Back to Journals » Journal of Pain Research » Volume 15
Impact of Informed Consent and Education on Care Engagement After Opioid Initiation in the Veterans Health Administration
Authors Avoundjian T , Troszak L , Cohen J, Foglia MB, Trafton J, Midboe A
Received 12 May 2021
Accepted for publication 6 September 2021
Published 25 May 2022 Volume 2022:15 Pages 1553—1562
DOI https://doi.org/10.2147/JPR.S317183
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Erica Wegrzyn
Tigran Avoundjian,1 Lara Troszak,1,2 Jennifer Cohen,3,4 Mary Beth Foglia,3,5 Jodie Trafton,1,6,7 Amanda Midboe1,2
1Center for Innovation to Implementation, Va Palo Alto Health Care System, Palo Alto, CA, USA; 2School of Medicine, Stanford University, Stanford, CA, USA; 3National Center for Ethics in Health Care, Veterans Affairs, Seattle, WA, USA; 4Department of Epidemiology, University of Washington, Seattle, WA, USA; 5Department of Bioethics and Humanities, School of Medicine, University of Washington, Seattle, WA, USA; 6Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University, Stanford, CA, USA; 7VA Office of Mental Health and Suicide Prevention, VA Palo Alto Healthcare System, Menlo Park, CA, USA
Correspondence: Tigran Avoundjian, Center for Innovation to Implementation, 745 Willow Road, Menlo Park, CA, 94205, USA, Fax +206-296-4803, Email [email protected]
Objective: To ensure all patients receiving long-term opioid therapy (LTOT) understand the risks, benefits and treatment alternatives, the Veterans Health Administration (VHA) released a national policy in 2014 to standardize a signature informed consent (SIC) process. We evaluated the impact of this policy on medical follow-up after LTOT initiation, a guideline recommended practice.
Methods: Using VHA administrative data, we identified patients initiating LTOT between May 2013 and May 2016. We used an interrupted time series design to compare the monthly rates of medical follow-up within 30 days and primary care visits within 3 months after LTOT initiation across three periods: 12 months before the policy (Year 1); 12 months after policy release (Year 2); and 12– 24 months after policy release, when the SIC process was mandatory (Year 3).
Results: Among the 409,895 patients who experienced 758,416 LTOT initiations, medical follow-up within 30 days and primary care engagement within 3 months increased by 4% between Year 1 and Year 3. Compared to Year 1, patients in Year 3 were 1.12 times more likely to have any medical follow-up (95% CI: 1.10, 1.13) and 1.13 times more likely to have a primary care visit (95% CI: 1.12, 1.15). Facilities with a greater proportion of patients receiving SIC had increased medical follow-up (RR: 1.04, 95% CI: 1.01, 1.07) and primary care engagement (RR: 1.06, 95% CI: 1.03, 1.10).
Conclusion: The VHA’s SIC policy is associated with increased medical follow-up among patients initiating LTOT, which may result in improved patient safety and has implications for other healthcare settings.
