Back to Journals » ClinicoEconomics and Outcomes Research » Volume 10
Impact of comorbidity on the risk and cost of hospitalization in HIV-infected patients: real-world data from Abruzzo Region
Authors Cammarota S , Citarella A , Manzoli L, Flacco ME , Parruti G
Received 16 January 2018
Accepted for publication 11 April 2018
Published 23 July 2018 Volume 2018:10 Pages 389—398
DOI https://doi.org/10.2147/CEOR.S162625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Dean Smith
Simona Cammarota,1 Anna Citarella,1 Lamberto Manzoli,2,3 Maria Elena Flacco,4 Giustino Parruti5
1LinkHealth s.r.l., Health Economics, Outcomes & Epidemiology, Naples, Italy; 2Department of Medicine Sciences, University of Ferrara, Ferrara, Italy; 3Regional Healthcare Agency of Abruzzo, Pescara, Italy; 4Local Health Unit of Pescara, Pescara, Italy; 5Infectious Diseases Unit, Pescara General Hospital, Pescara, Italy
Background: Due to the success of antiretroviral therapy, human immunodeficiency virus (HIV) infection has been transformed into a lifelong condition. In Italy, little is known about the impact of comorbidities (CMs) on the risk of hospitalization and related costs for people who live with HIV (PWLHIV). The objective of the study was to quantify the risk of hospitalization and costs associated with CMs in an Italian cohort of PWLHIV.
Methods: The study population included subjects aged ≥18 years with HIV infection, identified in the Abruzzo’s hospital discharge database among files stored from 2004 until 2013 and then followed up until December 2015. Patients’ CMs (Charlson Comorbidity Index [CCI)] were extracted from International Classification of Diseases, Ninth Revision, Clinical Modification codes in the hospital discharge abstracts. Poisson regression was used to compare the incidence rate of hospital admissions in patients with and without each CM class. Incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were adjusted for age, sex and the other CMs. A generalized linear model under gamma distribution was used to estimate adjusted mean hospital costs. Costs were derived from official Italian Diagnosis-related group (DRG) based reimbursements.
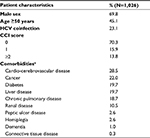
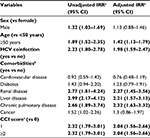
Results: Among 1,026 HIV patients identified (mean age 47 years), 30% had at least one CM and 14.5% underwent hospital admission during the follow-up period. The risk of acute hospitalization significantly increased among patients with hepatitis C virus (HCV) coinfection (adjusted IRR 1.98; 95% CI: 1.59–2.47), renal (adjusted IRR 2.27; 95% CI: 1.45–3.56), liver (adjusted IRR 2.21; 1.57–3.13) and chronic pulmonary CMs (adjusted IRR 2.31; 1.63–3.32). Adjusted mean hospital costs were €2,494 in patients without CMs and €4,422 and €9,734 in those with CCI=1 or CCI ≥2, respectively.
Conclusion: The presence of renal, liver and chronic pulmonary CMs, as well as HCV coinfection doubled the risk of hospitalization in the PWLHIV cohort. A CCI ≥2 is associated with a fourfold increase in hospitalization costs. Our study provides new evidence that CMs in PWLHIV increase the risk of hospitalization and local health service facilities.
Keywords: HIV, comorbidity, hospitalization, real-world data, inpatient cost, administrative data
Corrigendum for this paper has been published
Introduction
During the last 20 years, the advent and the therapeutic success of highly active antiretroviral therapy (HAART) transformed human immunodeficiency virus (HIV) infection into a lifelong condition.1–4 Mortality rates among people who live with HIV (PWLHIV), however, remain 3–15 times higher than those recorded in the general population.5–8 Furthermore, more than half of the deaths observed in PWLHIV are at present attributable to noninfectious comorbidities (CMs), as PWLHIV are more likely to experience age-related CMs including cardiovascular disease, diabetes mellitus and renal failure, compared to uninfected individuals.9,10 Results from several studies have shown that PWLHIV might have a higher prevalence of many noninfectious CMs at an earlier age than age-matched uninfected subjects.11,12 This effect may be caused by HIV infection itself, antiretroviral toxicity or coinfections, such as hepatitis C virus (HCV) coinfection.13–15 CMs complicate caring infrastructures for HIV-infected patients, because patients with multiple diseases and on complex pharmacological treatments are particularly difficult to manage, both in chronic and in acute phases.16,17
The increasing prevalence of CMs may enlarge the clinical, social and financial burden of HIV infection, since CMs may well impact both patients’ well-being and the resources used. So, gathering better knowledge of factors that may affect the risk of hospital utilization by PWLHIV in the HAART era may well be an important goal both for clinicians and policy makers in the strive to understand how health care needs are probably changing in this population.18–20 However, little is known at present on such a crucial issue. The aim of this study was to quantify the risk of hospitalization and costs associated with CMs in HIV-infected patients in a regional Italian cohort.
