Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Impact of Chronic Obstructive Pulmonary Disease on the Mortality of Patients with Small Cell Lung Cancer
Authors Liao KM , Hung CM, Shu CC, Lee HS , Wei YF
Received 18 July 2021
Accepted for publication 23 November 2021
Published 1 December 2021 Volume 2021:16 Pages 3255—3262
DOI https://doi.org/10.2147/COPD.S328938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Kuang-Ming Liao,1 Chao-Ming Hung,2 Chin-Chung Shu,3,4 Ho-Sheng Lee,5 Yu-Feng Wei6,7
1Department of Internal Medicine, Chi Mei Medical Center, Chiali, Tainan, Taiwan; 2Department of Surgery, E-Da Cancer Hospital, Kaohsiung, Taiwan; 3Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan; 4National Taiwan University College of Medicine, Taipei, Taiwan; 5Division of Chest Medicine, Department of Internal Medicine, E-Da Hospital, Kaohsiung, Taiwan; 6School of Medicine for International Students, College of Medicine, I-Shou University, Kaohsiung, Taiwan; 7Department of Internal Medicine, E-Da Cancer Hospital, Kaohsiung, Taiwan
Correspondence: Yu-Feng Wei
Department of Internal Medicine, E-Da Hospital, No. 1, Yida Road, Jiao-su Village, Yan-chao District, Kaohsiung, 824, Taiwan
Tel +886-7-6150011
Fax +886-7-6150927
Email [email protected]
Background: Limited studies have focused on the impact of the coexistence of small cell lung cancer (SCLC) and chronic obstructive pulmonary disease (COPD). The study was to examine the impact of COPD on mortality in SCLC patients.
Methods: We analyzed SCLC patients from the Taiwan Cancer Registry Database between January 1, 1997, and December 31, 2015. The COPD population was composed of patients with a COPD diagnosis before the diagnosis of SCLC. The control group was composed of randomly selected SCLC patients without COPD who were propensity score matched with those with concomitant COPD according to age, sex, index date, cancer staging and comorbidities at a 1:1 ratio.
Results: Among 9425 SCLC patients in the database, eligible subjects were divided into the COPD group (n = 4235) and the non-COPD group (n = 2334). Compared to patients in the non-COPD group, the patients in the COPD group were older (71.4 versus 65.7 years, p< 0.0001), had a lower percentage of stage IV disease (60.1% versus 68.3%, p< 0.0001) and had more comorbidities. After matching, there were 1457 patients in each group. Older age, lower body mass index (BMI), and some comorbidities were associated with higher mortality, and comorbid COPD was associated with lower 1-year mortality in SCLC patients. Multivariate analysis identified older age, lower BMI, and concomitant congestive heart failure or diabetes as risk factors for OS.
Conclusion: A diagnosis of COPD was associated with reduced 1-year mortality in SCLC patients, but no significant difference after 1-year in this population.