Keywords: chronic pain, opioid therapy, informed consent, care engagement
Introduction
The increased morbidity and mortality risks associated with long-term opioid therapy (LTOT) are well established in the clinical and scientific literature.1,2 A recent systematic review found in the Cochrane library estimates that 78% of patients using opioids for chronic pain management experience adverse events, such as nausea, constipation, and dizziness, and 7.5% experience serious adverse events such as addiction, suicide, overdose, and death.2 Between 1999 and 2015, more than 183,000 people died from overdoses related to prescription opioids. While the overall opioid prescription rate in the US has decreased almost 20% in the past 10 years, the LTOT prescription rate has increased by 40%.3
The opioid epidemic in the US has particularly impacted Veterans.4 Although opioid prescribing has declined in the VHA since 2015, at the end of 2018, 3.9% of Veterans in VHA received LTOT in the previous year.5 Veterans seeking VHA care have higher rates of chronic pain, mental health, and substance abuse conditions than the general US population, which may put them at comparatively higher risk of opioid-related adverse events.6–12 The opioid overdose prevalence among VHA patients is about 7 times greater than that of commercial health plan patients.13 Additionally, VHA patients have higher rates of suicide than the US population, and opioids may be used for intentional overdoses.14
To reduce opioid-related morbidity and mortality among Veterans, the VHA has implemented a multifaceted, patient-centered approach.15 This approach includes (1) improving patient and provider education on safe pain management strategies; (2) emphasizing an interdisciplinary team model for pain management; (3) promoting evidence-based nonpharmacologic treatment strategies; (4) monitoring risky prescribing practices; and (5) improving access to medication for addiction treatment for patients with opioid use disorder. Furthermore, in 2014, a VHA directive was released to implement a policy requiring a signature informed consent (SIC) process for patients initiating LTOT.16 The policy requires that, prior to initiating LTOT for pain, VHA prescribers provide standardized patient education and a conversation with the patient about risks and benefits of opioid therapy, as well as nonpharmacologic and non-opioid treatment options. The patient and provider work together to develop a treatment plan, which can include non-pharmacologic (eg, cognitive-behavioral therapy) and pharmacologic treatment for pain. Consistent with VHA policy regarding treatments with significant risk of complications or morbidity, the policy also requires the prescriber to obtain the patient’s written informed consent for the treatment plan.16–18
The policy prohibits the use of opioid pain care agreements in VHA and requires clinicians to replace existing opioid pain care agreements with the SIC process. The former is not preferred because it is based on a legalistic model that may seem more adversarial than therapeutic. Patient care contracts, such as an opioid pain care agreement, may use coercive language that patients perceive as intimidating or threatening, leading to distrust between patients and providers and decreased likelihood of seeking needed care.19–23 Their potential for stigmatizing patients may result in undertreatment of pain, physician refusal to prescribe opioids, and patient refusal to agree to perceived unfair and one-sided terms. Furthermore, the efficacy of pain care agreements on reducing aberrant opioid behaviors is uncertain; indicating that they may greatly harm the patient–provider relationship, while providing little benefit to patient safety. The ethical basis for the SIC requirement for LTOT is to show respect for the patient’s values and preferences, their right to accept or refuse any treatment or procedure, and promote shared decision-making – an approach where clinicians and patients work together to make decisions and select tests, treatments, and plans based on clinical evidence that balances risks and expected outcomes with patient preferences and values.24 Shared decision-making promotes patients’ trust in their caregivers and engagement with the healthcare system, which has been shown to improve health outcomes.25–30
Primarily, the SIC policy aims to encourage a patient–provider relationship characterized by respect, trust, and shared decision-making through a high-quality informed consent communication process. Improving patient–provider communication may also strengthen guideline-concordant opioid therapy. Both the Veterans Affairs/Department of Defense (VA/DoD) and Centers for Disease Control and Prevention (CDC) clinical guidelines for prescribing opioids for chronic pain recommend medical follow-up every 3 months after opioid initiation to reassess the benefits and risks of continued opioid therapy.1,31 We hypothesized that the SIC process would increase patient engagement, including medical follow-up after opioid initiation, which contributes to increased impact of risk mitigation strategies and reduces risk of serious adverse events.1
In order to assess the impact of the SIC policy on medical follow-up while minimizing the impact of other competing quality improvement initiatives, we selected an interrupted time series (ITS) design.32 This design allows comparison of outcomes in the same population before and after the implementation of a policy change while controlling for secular trends, seasonal patterns, and time-invariant confounders. We evaluated the extent to which the SIC policy for LTOT impacted patient engagement, as measured by any medical follow-up and primary care follow-up after opioid initiation.