Methods
Data source and study population
The team carried out a longitudinal retrospective cohort study using data extracted from the Abruzzo’s hospital discharge database from January 1, 2004 to December 31, 2015. All admission data in any Italian hospital related to Abruzzo’s residents were collected. The hospital discharge database was linked to the civil registry through a unique encrypted personal identification code to collect demographic information (ie, date of birth, sex and date of death). Because this automated system is anonymous, according to the Italian Data Protection Authority, neither ethical committee approval nor informed consent was required for this study. The Regional health authorities routinely use anonymous data file for epidemiological and administrative purposes.21,22
The study population included Abruzzo Region residents, 18 years of age and older, who were admitted in any Italian hospital with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for HIV-infection (ICD-9 042, V08, 795.71 or 079.53), in primary or secondary diagnoses, from 2004 to 2013.
Each individual accumulated person-years of follow-up from January 1, 2014 until the date of death or to December 31, 2015.
Covariates
For each patient, the following variables were assessed at baseline (January 1, 2014): age, sex, HCV coinfection presence (ICD-9 070.44, 070.54, 070.70, 070.71 in the primary or secondary diagnoses) and CMs by using Charlson Comorbidity Index (CCI).23 Age was categorized as <50 and ≥50 years considering that the proportion of HIV patients aged 50 years and older is increasing consequently to the therapeutic success of HAART.24 Additionally, HIV patients aged 50 years and older had a higher percentage of CMs compared to the younger group (<50 years).25 CCI includes myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, diabetes as well as other illnesses such as dementia, liver disease, pulmonary disease, rheumatic disease, etc (AIDS/HIV condition is not included among CMs). To evaluate the presence of each CM, we used the diagnostic information recorded in all hospital discharges (ordinary and day hospital admissions) over the 10-year period (from 2004 to 2013). Finally, according to CCI score, patients were classified into three groups: subjects without CMs (CCI=0), those with a CCI=1 and those with a CCI ≥2.
Clinical and economic outcomes
The primary outcome of this study was the number of all-cause hospitalizations during the follow-up period (ie, from January 1, 2014 to December 31, 2015). To evaluate the number of hospitalizations for acute events, we used only ordinary hospital admissions (day hospital admissions were excluded). We also investigated the specific cause of hospitalization using the diagnostic categories identified by the Agency for Healthcare Research and Quality as reference.26 To determine the cause of hospitalization among PWLHIV, we first identified the primary diagnosis for the hospitalization using the first-listed ICD-9 code that did not refer to HIV (ICD-9 042, V08, 079.53, 795.71) or HCV (ICD-9 070.44, 070.54, 070.70, 070.71). Then, we assigned the primary diagnosis to the following mutually exclusive categories: AIDS-defining illnesses, non-AIDS-defining infections, non-AIDS-defining cancers, cardiovascular, gastrointestinal or liver, pulmonary, endocrine or metabolic, renal or genitourinary, psychiatric, injury or poisoning.27,28
The secondary outcome was the estimation of the inpatient hospital cost predictors. For this analysis, the third-party payer perspective was adopted and therefore both ordinary and day hospital admissions were evaluated. Costs were calculated as the sum of all the claims related to HIV subjects recorded during the identified biennium (ie, from January 1, 2014 to December 31, 2015). The hospital costs were calculated using the CMS-DRG version 24 grouping algorithm.29 Costs were expressed in euro, as average cost per hospitalized patient per year.
