Back to Journals » Research and Reports in Tropical Medicine » Volume 10
Impact of annual preventive mass chemotherapy for soil-transmitted helminths among primary school children in an endemic area of Gurage zone: a prospective cross-sectional study
Authors Shumbej T , Menu S, Girum T, Bekele F , Gebru T, Worku M , Dendir A, Solomon A, Kahase D , Alemayehu M
Received 12 March 2019
Accepted for publication 19 May 2019
Published 5 July 2019 Volume 2019:10 Pages 109—118
DOI https://doi.org/10.2147/RRTM.S208473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Teha Shumbej,1 Sofia Menu,2 Tadele Girum,3 Fitsum Bekele,1 Teklemichael Gebru,3 Meron Worku,3 Andamlak Dendir,3 Absra Solomon,1 Daniel Kahase,1 Mihret Alemayehu1
1Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Wolkite University, Wolkite, Ethiopia; 2Department of Medicine, College of Medicine and Health Sciences, Wolkite University, Wolkite, Ethiopia; 3Department of Public Health, College of Medicine and Health Sciences, Wolkite University, Wolkite, Ethiopia
Background and aim: School-based preventive mass chemotherapy has been a key component of Ethiopia’s national plan for the control of soil-transmitted helminths. Without an impact evaluation on the impact of a deworming program on infection levels, it is unclear whether the deworming program warrants levels of environmental transmission of infection. This study aimed to determine the impact of annual preventive mass chemotherapy for soil-transmitted helminths among schoolchildren in an endemic area of Gurage zone, south-central Ethiopia.
Methods: A repeated school-based quantitative prospective cross-sectional method was employed. Data were collected from study participants selected using systematic sampling with probability proportional to size at baseline and after annual treatment. Fresh stool samples were collected and processed using the Kato─Katz technique at the Wolkite University parasitology laboratory. SPSS-21 was used for data management and analysis. Changes in parasitological variables after treatment were estimated.
Results: Overall, 41.1% prevalence and 22.3% mean geometric infection-intensity reduction were found. Reductions in prevalence of Schistosoma mansoni and hookworms were 13.2% and 15.3%, respectively. Similarly, decreases in prevalence were seen in Ascaris lumbricoides and Trichuris trichiura, representing 94.4% and 80.0% reduction rates, respectively, while 25.9% of the children had heavy S. mansoni (≥400 eggs per gram) infections at baseline, which were reduced to 4.5% after annual treatment. Geometric mean infection intensity–reduction rates for hookworms, A. lumbricoides, and T. trichiura were 80.8%, 20.2%, and 96.7%, respectively.
Conclusion: Annual mass chemotherapy failed to clear soil-transmitted helminths completely in the present study. However, it resulted in a substantial reduction in overall prevalence and infection intensity. Therefore, other than deworming for school children, interventions such as access to improved personal hygiene and environmental hygiene in school should be emphasized to interrupt transmission.
Keywords: mass chemotherapy, STHs, Gurage zone, Ethiopia
Background
Neglected tropical parasitic diseases (NTPDs) are a cluster of diseases that affect the poorest populations.1 NTPDs are highly prevalent in developing countries. Most middle-income countries and low-income countries are affected simultaneously by more than one neglected tropical diseases (NTDs).2 Soil-transmitted helminths (STHs) and Schistosomia mansoni infections are among the NTPDs that cause suffering and delays in physical and cognitive growth, perpetuating the poverty of those infected by hindering their productivity.3
An estimated 300 million people are severely ill with STHs and S. mansoni. Of these, at least 50% are schoolage children (SAC) aged 5–15 years.4 It is estimated that 25%–35% of SAC are infected with one or more of the major species of helminth infection.5 In Ethiopia, there are an estimated 37.3 million people living in schistosomiasis-endemic areas, comprising 12.3 million SAC.6 The number of people living in STH-endemic areas of Ethiopia is estimated to be 79 million, which comprises 25.3 million SAC. Around 741 districts are known to be endemic for both STHs and S. mansoni in Ethiopia.7 According to a Gurage Zone Health Department report, most NTDs identified by the Federal Ministry of Health of Ethiopia are prevalent and endemic in some districts located in the zone.
In recognition of their significance to public health and economies, many countries, including Ethiopia, are investing in preventing and controlling STHs and S. mansoni.8 The World Health Organization (WHO) policy for NTD control focuses on mass chemotherapy to control and ultimately eliminate STHs and S. mansoni.9 Current strategies used to control STH and S. mansoni infections focus on SAC due to the most intense infections and related illness occurring in SAC. The WHO guidelines for controlling STHs and S. mansoni recommend treating SAC annually where any STH- and S. mansoni-infection prevalence of the targeted area is 20%–50% and twice a year where prevalence is >50%. The guideline also recommends focusing on SAC as a target for treatment and monitoring STHs and S. mansoni.10
Today, there is renewed global commitment to and funding opportunities for the control of STH and S. mansoni infections.11 In Ethiopia, regular mass chemotherapy has been in place since 2015, targeting SAC and pre-SAC.12 Since the launch, mass-chemotherapy coverage for STH and S. mansoni infections is 77% and 78.25%, respectively,6 aiming to eliminate STH- and S. mansoni infection–related morbidity by 2020.13
Mass chemotherapy in Ethiopia embraces different monitoring and evaluation measures like process monitoring and performance monitoring for quality of program delivery, but not impact monitoring.6 Unless monitoring the impact of mass chemotherapy on infection levels is conducted, it is unclear whether levels of environmental transmission of infectionwarrant the program. As such, this study was proposed to assess the impact of annual mass chemotherapy for STH infections among primary SAC in an endemic area of Gurage zone.