Methods
Data Sources and Study Population
We identified patients who initiated LTOT between May 6, 2013 and May 5, 2016 using VHA’s Corporate Data Warehouse (CDW) – an administrative health data repository. Accordant with the SIC policy, we defined LTOT as receipt of any long-acting opioid prescription or receipt of short-acting opioid (excluding Schedule IV and V opioids) prescriptions providing at least 90 days of opioid supply within a 365-day period. We defined LTOT initiation as the first release date of an LTOT prescription, with no record of opioid receipt in the prior 90 days. We excluded patients who, in the year prior to initiation, had (1) a diagnosis code for cancer and an oncology visit or (2) a hospice care visit (see Supplementary Materials). These criteria were operationalized based on the SIC policy. Additionally, sex, age at LTOT initiation, race, ethnicity, and facility region were captured using CDW. Moreover, a patient’s most frequently self-reported race and ethnicity were mapped to a combined variable based on methods in the National Veterans Health Equity Report – FY13 Technical Appendix.33
Study Periods
Since VHA facilities were given one year to implement the SIC process after the policy was issued, we divided the study period into three periods, which we refer to as Year 1, Year 2, and Year 3. Year 1 (May 6, 2013 through May 5, 2014) was the year before the policy was issued. Year 2 (May 6, 2014 through May 5, 2015) was the year after the policy was issued when VHA facilities were to begin implementing the process for all eligible patients. Year 3 (May 6, 2015 through May 5, 2016) was the year in which the policy was to be fully implemented, when SIC was mandatory for all eligible patients.
Outcome Definitions
We considered two definitions of care engagement: (1) any medical follow-up within 30 days after LTOT initiation and (2) primary care follow-up within 3 months after LTOT initiation. These outcomes align with the VA/DoD and CDC clinical guideline recommendations for medical follow-up after opioid initiation and national performance metrics for measuring adherence to the VA/DoD guidelines.1,31,34 Any follow-up 30 days after opioid initiation was defined as any in-person or telephone visit to a VHA facility in the 30 days after opioid initiation. Primary care follow-up was defined as a visit to a clinic classified by a stop code for primary care (including home-based and telephone primary care), women’s health, geriatric care, general internal medicine, or mental health primary care.
Statistical Analyses
We used an ITS design to assess the impact of the SIC policy on study outcomes. In this study design, the study population (ie, patients initiating LTOT) is compared to itself during each study period (Year 1, Year 2, and Year 3). A strength of the ITS design is that it allows for the control of unmeasured time-invariant confounding and secular trends, including the effect of competing interventions already present during the entire study period.32 In addition, because the ITS design compares outcomes at the population-level, there is limited concern about bias by individual-level factors.
Patient-level data were aggregated to obtain counts of LTOT prescriptions initiated each month. For each month, we calculated the proportion of patients initiating LTOT with any medical follow-up within 30 days after initiation, and the proportion with a primary care visit within 3 months after initiation.
We hypothesized that there would be both an immediate increase (“level change”) and gradual increase over time in the outcomes of interest in Year 2 compared to Year 1, in response to the issuing of the SIC policy and the gradual adoption of the policy over the course of the year. Similarly, when comparing Year 3 to Year 1, we expected an increase in both the level and slope as a result of the policy becoming mandatory for all facilities in Year 1. We hypothesized that there would be an immediate increase in any medical follow-up within 30 days and primary care visits within 3 months after LTOT initiation between Years 2 and 3, but a less gradual increase over time in both outcomes in Year 3 compared to Year 2, corresponding to a negative slope change.
To test these hypotheses, we fit Poisson segmented regression models for each outcome. The models included time since the start of Year 1 as a continuous variable, indicator variables for Year 2 and Year 3, and interaction terms between continuous time and each indicator variable. The indicator variables for Year 2 and Year 3 were used to assess the SIC policy’s immediate impact in these years (ie, the “level change”), and the interaction terms between each indicator and continuous time were used to assess the impact of the SIC policy over time (“slope change”). Using these terms, we compared the SIC policy’s immediate impact and impact over time on care engagement in Years 2 and Years 3 compared to Year 1, and in Year 3 compared to Year 2. In addition, we assessed the effect of facility-level SIC coverage, the proportion of patients initiating LTOT in each month who received SIC at any time before or during their LTOT episode, on each outcome of interest.