Statistical analysis
The effect of each CM class and CCI group on the risk of hospitalizations was estimated through the Poisson regression analyses. The models compared the number of hospital admissions in patients with and without each CM class and in patients with CCI=1 and CCI ≥2, relative to those without CMs. The results are shown as unadjusted and adjusted incidence rate ratios (IRRs) with 95% CIs. Age at the start of follow-up (≤50 and >50 years), sex and HCV coinfection were included as potential confounders for adjusted IRRs.
In order to determine hospital costs predictors, we used multivariable generalized linear models with a logarithmic link and gamma distribution. The advantage of this type of model is that it considers the skewed nature of cost data and it produces β-coefficients that are interpreted as the relative increase in mean cost for each increment in the covariate. Covariates included in the model were age, sex, HCV coinfection and CCI group. All analyses were conducted using STATA software, version 11 (Stata Corp, College Station, TX, USA).
Results
Characteristics of study cohort
Between 2004 and 2013, a total of 1,026 patients who met our study criteria were identified. Of these, 67.8% were males and 45.1% were aged 50 years and older. As shown in Table 1, 23.1% of HIV patients had HCV coinfection and 29.7% experienced at least one CM (15.9% CCI=1 and 13.8% CCI ≥2). Among patients with a CCI score >0, 28.5% had cardiovascular disease, 22% cancer, 19.7% diabetes, 19.7% liver disease, 18.7% chronic pulmonary disease and 10.5% renal disease (Table 1, Table S1).
Outcomes
During the 2-year follow-up, 17.0% of HIV-infected patients underwent hospital admission(s): 12.3% of patients without CMs, 25.2% with CCI score=1 and 31.7% with a CCI score ≥2 (p<0.0001). Among those aged >50 years, these proportions rose to 14.2%, 26.7% and 36.3%, respectively (Figure 1).
In total, 341 hospital admissions for acute events occurred during the follow-up period. Of these, 206 (~60%) were HIV-related hospitalizations. Figure 2 shows the distribution of HIV-related hospitalizations stratified by specific diagnostic categories. AIDS-defining illnesses (26.7%) were the most common diagnoses, followed by non-AIDS-defining infections (15.0%), cardiovascular disease (14.1%) and pulmonary disease (12.1%).
  | Figure 2 Percentage of hospital admissions during the 2-year follow-up stratified by diagnostic category. |
Table 2 presents the results of the search for factors associated with all-cause hospitalization. The presence of at least one CM in HIV patients was the strongest predictor of all-cause hospitalization with an adjusted IRR of 2.68 (95% confidence interval [CI]: 1.94–3.70) for patients with CCI score=1 and 2.99 (95% CI: 2.15–3.70) for patients with CCI score ≥2, compared with those without CMs. The risk of hospitalization significantly increased among patients with renal (adjusted IRR 2.27; 95% CI: 1.45–3.56), liver (adjusted IRR 2.21; 95% CI: 1.57–3.13) and chronic pulmonary CMs (adjusted IRR 2.31; 95% CI: 1.63–3.32). In addition, the risk of hospitalization increased in subjects with HCV coinfection (adjusted IRR=1.98: 95% CI: 1.59–2.47) compared with HIV-mono-infected patients. Finally, age >50 years was an independent risk factor associated with hospitalization (adjusted IRR=1.42: 95% CI: 1.13–1.79). The analysis of factors associated with HIV-related hospitalization produced similar results (Table S2).
Predictors of hospital costs
Table 3 presents the unadjusted and adjusted hospital costs estimated by general linear model for the study cohort. HIV patients with CMs had higher adjusted hospital costs compared with those without CMs. Adjusted mean annual costs per hospitalized patients were €2,494 in patients without CMs versus €4,422 and €9,734 in those with CCI=1 or CCI ≥2, respectively. Other factors including age, sex and HCV coinfection were not statistically significant for hospital cost evaluation. Adjusted annual costs per hospitalized patient for HIV-related conditions are reported in Table S3.
