomization device for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7(5):488–493.
81. Mohd Zain A, Md Noh UK, Hussein S, Che Hamzah J, Mohd Khialdin S, Md Din N. The Relationship between Long-term Use of Intranasal Corticosteroid and Intraocular Pressure. J Glaucoma. 2019;28(4):321–324.
82. Douglas RG, Psaltis AJ, Rimmer J, Kuruvilla T, Cervin A, Kuang Y. Phase 1 clinical study to assess the safety of a novel drug delivery system providing long-term topical steroid therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(4):378–387.
83. Forwith KD, Chandra RK, Yun PT, Miller SK, Jampel HD. ADVANCE: a multisite trial of bioabsorbable steroid-eluting sinus implants. Laryngoscope. 2011;121(11):2473–2480.
84. Seiberling KA, Chang DF, Nyirady J, Park F, Church CA. Effect of intranasal budesonide irrigations on intraocular pressure. Int Forum Allergy Rhinol. 2013;3(9):704–707.
85. Şimşek A, Bayraktar C, Doğan S, Karataş M, Sarıkaya Y. The effect of long-term use of intranasal steroids on intraocular pressure. Clin Ophthalmol. 2016;10:1079–1082.
86. Spiliotopoulos C, Mastronikolis NS, Petropoulos IK, Mela EK, Goumas PD, Gartaganis SP. The effect of nasal steroid administration on intraocular pressure. Ear Nose Throat J. 2007;86(7):394–395.
87. Yenigun A, Elbay A, Dogan R, Ozturan O, Ozdemir MH, Pilot Study A. Investigating the Impact of Topical Nasal Steroid Spray in Allergic Rhinitis Patients with Dry Eye. Int Arch Allergy Immunol. 2018;176(2):157–162.
88. Adriaensen GFJPM, Lim KH, Fokkens WJ. Safety and efficacy of a bioabsorbable fluticasone propionate–eluting sinus dressing in postoperative management of endoscopic sinus surgery: a randomized clinical trial. Int Forum Allergy Rhinol. 2017;7(8):813–820.
89. Berger WE, Shah S, Lieberman P, et al. Long-term, randomized safety study of mp29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) in subjects with chronic rhinitis. J Allergy Clin Immunol Pract. 2014;2(2):179–185. doi:10.1016/j.jaip.2013.09.019
90. Bross-Soriano D, Hanenberg-Milver C, Schimelmitz-Idi J, Arrieta-Gomez JR, Del Toro RA, Bravo-Escobar G. Effects of three nasal topical steroids in the intraocular pressure compartment. Otolaryngol. 2004;130(2):187–191.
91. Chervinsky P, Kunjibettu S, Miller DL, et al. Long-term safety and efficacy of intranasal ciclesonide in adult and adolescent patients with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2007;99(1):69–76. doi:10.1016/S1081-1206(10)60624-2
92. Han JK, Forwith KD, Smith TL, et al. RESOLVE: a randomized, controlled, blinded study of bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis. Int Forum Allergy Rhinol. 2014;4(11):861–870.
93. Igarashi T, Nakazato Y, Kunshige T, et al. Mometasone Furoate Nasal Spray Relieves the Ocular Symptoms of Seasonal Allergic Rhinoconjunctivitis. J Nippon Med Sch. 2012;79(3):182–190.
94. Kim K, Weiswasser M, Nave R, et al. Safety of Once-Daily Ciclesonide Nasal Spray in Children 2 to 5 Years of Age with Perennial Allergic Rhinitis. Pediatr Asthma Allergy Immunol. 2007;20(4):229–242.
95. Kothiwala M, Samdani S, Grover M, Gurjar V. Efficacy of Topical High Volume Budesonide Nasal Irrigation in Post FESS Patients of Chronic Rhinosinusitis With or Without Nasal Polyposis. Indian J Otolaryngol Head Neck Surg. 2021. doi:10.1007/s12070-021-02509-9
96. Marple BF, Smith TL, Han JK, et al. Advance II: a Prospective, randomized study assessing safety and efficacy of bioabsorbable steroid-releasing sinus implants. Otolaryngol Head Neck Surg. 2012;146(6):1004–1011.
97. Ratner PH, Meltzer EO, Teper A. Mometasone furoate nasal spray is safe and effective for 1-year treatment of children with perennial allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2009;73(5):651–657.
98. Rosenwasser LJ, Mahr T, Abelson MB, Gomes PJ, Kennedy K. A comparison of olopatadine 0.2% ophthalmic solution versus fluticasone furoate nasal spray for the treatment of allergic conjunctivitis. Allergy Asthma Proc. 2008;29(6):644–653.
99. Yuen D, Buys YM, Jin YP, Alasbali T, Trope GE. Effect of beclomethasone nasal spray on intraocular pressure in ocular hypertension or controlled glaucoma. J Glaucoma. 2013;22(2):84–87.
100. Bui CM, Chen H, Shyr Y, Joos KM. Discontinuing nasal steroids might lower intraocular pressure in glaucoma. J Allergy Clin Immunol. 2005;116(5):1042–1047.
101. Davis KJ, Hinds D, Motsko SP, Goehring E, Jones JK. Intranasal Fluticasone Propionate Observational Cohort Safety Studies: reviewing Evidence from Databases on Two Continents. Drugs - Real World Outcomes. 2016;3(1):53–60.
102. Martino BJ, Church CA, Seiberling KA. Effect of intranasal dexamethasone on endogenous cortisol level and intraocular pressure. Int Forum Allergy Rhinol. 2015;5(7):605–609.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