We adjusted all models for VHA facility, opioid tier, and study month (to adjust for seasonality – patterns in the outcome that repeat according to time of year, and secular trends – global changes in the outcome that occur over a long period of time). Opioid tier was defined as the type of LTOT initiated – long acting, if any opioid prescriptions during a patient’s LTOT episode were for a long-acting opioid, otherwise, chronic short acting. This distinction was made because the SIC process and its impact on care engagement may vary between patients who initiated a long-acting opioid or who were initially started on a short-acting opioid and switched to a long-acting opioid, and patients who continued to stay on a short-acting opioid after LTOT was initiated. Because patients could receive care from multiple VHA facilities, we assigned each patient to a “home” facility in each study year, defined as their most frequently visited facility during that year. To address temporal autocorrelation due to seasonality and secular trends, we included a Fourier term for time (in months), which is a common approach for modelling seasonal patterns in time series data.32 The Durbin–Watson test was used to test for residual autocorrelation. To account for correlated observations within the same facility, we estimated cluster robust standard errors and 95% confidence intervals using a cluster bootstrap and the sandwich estimator (clustering on facility).
Our study was designed as a quality improvement project to evaluate a previously implemented VHA policy using administrative data. Thus, our evaluation was determined by the Institutional Review Board of Record, Stanford University, to not meet the definition of human subject research.
Results
Descriptive Results
During our study period, 409,895 unique patients experienced 758,416 SIC-eligible LTOT initiations. The number of LTOT initiations declined from 280,528 in Year 1 to 259,061 in Year 2 to 218,827 in Year 3 (Table 1). Distributions of age, sex, race/ethnicity, facility region, and opioid tier were similar across the three years. Most VHA patients initiating LTOT were male (92%), White (71%), and over 50 (84%). Eighty-six percent of LTOT initiations included only short-acting opioid prescriptions for the duration of therapy. Most initiations occurred at VHA facilities in the South (44%), followed by the West (27%), Midwest (21%), and Northeast (8%) regions. SIC coverage increased across the three study years. Since a few VHA facilities piloted the SIC process, 11% of patients initiating LTOT had a documented SIC in Year 1. In Year 2, 34% of patients had a documented SIC, and in Year 3 this increased to 59% of patients initiating LTOT. Care engagement also increased across the three years. In Year 1, 75% of patients had any medical follow-up in the 30 days after LTOT initiation, compared to 77% of patients in Year 2, and 78% in Year 3. The proportion of patients initiating LTOT who had a primary care visit in the 3 months after LTOT initiation increased from 75% in Year 1 to 78% in Year 2, and 80% in Year 3.
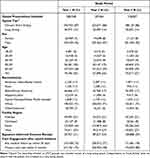 |
Table 1 Patient and Opioid Prescription Initiation Characteristics |
Facility-level SIC coverage increased after the policy was issued and continued to increase after full implementation (Figure 1). During Year 1, SIC coverage was mostly stable: the median SIC coverage was 9% (IQR: 6%, 16%) at the start of Year 1, and about 16% (IQR: 10%, 23%) at the end of that year. At the end of Year 2, the median SIC coverage increased to 50% (IQR: 34%, 64%), and at the end of Year 3, the median SIC coverage increased further to 63% (IQR: 45% 79%).
Interrupted Time Series Results
Any Medical Follow-Up Within 30 Days
There was a positive level-change in any medical follow-up within 30 days after LTOT initiation at the start of Year 2 and Year 3 after adjusting for opioid tier, facility, facility-level SIC coverage, and seasonality (Table 2). Compared to Year 1, there was an 8% increase in this outcome in Year 2 (95% CI: 1.07, 1.09), and an 12% increase in Year 3 (95% CI: 1.10, 1.13). Compared to Year 2, any medical follow-up increased by 4% in Year 3 (95% CI: 1.03, 1.05).
 |
Table 2 Association Between SIC Policy Implementation and Engagement After Opioid Initiation |
In Year 1, the proportion of patients initiating LTOT who had any medical follow-up within 30 days decreased by 0.8%/month (Figure 2A). Compared to Year 1, the slope for any medical follow-up increased by 0.7%/month in Year 2 (95% CI: 1.005, 1.008), and 0.4%/month in Year 3 (95% CI: 1.003, 1.005). However, compared to Year 2, the slope in Year 3 decreased by 0.3%/month (RR: 0.997, 95% CI: 0.996, 0.998), suggesting that the effect of the SIC policy waned over time.