Discussion
HIV-infected patients may have a near-normal life expectancy in the modern HAART era.3,4 For these patients, in 2010, the Italian Government and associated Health Care Agencies stated that inpatient hospitalization may be at high risk of not being appropriate.30 Nonetheless, nationwide, Italy has high rates of late presentation and many patients still suffer from AIDS-related clinical complications at presentation or in the following months until immune recovery.31,32 Guaraldi et al showed that late presentation for HIV diagnosis is associated with higher prevalence of non-infectious CMs and multimorbidity when compared to uninfected HIV people and it is also associated with higher total care costs.33 Seng et al emphasize late presentation as an important predictor of hospitalizations especially for HIV-related events in the French context.34 Moreover, it is well known that aging with HIV is a complex clinical problem, as CMs in HIV infection increase with age.16,17,35,36 In fact, HIV patients have documented accelerated aging at the vascular level; they often present with increased levels of blood atherogenic lipids due to antiretrovirals and frequently suffer from renal and bone toxicities due to both HIV and HAART drugs.9–12,37–40 Chronic pulmonary diseases have been lately documented as frequent CMs in people aging with HIV, and end-stage liver disease, often associated with HCV coinfection, is definitely more recurrent and severe among these patients.41–44 Finally, HIV unrelated neoplasms have been reported with increased frequency in recent years.5,45
With regard to this background, we wondered to what extent acute hospitalizations occur for patients with chronic HIV infection in the Abruzzo Region, which are the main causes of hospital admission, and what is the burden of CMs in our HIV population.
Although hospital admissions of HIV-infected patients are deemed potentially inappropriate by Regulatory Agencies, our findings show that 17% of HIV-infected patients are hospitalized during the 2 years of follow-up and the main cause of hospital admissions is for an AIDS-defining illness. Acknowledging the paucity of information about this issue, in our country as well as in other countries, our analysis may be relevant to understand how HIV infection may still be causing health care resources utilization as well as health care expenditure in the present setting, beyond HIV testing, HAART expenditure and continuous efforts to improve high quality retention in care.18–20,46
First, this study provides a new line of evidence, which is that patients with at least one comorbid condition represent approximately one-third of our sample (ie, patients with at least one hospitalization for HIV between 2004 and 2013), with an even higher proportion among those aged 50 years or more. In line with many other reports showing that the burden of CMs is on the rise in the aging HIV population, this evidence indicates that health care resources will still be necessary in the near future, likely in growing amount, to assist PWLHIV in the ordinary settings of care.35,36,40,42,47
Second, we found that cardiovascular, renal and pulmonary CMs, as well as HCV coinfection, are significantly associated with an increased risk of acute hospitalizations among PWLHIV, in line with other reports in the literature.18,20,46,48 These findings contribute to the idea that a more stringent control of such CMs may well be necessary in the setting of chronic management of HIV infection. Although much has been done in terms of research and development of newer antiretrovirals, with a more favorable profile of toxicity in all lines of HAART, efforts to reduce the prevalence of CMs in HIV patients need to be increased rather than reduced.20,48 Furthermore, these findings also reinforce the view that treating HCV coinfection, as a priority in the setting of PWLHIV in the era of new direct acting antivirals, is a mandatory and urgent task.49
Finally, our hospitalizations cost analysis suggests that any effort and cost to accomplish the above-mentioned priorities may, at least partially, be defrayed by a likely reduction in increased costs paid by health services to assist PWLHIV in the setting of acute hospitalizations. Indeed, our analyses reveal that acute hospitalizations for comorbid HIV patients are costly, with up to four times higher expenditure compared with acute hospitalizations of non-comorbid PWLHIV, in line with other lines of evidence.18,20,48 This evidence highlights that appropriate tailoring of antiretroviral therapy in accordance with HIV-associated CMs needs to be considered in order to reduce such acute events.