Keywords: chronic obstructive pulmonary disease, small cell lung cancer
Introduction
Chronic obstructive pulmonary disease (COPD) is a common and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation. Data from the World Health Organization indicated that COPD was the third leading cause of death worldwide in 2019.1 COPD is not only a pulmonary inflammation-related disease but also a systemic inflammatory disease and is associated with many systemic comorbidities. Previous studies indicated the significantly higher risk of cancer in patients with COPD than in those without COPD, and the most common cancer in this population was lung cancer.2,3 Our previous study showed that COPD status was associated with a twofold increase in lung cancer incidence.3 The tight association between COPD and lung cancer results from some common risk factors, such as age and cigarette smoking. Other mechanisms, including chronic inflammation in COPD, lead to tumorigenesis through increased expression of growth factors and overexpression of the transcription factor STAT3.4
Lung cancer, including small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC), is the leading cause of cancer death worldwide. The prevalence of COPD is high in both SCLC and NSCLC patients and vice versa.5,6 COPD has been reported to play an important role in the prognosis of SCLC and NSCLC. Lee et al compared the clinical characteristics of patients with NSCLC concomitant with COPD and found that there was no impact of COPD on the mortality of patients with NSCLC.7 Tammemagi et al found that COPD was a significant predictor of survival variation in early- and late-stage SCLC and NSCLC patients.8 A meta-analysis conducted by Gao et al concluded that the presence of COPD and emphysema were predictors of poor prognosis in SCLC and NSCLC patients, but significant heterogeneity across their studies included patient characteristics, tumor staging, histologic types and different study designs and did not enroll patients with advanced (III–IV) stages.9 Tan et al conducted another systematic review and meta-analysis to evaluate the prognosis in patients with COPD and NSCLC following pulmonary resection and found that COPD increased mortality in patients with lung cancer following resection.10 Wu et al investigated the effect of NSCLC concomitant with COPD on prognosis and indicated that COPD has a deleterious effect on survival.11 Mostly of those studies were focused on NSCLC, and studies investigating the prognostic significance of COPD for SCLC patients are limited. In addition, previous studies focused on the association of lung cancer and COPD were mainly in Caucasian populations, relevant data in Asian populations was scanty. Thus, we conducted a nationwide population-based study from the cancer registry of Taiwan’s National Health Insurance Research Database (NHIRD) to investigate the impact of COPD on the survival of patients with SCLC in Asian populations.
Methods
Database
Study Cohort and Observation of Outcomes
Adult patients aged 20 years and older who were diagnosed with SCLC between 1 January 2000 and 31 December 2015 were identified from the cancer registry database of the NHIRD in Taiwan. Patients were excluded from this study if they had been diagnosed with other types of cancer, other catastrophic illnesses (defined by the Ministry of Health and Welfare in Taiwan),12 or COPD within 3 months or after their lung cancer diagnosis. The index date was defined as the date of lung cancer diagnosis. These patients were divided into two cohorts on the basis of COPD. The COPD cohort was composed of patients with a COPD diagnosis evidenced by at least three outpatient claims or at least one inpatient claim who needed at least one bronchodilator prescription at least 3 months before the diagnosis of SCLC. Patients without a diagnosis of COPD composed the “non-COPD cohort”. The comorbidities of the subjects in both cohorts were recorded using the ICD-10 version of Charlson comorbidity index before or on the index day. This study was approved by the institutional review board of E-Da Hospital (EMRP-108-061). Because patients’ data could not be identified, the requirement for informed consent was waived.
Statistical Analysis
Clinical demographic data of the COPD and non-COPD cohorts were analyzed. These data included age, sex, cancer stage, body mass index (BMI), smoking (including former and current smokers), and associated comorbidities. Time to death was defined as the period from the index date to the death date. The results of subjects who were still living at the end of the follow-up period or were excluded from the database within the follow-up period were censored. The control group was composed of randomly selected SCLC patients without COPD after propensity score matching with SCLC patients with COPD according to age, sex, index date, cancer stage and comorbidities at a 1:1 ratio. After adjusting for age, sex, cancer stage, BMI, smoking, and associated comorbidities, the cumulative survival hazard ratios (HRs) for the two cohorts were calculated in accordance with the Cox proportional hazards regression model. Data were analyzed with SAS software for Windows, version 9.4 (SAS institute Inc., Cary, NC, USA). A two-sided p-value of 0.05 was considered statistically significant.
Results
A total of 9425 SCLC patients were identified in the database. As shown in Figure 1, after excluding ineligible subjects, we included 4235 patients in the COPD cohort and 2334 patients in the non-COPD cohort. The baseline characteristics of the study population are presented in Table 1. Compared to the non-COPD patients, the COPD patients were older (71.4 versus 65.7 years, p<0.0001), had a lower percentage of stage IV disease (60.1% versus 68.3%, p<0.0001) and had more comorbidities. After propensity score matching, as shown in Table 2, 1457 patients in each group were included in further analyses. Table 3 demonstrates the factors associated with survival after matching. Older age (HR: 1.02, CI: 1.03–1.04, p<0.0001), stage IV disease (HR: 2.80, CI: 1.05–7.48, p=0.0397), lower BMI (HR: 0.94, CI: 1.03–1.04, p<0.0001), and certain comorbidities were associated with higher mortality. Comorbid COPD was associated with lower mortality in SCLC patients (HR: 0.89, CI: 0.83–0.96, p=0.0018). However, multivariable Cox proportional analysis identified that only older age, lower BMI, and comorbidities of congestive heart failure (CHF) or diabetes were risk factors for OS.