Primary Care Visit Within 3 Months
There was a positivelevel-change in primary care visits within 3 months after LTOT initiation at the start of Year 2 and Year 3, after adjusting for opioid tier, facility, facility-level SIC coverage, and seasonality (Table 2). Compared to Year 1, the proportion of patients initiating LTOT who had a primary care visit in the 3 months after initiation increased by 8% in Year 2 (95% CI: 1.08, 1.09) and 13% in Year 3 (95% CI: 1.12, 1.15). Compared to Year 2, there was a 5% increase in primary care follow-up at the start of Year 3 (95% CI: 1.04, 1.06).
In Year 1, the proportion of patients initiating LTOT who had a primary care visit was decreasing by 0.8% per month (Figure 2B). Compared to Year 1, the slope for this outcome increased by 0.6%/month in Year 2 (95% CI: 1.005, 1.007), and 0.3% in Year 3 (95% CI: 1.001, 1.004). However, when compared to Year 2, the slope in Year 3 decreased by 0.3%/month (95% CI: 0.996, 0.998), again, suggesting that the effect of the policy waned over time.
SIC Coverage
After adjusting for study period, facility, opioid tier, and seasonality, SIC coverage was associated with significant increases in any medical follow-up within 30 days after LTOT initiation, and primary care visits within 3 months after LTOT initiation. A 1% increase in SIC coverage among patients from the same facility, in the same opioid tier, and in the same month was associated with a 4% increase in medical follow-up (95% CI: 1.01, 1.07), and a 6% increase in primary care visits (1.03, 1.10).
Residual Autocorrelation
The Durbin Watson test was not statistically significant for residual autocorrelation after including Fourier terms for time, facility, facility-level SIC coverage, and opioid tier for both outcomes (all p > 0.4).
Discussion
Implementation of the VHA’s SIC policy was associated with increased medical follow-up and primary care engagement. Our findings suggest that informing and engaging patients in the decision-making process regarding their pain management may increase their care engagement. The SIC policy is grounded in patient-driven health care, and encourages providers and patients to see “informed consent” as a means of ensuring shared decision-making through iterative communication rather than a one-time event.16 This emphasis on process is an important shift away from the varying opioid pain care agreements, which have the potential to stigmatize patients, and may be perceived as coercive, punitive, demeaning, or intimidating, straining the patient–provider relationship and resulting in decreased quality of care.19,20,35
It appears that providers who provide SIC and educational materials about taking opioids responsibly may have patients who are more likely to return for medical follow-up after opioid initiation, which is in line with opioid clinical practice guidelines. Our findings may relate to improvements in the patient–provider relationship due to the SIC process. Previous research demonstrates that when patients and providers have shared goals and mutual trust, the patient–provider relationship is strengthened.36–39 In the context of LTOT initiation, engaging patients in a shared decision-making process like SIC can demonstrate that the provider’s therapy recommendations encompass respect for patient autonomy, their right to accept or refuse any medical treatment, and concern for the patient’s well-being, rather than mistrust.39 This may lead to patients who are more likely to implement and maintain treatment.
Importantly, despite initial increases in patient engagement after implementation of the SIC policy, the effect of the policy on engagement waned over time, which has implications for future iterations of the SIC policy for LTOT. The current SIC policy only mandates additional informed consent conversations if (1) there is significant deviation from the treatment plan to which the patient originally consented, or (2) there is a change in the patient’s condition or diagnosis that would reasonably be expected to alter the original informed consent. Our findings suggest that it may be necessary to re-visit SIC periodically to continue to realize the patient engagement benefits of the policy. Additionally, our findings indicate that facilities with higher proportions of patients with SIC also had higher proportions of patients who had any medical follow-up within 30 days and primary care visits within 3 months after opioid initiation. This suggests that increasing facility-level SIC coverage may improve facility-level rates of care engagement after opioid initiation. At the end of our study period, only 59% of VHA patients who had a long-acting or chronic short-acting opioid prescribed at the VHA in the past year had documented receipt of SIC, and as of 2019, 89% of these patients had a documented SIC in their EHR.5 In addition, further research is needed to assess the impact of the SIC policy on longer-term care engagement, measured by receipt of multiple follow-up visits at recommended intervals after LTOT intervals. Finally, this study sought to examine the effects of the SIC policy on provider’s adherence to guidelines for medical follow-up following LTOT initiation and our findings reflect the impact of the SIC policy on prescribing practices. Future research is needed to understand the impact of the SIC policy change on patient perspectives as well. Additional evaluation of the impact of the SIC policy on no-shows and appointments cancelled by patients could provide an additional patient-centric context for the SIC policy’s effect on patient engagement following LTOT initiation.