The strengths of our study are the use of a large-scale, population-based cohort over an extended period and the ability to draw information from a real-world setting through the analysis of administrative data. Nonetheless, our study has also some limitations, mainly inherent to the use of health care administrative data. We based our analysis on administrative discharge data that are routinely collected and are not specifically focused on the study aims. Therefore, it is possible that ICD-9 codes for CMs or HCV coinfection are misreported or not reported, leading us to underestimate the number of HIV patients with CMs or coinfection. However, with regard to CMs, the cohort of HIV patients who had at least one CM at baseline represents about 30% of the overall sample in line with the figure depicted by a previous population-based study performed in a province of North Italy with high prevalence of HIV.50 Although multivariable analyses controlled for potentially confounding variables, it is possible that unmeasured confounding effects (ie, antiretroviral treatment, smoking and BMI) may have influenced the results. In addition, direct information on the duration of HIV infection, as well as on the proportion of patients with low (<350/mmc) or very low (<200/mmc) CD4 T-cell lymphocytes at the time of enrollment in our cohort, which influence the risk and the cost of hospitalizations, are not available in our data sources.50,51 However, late presentation in our local context was constantly at least as high as 50% in the study period (unpublished data), and systematic efforts on its prevention, actively started with a definite investment fund by our local Government since 2014, will likely positively impact the rate and cost of hospitalizations of HIV patients in the next years.52,53 It will also be of interest to gauge with the same method how such costs evolve in parallel in different Italian regions. Finally, generalizations of the study’s findings should be made with caution, particularly because data are limited to a single Italian region and to the hospital setting.
Conclusion
In conclusion, our evidence highlights the substantially increased risk of hospitalizations among HIV patients with one or more CMs and their subsequent relevant economic impact. Clinicians and policy makers should be aware of the clinical and economic implications of CMs among PWLHIV, as they may well drive decision making on how to allocate scarce health care resources on the most effective interventions for the prevention and the treatment of non-infectious CMs.
Acknowledgments
An abstract of this study was published in the Journal of The International Society for Pharmacoeconomics and Outcomes Research (http://dx.doi.org/10.1016/j.jval.2017.08.2260). Research funding from Gilead Sciences supported the present study, however, the funders had no role in the data collection and analysis and were not involved in the interpretation of the results.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. | ||
Bhaskaran K, Hamouda O, Sannes M, et al; CASCADE Collaboration. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300(1):51–59. | ||
Lewden C, Chene G, Morlat P, et al; Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO8 APROCO-COPILOTE Study Group; Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO3 AQUITAINE Study Group. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46(1):72–77. | ||
van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F; ATHENA national observational cohort study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24(10):1527–1535. | ||
Zucchetto A, Suligoi B, De Paoli A, et al. Excess mortality for non-AIDS-defining cancers among people with AIDS. Clin Infect Dis. 2010;51(9):1099–1101. | ||
Serraino D, Bruzzone S, Zucchetto A, et al. Elevated risks of death for diabetes mellitus and cardiovascular diseases in Italian AIDS cases. AIDS Res Ther. 2010;7:11. | ||
Suligoi B, Zucchetto A, Grande E, et al. Risk factors for early mortality after AIDS in the cART era: a population-based cohort study in Italy. BMC Infect Dis. 2015;15:229. | ||
Grande E, Zucchetto A, Suligoi B, et al. Multiple cause-of-death data among people with AIDS in Italy: a nationwide cross-sectional study. Popul Health Metr. 2017;15(1):19. | ||