 |
Table 1 Baseline Characteristics of the Study Population |
 |
Table 2 Baseline Characteristics of the Matched Study Population |
 |
Table 3 Multivariable Cox Proportional Hazards Model Analysis for the Risk of Death |
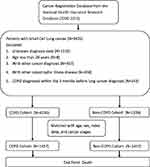 |
Figure 1 Flowchart of the study patients with newly diagnosed small cell lung cancer with and without COPD. |
Figure 2 illustrates the survival curves of SCLC patients with and without COPD. The cumulative incidences of mortality between the non-COPD and COPD cohorts were statistically significant (74.0% vs 70.0%, p = 0.0011) at 1 year but no more significant after 1 year (93.0% vs 91.0% at 2 years, 96.4% vs 95.0% at 3 years and 97.7% vs 97.0% at 5 years, respectively).
 |
Figure 2 Survival probability of COPD and non-COPD cohorts. |
Discussion
Our study indicated that comorbid COPD was associated with higher 1-year survival in patients with SCLC, whereas older age, lower BMI, stage IV disease, and comorbidities of CHF or diabetes were associated with a lower survival in these patients. After multivariate analysis, however, only age, BMI, and comorbid CHF or diabetes were risk factors for mortality.
Previous studies have examined the effect of COPD on the prognosis of lung cancer; however, most of those studies focused on operable lung cancer or NSCLC.7–11,13 A meta-analysis of 26 studies conducted by Gao et al indicated that the presence of COPD and emphysema are robust predictors of poor survival in patients with lung cancer.9 Recently, Wu et al conducted a meta-analysis with 12 case-control studies and showed that patients with NSCLC concomitant COPD was associated with poorer OS than the absence of COPD.11
Few studies have investigated the impact of COPD on SCLC. Aarts et al conducted a large-scale, population-based study in Netherlands to survey the trends of comorbidities and their prognostic impact in patients with SCLC from 1995 to 2012.14 They reported that pulmonary diseases (included obstructive, restrictive pulmonary disease, lung fibrosis, and lung transplantation) were one of the most prevalent and increasingly common comorbid diseases. However, after multivariate analysis, they concluded that only digestive and cardiac disease in the limited stage and cardiac and cerebrovascular disease in the extensive-stage disease affected the prognosis. The results were consistent with our current study, which demonstrated that comorbid CHF and diabetes were associated with a poor outcome.
Ju et al retrospectively enrolled 110 Korean SCLC patients and categorized them into COPD (n = 57) or non-COPD (n = 53) groups according to the results of pulmonary function testing. Their multivariate analysis showed that only extensive-stage SCLC and poor performance status were independent risk factors for shorter OS. They concluded that coexisting COPD had no negative impact on the survival of patients with SCLC.15 In this nationwide, large-scale cohort study, although comorbid COPD was associated with lower mortality at 1 year, we observed a relative lower percentage of stage IV disease (60% versus 68% in non-COPD group) in this population, probable due to the relatively earlier diagnosis of SCLC in COPD patients who already receive treatment and regular follow-up in health facilities. Nevertheless, the mortality of SCLC patients with COPD was similar to that of patients without COPD after 1 year of diagnosis, probably due to the high mortality of SCLC, and less than 10% of patients survived 2 years or longer.