In this study, we conducted a pragmatic evaluation of the real-world impact of a SIC policy on patient care engagement after opioid initiation in a large national healthcare system. Due to the urgency surrounding the opioid epidemic, particularly for veterans on LTOT, the VHA implemented the SIC process for LTOT for chronic pain without a rigorous research design, such as an RCT or staged roll-out. We developed an analytic approach using the interrupted time series design that would allow us to examine this national quality improvement initiative, since more rigorous research designs were not possible. A major concern with studying the impact of opioid therapy-related policies in the VHA or any healthcare system is confounding by competing policies and interventions. There may have also been variation in the implementation of competing interventions across facilities. For example, the Opioid Safety Initiative (OSI) was introduced in VHA in 2013, prior to the start of our study period, and was primarily focused on reducing risky opioid prescribing practices.15 In addition, the CDC updated the Guideline for Prescribing Opioids for Chronic Pain in March 2016, near the end of our study period. Competing interventions that were present prior to the start of the study period are not likely to have affected the study results, because their effect would have been included in the baseline effect estimates in Year 1 of the study. However, as is the case of the OSI, if the effect of competing interventions changed over time, especially if these changes coincided with the break points between the three study periods, we cannot adequately separate the impacts of other policies from the impacts of the SIC policy on LTOT follow-up. Since the updated CDC guidelines only overlapped our study period by three months, their confounding effects are likely minimal. In addition, because the ITS design compares the study population to itself across time, there is little concern about individual-level or time-invariant confounding. By adjusting for facility, opioid tier, and facility-level care utilization, we accounted for potential sources of confounding and heterogeneity in the impact of the SIC policy across facilities and across opioid tiers. Because of these strengths, we chose to use an ITS design to conduct our evaluation rather than an alternative individual-level observational design (eg, a cohort or case–control study). Although further evaluation (eg, a randomized control trial) would be needed to estimate a causal effect, our evaluation provides evidence from a pragmatic clinical setting that the SIC process may indeed improve patient engagement.
Our study has several limitations. First, the ITS design evaluates population-level impacts and cannot be used to draw conclusions about individual-level impacts. Further research is needed to understand mechanisms by which the SIC process impacts care engagement for an individual. Second, other initiatives aimed at improving safe opioid prescribing coincided with our evaluation and may have affected care engagement after opioid initiation. Furthermore, it is possible that with the decreases in opioid prescribing over the course of our study period, the population of patients initiating LTOT may have changed in complexity. Over time, providers may have reduced their LTOT prescribing so that only those with strong indications for LTOT or those who exhausted all other treatment options were initiated. Such a change may result in a more complex LTOT population with greater need for follow-up regardless of the impacts of the SIC policy. We address this by adjusting for opioid tier and facility – to account for differences in the reasons for initiating LTOT and facility-level prescribing practices. Data to account for changes in patient indications for LTOT were not available in this analysis, so residual unmeasured confounding due to other factors that may affect reasons for LTOT initiation may exist. Third, care engagement is an intermediate outcome in patient safety and there may be other factors associated with provision of safe opioid therapy. However, medical follow-up after opioid initiation is a part of guideline-concordant opioid therapy and has been shown to improve patient safety and reduce serious adverse events. Additionally, our study outcomes were defined as any medical follow-up within 30 days or primary care follow-up within 3 months. Although these definitions are consistent with VA/DoD Clinical Practice Guidelines, we were not able to discern how the follow-up visits were used by providers using administrative data. In order to assess what impact follow-up visits had on improving patient safety and reducing serious adverse events, future research should examine clinical follow-up notes, using medical chart abstraction or natural language processing. Fourth, our study population was restricted to VHA patients who initiated LTOT within the VHA. We may not have captured all Veterans receiving LTOT for chronic pain, as many Veterans likely access commercial insurance, Medicare, and Medicaid. The SIC policy within the VHA was specifically intended for patients receiving LTOT within the VHA, and it did not require that those receiving LTOT outside the VHA be included in the SIC requirement. Thus, any increased primary care engagement we might see is likely targeting those who received LTOT in the VHA. By restricting our study population to patients initiating LTOT within the VHA, we assessed the impact of the SIC policy on the population that it would have directly impacted. Finally, the policy implemented a patient-driven process involving shared decision-making to establish a mutual understanding of goals, risks, benefits and alternatives to opioid therapy, eventually resulting in completion of a SIC. Our definition of this, as evidence of SIC in administrative data, may imperfectly measure the entire informed consent process.