De Socio GV, Ricci E, Parruti G, et al. Chronological and biological age in HIV infection. J Infect. 2010;61(5):428–430. | ||
Troya J, Bascuñana J. Safety and tolerability: current challenges to antiretroviral therapy for the long-term management of HIV infection. AIDS Rev. 2016;18(3):127–137. | ||
Mazzotta E, Ursini T, Agostinone A, et al. Prevalence and predictors of low bone mineral density and fragility fractures among HIV-infected patients at one Italian center after universal DXA screening: sensitivity and specificity of current guidelines on bone mineral density management. AIDS Patient Care STDS. 2015;29(4):169–180. | ||
De Socio GV, Ricci E, Maggi P, et al; CISAI study group. Time trend in hypertension prevalence, awareness, treatment, and control in a contemporary cohort of HIV-infected patients: the HIV and Hypertension Study. J Hypertens. 2017;35(2):409–416. | ||
Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. 2017;14(3):93–100. | ||
Naggie S. Hepatitis C virus, inflammation, and cellular aging: turning back time. Top Antivir Med. 2017;25(1):3–6. | ||
Unsal AB, Mattingly AS, Jones SE, et al. Effect of antiretroviral therapy on bone and renal health in young adults infected with HIV in early life. J Clin Endocrinol Metab. 2017;102(8):2896–2904. | ||
Guaraldi G, Malagoli A, Theou O, et al. Correlates of frailty phenotype and frailty index and their associations with clinical outcomes. HIV Med. 2017;18(10):764–771. | ||
Calcagno A, Piconi S, Focà E, et al; GEPPO (GEriatric Patients living with HIV/AIDS: a Prospective Multidimensional cOhort) Study Group. Role of normalized T-cell subsets in predicting comorbidities in a large cohort of geriatric HIV-infected patients. J Acquir Immune Defic Syndr. 2017;76(3):338–342. | ||
Fleishman JA, Monroe AK, Voss CC, Moore RD, Gebo KA. Expenditures for persons living with HIV enrolled in Medicaid, 2006–2010. J Acquir Immune Defic Syndr. 2016;72(4):408–415. | ||
Berry SA, Fleishman JA, Moore RD, Gebo KA. Thirty-day hospital readmissions for adults with and without HIV infection. HIV Med. 2016;17(3):167–177. | ||
Catumbela E, Freitas A, Lopes F, et al. HIV disease burden, cost, and length of stay in Portuguese hospitals from 2000 to 2010: a cross-sectional study. BMC Health Serv Res. 2015;15:144. | ||
Cammarota S, Bruzzese D, Catapano AL, et al. Lower incidence of macrovascular complications in patients on insulin glargine versus those on basal human insulins: a population-based cohort study in Italy. Nutr Metab Cardiovasc Dis. 2014;24(1):10–17. | ||
Cammarota S, Falconio LM, Bruzzese D, et al. Lower rate of cardiovascular complications in patients on bolus insulin analogues: a retrospective population-based cohort study. PLoS One. 2013;8(11):e79762. | ||
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. | ||
Smit M, Brinkman K, Geerlings S, et al; ATHENA observational cohort. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–818. | ||
Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126. | ||
Clinical Classifications Software (CCS) cpR. Rockville, MD: U.S. Agency for Healthcare Research and Quality; 2013. | ||
Crowell TA, Gebo KA, Balagopal A, Fleishman JA, Agwu AL, Berry SA; HIV Research Network. Impact of hepatitis coinfection on hospitalization rates and causes in a multicenter cohort of persons living with HIV. J Acquir Immune Defic Syndr. 2014;65(4):429–437. | ||
Berry SA, Fleishman JA, Moore RD, Gebo KA, HIV Research Network. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr. 2012;59(4):368–375. | ||
Fattore G, Torbica A. Inpatient reimbursement system in Italy: how do tariffs relate to costs? Health Care Manag Sci. 2006;9(3):251–258. | ||
Allegato B. Gazzetta Ufficiale della repubblica italiana del 5-1-2010; DRG ad alto rischio di non appropriatezza in regime di degenza ordinaria. [Official Journal of the Italian Republic; DRG at high risk of no fitness in the ordinary hospitalization]. Available from: http://www.gazzettaufficiale.it/eli/gu/2010/01/05/3/sg/pdf. Accessed June 29, 2018. | ||
Camoni L, Raimondo M, Dorrucci M, Regine V, Salfa MC, Suligoi B; CARPHA Study Group. Estimating minimum adult HIV prevalence: a cross-sectional study to assess the characteristics of people living with HIV in Italy. AIDS Res Hum Retroviruses. 2015;31(3):282–287. | ||