The stage at presentation is one of the most important prognostic factors in patients with SCLC. Other clinical parameters in present study, such as performance status (PS), BMI, and comorbidities, also have prognostic importance in SCLC patients. A retrospective study indicated that age, BMI, and stage, but not COPD, were associated with the mortality of Korean patients with NSCLC, which was similar to the findings of our study.7 The prevalence of comorbidities is also high and has a substantial impact on prognosis in SCLC patients. A retrospective study in Canada reviewed 174 patients with limited-stage SCLC and reported that increasing age was associated with decreased PS and increased comorbidity, but only poor PS was an independent prognostic factor associated with survival.16 The contrasting results might be explained by the relatively small sample size and enrollment of only limited-stage SCLC patients. In this large-scale study, age was still an independent risk factor for mortality.
Diabetes mellitus is one of the most common comorbidities in SCLC and NSCLC patients. However, the prognostic effect of diabetes in those lung cancer patients is inconclusive. An earlier study conducted in Norway reported prolonged survival in SCLC and NSCLC patients with diabetes mellitus; however, a low prevalence of diabetes in the study population (only 84 of 1852 patients) might indicate selection bias.17 Similar to our present study, two retrospective studies in China and Turkey demonstrated that diabetes was a poor prognostic factor for surgically treated and advanced NSCLC patients.18,19 In addition, Kurishima et al studied 1798 Japanese patients with lung cancer, and 338 (18.8%) were comorbid with diabetes; they concluded that poorer PS, stage IV disease, SCLC pathology, and diabetes were poor prognostic factors for lung cancer.20 However, a retrospective study in Turkey reviewed 161 SCLC patients with a focus on diabetes and other prognostic variables reported that diabetes at the time of diagnosis of SCLC did not have prognostic importance for survival.21 This results was disconcordant to our study, probably due to the relatively small sample size compared to ours. Physicians should increase awareness to recognize the associated comorbidities, including cardiovascular disease and diabetes, when providing patient care because the coexistence of comorbidities requires the integration of multiple disciplines and awareness of drug‐drug interactions during cancer treatment.
A major limitation of our study is the lack of information on pulmonary function tests in the cancer registry data of the NHIRD, which may limit the diagnostic accuracy of COPD. However, we identified COPD patients with at least three outpatient claims or at least one inpatient claim coded who needed at least one bronchodilator prescription to minimize the rate of misdiagnosis. We also included patients with a diagnosis of COPD at least 3 months before the diagnosis of SCLC to minimize the miscoding and prescription of bronchodilators simply for symptom relief. We were not able to perform a severity-adjusted comparison in patients according to COPD severity and PS due to the unavailability of data. However, this large-scale population-based study with a long duration period provided real-world data without selection bias.
Conclusion
In patients with SCLC, a diagnosis of COPD was associated with reduced 1-year mortality, but no significant difference after 1-year. Age, BMI, and some comorbidities, such as CHF and diabetes, play an important role in survival. After the diagnosis of SCLC, clinicians should actively screen for coexisting comorbidities because early detection and treatment may improve outcomes in those patients.
Acknowledgment
We thank the Center for Database Research, E-DA HEALTHCARE GROUP, and Health Data Science Center, National Cheng Kung University Hospital for providing administrative and technical support.
Funding
This study was supported by research grants from E-Da Hospital (EDAHP109064).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128.