In conclusion, our findings suggest that system-wide policies fostering shared decision-making and ethical healthcare practices, such as informed consent can improve patient engagement in a large, national healthcare system, which could improve patient safety. Further research is needed to focus on understanding the impact of the VHA’s SIC policy on other measures of guideline-concordant opioid therapy and on serious adverse events.
VA Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Disclosure
Dr Jodie Trafton reports additionally working for this non-profit Institute for Brain Potential that provide continuing medical education around behavioral health topics. Findings from this and other related work may be included in the education as they are useful to inform clinical care and understanding of behavioral health disorders and their treatment, outside the submitted work. The authors report no conflicts of interest in this work.
References
1. Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guidelines for opioid therapy for chronic pain; 2016.
2. Els C, Jackson TD, Kunyk D, et al. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;10:CD012509. doi:10.1002/14651858.CD012509.pub2
3. Centers for Disease Control and Prevention. 2018 annual surveillance report of drug-related risks and outcomes - United States; 2018.
4. Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the veterans health administration. Pain Pract. 2014;14(5):437–445. doi:10.1111/papr.12097
5. Patient Safety VHA. Opioid Therapy Guideline Adherence Report. Center of Inquiry; 2019.
6. Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the veterans health administration. Pain Pract. 2013;14(5):437–445. doi:10.1111/papr.12097
7. Toblin RL, Quartana PJ, Riviere LA, Walper KC, Hoge CW, Pain C. Opioid use in US soldiers after combat deployment. JAMA Intern Med. 2014;174(8):1400. doi:10.1001/jamainternmed.2014.2726
8. Seal KH, Cohen G, Waldrop A, Cohen BE, Maguen S, Ren L. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001–2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116(1–3):93–101. doi:10.1016/j.drugalcdep.2010.11.027
9. Hoglund MW, Schwartz RM. Mental health in deployed and nondeployed veteran men and women in comparison with their civilian counterparts. Mil Med. 2014;179(1):19–25. doi:10.7205/MILMED-D-13-00235
10. Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of operations enduring freedom and Iraqi freedom: table 1. Pain Med. 2006;7(4):339–343. doi:10.1111/j.1526-4637.2006.00146.x
11. Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage. 2002;23(2):131–137. doi:10.1016/S0885-3924(01)00396-7
12. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252–3257. doi:10.1001/archinte.160.21.3252
13. Blow FC, Bohnert ASB, Ilgen MA, et al. Suicide mortality among patients treated by the veterans health administration from 2000 to 2007. Am J Public Health. 2012;102(S1):S98–S104. doi:10.2105/AJPH.2011.300441
14. Bohnert ASB, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64–70. doi:10.1176/appi.ajp.2011.10101476
15. Gellad WF, Good CB, Shulkin DJ. Addressing the Opioid Epidemic in the United States. JAMA Intern Med. 2017;177(5):611. doi:10.1001/jamainternmed.2017.0147