Taborelli M, Virdone S, Camoni L, et al. The persistent problem of late HIV diagnosis in people with AIDS: a population-based study in Italy, 1999–2013. Public Health. 2017;142:39–45. | ||
Guaraldi G, Zona S, Menozzi M, et al. Late presentation increases risk and costs of non-infectious comorbidities in people with HIV: an Italian cost impact study. AIDS Res Ther. 2017;14(1):8. | ||
Seng R, Mutuon P, Riou J, et al; COREVIH. Hospitalization of HIV positive patients: significant demand affecting all hospital sectors. Rev Epidemiol Sante Publique. 2018;66(1):7–17. | ||
Jourjy J, Dahl K, Huesgen E. Antiretroviral treatment efficacy and safety in older HIV-infected sdults. Pharmacotherapy. 2015;35(12):1140–1151. | ||
Greene M, Covinsky KE, Valcour V, et al. Geriatric syndromes in older HIV-infected adults. J Acquir Immune Defic Syndr. 2015;69(2):161–167. | ||
Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69(7):833–842. | ||
De Socio GV, Ricci E, Parruti G, et al. Statins and Aspirin use in HIV-infected people: gap between European AIDS Clinical Society guidelines and clinical practice: the results from HIV-HY study. Infection. 2016;44(5):589–597. | ||
Mazzotta E, Agostinone A, Rosso R, et al. Osteonecrosis in human immunodeficiency virus (HIV)-infected patients: a multicentric case-control study. J Bone Miner Metab. 2011;29(3):383–388. | ||
Jotwani V, Atta MG, Estrella MM. Kidney disease in HIV: moving beyond HIV-associated nephropathy. J Am Soc Nephrol. 2017;28(11):3142–3154. | ||
Goussard P, Gie RP. HIV-related chronic lung disease in adolescents: are we prepared for the future? Expert Rev Respir Med. 2017;11(12):969–975. | ||
Calligaro GL, Gray DM. Lung function abnormalities in HIV-infected adults and children. Respirology. 2015;20(1):24–32. | ||
Wittkop L. Editorial commentary: end-stage liver disease in HIV infection: an avoidable burden? Clin Infect Dis. 2016;63(9):1168–1170. | ||
Klein MB, Althoff KN, Jing Y, et al; North American AIDS Cohort Collaboration on Research and Design of IeDEA; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis. 2016;63(9):1160–1167. | ||
Zucchetto A, Virdone S, Taborelli M, et al. Non-AIDS-defining cancer mortality: emerging patterns in the late HAART era. J Acquir Immune Defic Syndr. 2016;73(2):190–196. | ||
Liu P, Dillingham R, McManus K. Hospital days attributable to immune reconstitution inflammatory syndrome in persons living with HIV before and after the 2012 DHHS HIV guidelines. AIDS Res Ther. 2017;14:25. | ||
Camoni L, Regine V, Raimondo M, Salfa MC, Boros S, Suligoi B. The continued ageing of people with AIDS in Italy: recent trend from the national AIDS Registry. Ann Ist Super Sanita. 2014;50(3):291–297. | ||
Long LC, Fox MP, Sauls C, Evans D, Sanne I, Rosen SB. The high cost of HIV-positive inpatient care at an urban hospital in Johannesburg, South Africa. PLoS One. 2016;11(2):e0148546. | ||
Allyn PR, O’Malley SM, Ferguson J, Tseng CH, Chew KW, Bhattacharya D. Attitudes and potential barriers towards hepatitis C treatment in patients with and without HIV coinfection. Int J STD AIDS. 2018;29(4):334–340. | ||
Quiros-Roldan E, Magoni M, Raffetti E, et al. The burden of chronic diseases and cost-of-care in subjects with HIV infection in a Health District of Northern Italy over a 12-year period compared to that of the general population. BMC Public Health. 2016;16(1):1146. | ||
Guaraldi G, Zona S, Menozzi M, et al. Cost of noninfectious comorbidities in patients with HIV. Clinicoecon Outcomes Res. 2013;5:481–488. | ||
Polilli E, Sozio F, Di Stefano P, Clerico L, Di Iorio G, Parruti G. Preliminary evaluation of the impact of a Web-based HIV testing programme in Abruzzo Region on the prevention of late HIV presentation and associated mortality. Int J Infect Dis. 2018;69:44–46. | ||
Polilli E, Sozio F, Di Stefano P, et al. Web-based HIV testing in Abruzzo, Italy: analysis of 15-month activity results. AIDS Patient Care STDS. 2016;30(10):471–475. |
Supplementary materials
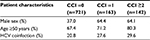  | Table S1 Characteristics of study population stratified by CCI score groups Abbreviations: CCI, Charlson Comorbidity Index; HCV, hepatitis C virus. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.