2. Mouronte-Roibas C, Leiro-Fernandez V, Fernandez-Villar A, Botana-Rial M, Ramos-Hernandez C, Ruano-Ravina A. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett. 2016;382(2):240–244. doi:10.1016/j.canlet.2016.09.002
3. Ho CH, Chen YC, Wang JJ, Liao KM. Incidence and relative risk for developing cancer among patients with COPD: a nationwide cohort study in Taiwan. BMJ Open. 2017;7(3):e013195. doi:10.1136/bmjopen-2016-013195
4. Qu P, Roberts J, Li Y, et al. Stat3 downstream genes serve as biomarkers in human lung carcinomas and chronic obstructive pulmonary disease. Lung Cancer. 2009;63(3):341–347. doi:10.1016/j.lungcan.2008.05.025
5. Balata H, Harvey J, Barber PV, et al. Spirometry performed as part of the Manchester community-based lung cancer screening programme detects a high prevalence of airflow obstruction in individuals without a prior diagnosis of COPD. Thorax. 2020;75(8):655–660. doi:10.1136/thoraxjnl-2019-213584
6. Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. doi:10.1183/09031936.00144208
7. Lee SJ, Lee J, Park YS, et al. Impact of chronic obstructive pulmonary disease on the mortality of patients with non-small-cell lung cancer. J Thorac Oncol. 2014;9(6):812–817. doi:10.1097/JTO.0000000000000158
8. Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103(6):792–802. doi:10.1002/ijc.10882
9. Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: a meta-analysis of observational studies. Respirology. 2016;21(2):269–279. doi:10.1111/resp.12661
10. Tan LE, Razak AM, Lim CS. Association of chronic obstructive pulmonary disease and postresection lung cancer survival: a systematic review and meta-analysis. J Investig Med. 2017;65(2):342–352. doi:10.1136/jim-2016-000059
11. Wu K, Wang J, Zhao L, Wang P, Duan Q. The prognosis of non-small cell lung cancer combined with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clin Respir J. 2020;14(4):389–396. doi:10.1111/crj.13144
12. Hsu YC. Analyzing Taiwan's National Health Insurance Research Database to explicate the allocation of health-care resources. Advances in Digestive Medicine. 2015;2:41-43. doi:10.1016/j.aidm.2015.04.001.
13. Sekine Y, Suzuki H, Yamada Y, Koh E, Yoshino I. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac Cardiovasc Surg. 2013;61(2):124–130. doi:10.1055/s-0032-1304543
14. Aarts MJ, Aerts JG, van den Borne BE, Biesma B, Lemmens VE, Kloover JS. Comorbidity in patients with small-cell lung cancer: trends and prognostic impact. Clin Lung Cancer. 2015;16(4):282–291. doi:10.1016/j.cllc.2014.12.003
15. Ju S, Lee HR, Kim JY, et al. Impact of coexistent chronic obstructive pulmonary disease on the survival of patients with small cell lung cancer receiving chemotherapy. Thorac Cancer. 2018;9(10):1271–1278. doi:10.1111/1759-7714.12832
16. Ludbrook JJ, Truong PT, MacNeil MV, et al. Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? A community-based population analysis. Int J Radiat Oncol Biol Phys. 2003;55(5):1321–1330. doi:10.1016/S0360-3016(02)04576-5
17. Hatlen P, Gronberg BH, Langhammer A, Carlsen SM, Amundsen T. Prolonged survival in patients with lung cancer with diabetes mellitus. J Thorac Oncol. 2011;6(11):1810–1817. doi:10.1097/JTO.0b013e31822a75be
18. Wang G, Li X, Xiong R, Wu H, Xu M, Xie M. Long-term survival analysis of patients with non-small cell lung cancer complicated with type 2 diabetes mellitus. Thorac Cancer. 2020;11(5):1309–1318. doi:10.1111/1759-7714.13398
19. Inal A, Kaplan MA, Kucukoner M, Urakci Z, Kilinc F, Isikdogan A. Is diabetes mellitus a negative prognostic factor for the treatment of advanced non-small-cell lung cancer? Rev Port Pneumol. 2014;20(2):62–68. doi:10.1016/j.rppneu.2013.09.001
20. Kurishima K, Watanabe H, Ishikawa H, Satoh H, Hizawa N. Survival of patients with lung cancer and diabetes mellitus. Mol Clin Oncol. 2017;6(6):907–910. doi:10.3892/mco.2017.1224
21. Inal A, Kaplan MA, Kucukoner M, et al. Is diabetes mellitus a prognostic factor for survival in patients with small cell lung cancer? APJCP. 2012;13(4):1491–1494. doi:10.7314/APJCP.2012.13.4.1491
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