16. Veterans Health Administration. VHA directive 1005 informed consent for long-term opioid therapy for pain; 2014.
17. Veterans Health Administration. VHA handbook 1004.01: informed consent for clinical treatments and procedures; 2009.
18. Veterans Health Administration. 38 CFR § 17.32 - informed consent and advance care planning; 2009.
19. Arnold RM, Han PKJ, Seltzer D. Opioid contracts in chronic nonmalignant pain management: objectives and uncertainties. Am J Med. 2006;119(4):292–296. doi:10.1016/j.amjmed.2005.09.019
20. Tobin DG, Keough Forte K, Johnson S. Breaking the pain contract: a better controlled-substance agreement for patients on chronic opioid therapy. Cleve Clin J Med. 2016;83(11):827–835. doi:10.3949/ccjm.83a.15172
21. Fishman SM, Bandman TB, Edwards A, Borsook D. The opioid contract in the management of chronic pain. J Pain Symptom Manage. 1999;18(1):27–37. doi:10.1016/S0885-3924(99)00035-4
22. Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioeth. 2010;10(11):5–12. doi:10.1080/15265161.2010.519425
23. Buchman DZ, Ho A. What’s trust got to do with it? Revisiting opioid contracts. J Med Ethics. 2014;40(10):673–677. doi:10.1136/medethics-2013-101320
24. Department of Health and Human Services National Learning Consortium. Shared decision making fact sheet - December 2013; 2013.
25. Pergolizzi JV, Curro FA, Nanada C, et al. A multicentre evaluation of an opioid patient-provider agreement. Postgrad Med J. 2017;93(1104):613–617. doi:10.1136/postgradmedj-2016-134607
26. Chittle M, Oklu R, Pino RM, et al. Sedation shared decision-making in ambulatory venous access device placement: effects on patient choice, satisfaction and recovery time. Vasc Med. 2016;21(4):355–360. doi:10.1177/1358863X16643602
27. Lé Garé F, Thompson-Leduc P. Twelve myths about shared decision making. Patient Educ Couns. 2014;96(3):281–286. doi:10.1016/j.pec.2014.06.014
28. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2021;27:1361–1367. doi:10.1007/s11606-012-2077-6
29. Rubin M. Shared medical decision making in consideration of opioid therapy in a patient with restless legs syndrome. Contin Lifelong Learn Neurol. 2017;23(4,Sleep Neurology):1151–1155. doi:10.1212/con.0000000000000493
30. Clark MR. Chronic opioid therapy for chronic pain: an e-learning program to develop shared decision-making and communication skills. Curr Pain Headache Rep. 2011;15(2):88–90. doi:10.1007/s11916-011-0175-5
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi:10.15585/mmwr.rr6501e1er
32. Lopez Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2016;dyw098. doi:10.1093/ije/dyw098
33. VA Office of Health Equity. National veteran health equity report - FY2013; 2013.
34. Midboe AM, Lewis ET, Paik MC, et al. Measurement of adherence to clinical practice guidelines for opioid therapy for chronic pain. Transl Behav Med. 2012;2(1):57–64. doi:10.1007/s13142-011-0104-5
35. Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712. doi:10.7326/0003-4819-152-11-201006010-00004
36. Légaré F, Ratté S, Stacey D, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. In: Légaré F, editor. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2010:CD006732. doi:10.1002/14651858.CD006732.pub2
37. Matthias MS, Salyers MP, Frankel RM. Re-thinking shared decision-making: context matters. Patient Educ Couns. 2013;91:176–179.
38. Montori VM, Gafni A, Charles C. A shared treatment decision-making approach between patients with chronic conditions and their clinicians: the case of diabetes. Health Expect. 2006;9(1):25–36. doi:10.1111/j.1369-7625.2006.00359.x
39. Matthias MS, Krebs EE, Bergman AA, Coffing JM, Bair MJ, Matthias S. Communicating about opioids for chronic pain: a qualitative study of patient attributions and the influence of the patient-physician relationship. Eur J Pain. 2014;18(6):835–843. doi:10.1002/j.1532-2149.2013.00426.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