Methods
Study setting
This study was conducted in Gurage zone in the Southern Nations, Nationalities, and People’s Region. It is located 155 kilo-meters south of Addis Ababa, the capital of Ethiopia. The zone is found at an average altitude of 100–3,300 m above sea level. About 65.3% the climatic zone lies in Woina Dega. The rest — 27.5%, 3.1%, and 4.1% — lie in the Dega, Kolla, and Wereche climates, respectively. The average annual temperature of the zone is 18°C, and annual is 800–1,400 mm.
Study design and procedures
A repeated school-based quantitative prospective cross-sectional method was employed at baseline (April–May 2017) and after annual treatment (May–June 2018). Pre- and posttreatment-impact indicators were assessed through parasitological examinations. The sample size was calculated with an assumption of estimated prevalence of 23.3%,14 95% confidence level, 5% margin of error, 10% non-response rate, and design effect of 2 for the baseline survey and estimated prevalence of 23.3%,14 95% confidence level, 5% margin of error, and 10% non-response rate for the final post-treatment parasitological survey. For each class, children were selected using systematic sampling from class registers provided by the class teachers (Figures 1 and 2).
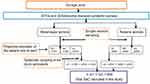 |
Figure 1 Flowchart of study participant recruitment hierarchy at baseline survey. Abbreviations: STHs, soil-transmitted helminths; SAC, school-age children; n, number of children; S, school. |
Inclusion and exclusion criteria
All children aged 5–15 years who are available in their school at the survey visit were included. Children who were on antihelminthic drug or treated within 1 month prior to data collection were excluded for the baseline study.
Data collection and drug administration
A pre-tested, semistructured questionnaire was used to gather information on socio-demographic and other factors related to STH and S. mansoni infections. The questionnaire was initially prepared in English and then translated to Amharic by a fluent speaker of both languages to ensure its consistency. Then, data collectors interviewed the children’s parents/guardians. Before the administration of 500 mg mebendazole (expired in November 2021), baseline data were collected between April and May 2017, and on day 21 post-–annual treatment data were collected between May and June 2018. Data collection and drug administration were done by health extension workers who can speak both Amharic and the local language (Guraghaegna). For children who were not at school at the time of the survey visit, the next students in the sampling frame were considered.
Sample collection and laboratory analyses
Single fresh stool samples were collected before and after annual treatment from each study participant using clean, dry, wide-mouthed labeled stool cups, after which the samples were transported to the Wolkite University parasitology laboratory. The collected stool samples were processed by the Kato–Katz egg counting technique, as described elsewhere,15 for which single slides were examined by a trained laboratory technician. Briefly, the technique depends on microscopic counting of STHs and S. mansoni eggs in prepared slides with special staining of known weight (41.7 mg) of stools. Infection intensities of the STHs and S. mansoni were recorded and graded as light, moderate, or heavy, based on the number of eggs per gram (EPG) according to the WHO threshold.16 For Ascaris lumbricoides, 1–4,999, 5,000–49,999, and ≥50,000 EPG was considered light, moderate, and heavy, respectively. For Trichuris trichiura, these figures were 1–999, 1,000–9,999, and ≥10,000 EPG, respectively, and for hook worms 1–1,999, 2,000–3,999, and ≥4,000 EPG, respectively. On the other hand, infection intensity of S. mansoni 1–1,999, 2,000–3,999, and >4,000 was considered light, moderate, and heavy, respectively.
Data-quality assurance
Refreshment training was given to data collectors and laboratory technicians on Kato–Katz smears by experienced personnel in the field. From both positive and negative Kato–-Katz smears, 10% were randomly selected and re-read by two independent medical laboratory experts who were blinded to the primary result. A fresh working solution of malachite -green was used routinely to maintain the quality of the smear. Every laboratory step of the parasitological examination was conducted based on standard operating procedures.
Data processing and statistical analysis
The collected data were checked for completeness, cleared, and entered into SPSS version 21 for management and analysis. Descriptive statistics were used to summarize sociodemographic characteristics and indicator variables. Changes in prevalence and infection intensity were estimated. The quantity of STHs and S. mansoni eggs was determined before and after annual treatment to determine the intensity of infection according to the WHO threshold.16 Binary logistic regression was used to test for linear associations in the prevalence of STH and S. mansoni infections over the study period. Trends of geometric mean intensity were calculated. Predictors of STH and S. mansoni infections were identified using multivariate logistic regression analysis. Statistical significance was set at P<0.05. Relative reduction (RR) between pre-and posttreatment was calculated:
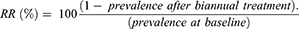
Study variables
Study variables were STH and S. mansoni infections, sociodemographic characteristics (age, sex, residence, family size), family status (occupation of mother, educational status of mother, occupation of father, educational status of father), hygienic factors (shoe wearing, washing habits, habit of nail biting, habit of playing in the soil), and source of water.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. Study protocols were reviewed and approved by the Ethical Review Committee of Wolkite University (wku/53/10). Letters of permission were obtained from the Guragea Zone Health Department and each district health office. The objectives and nature of the study were explained to school directors and written informed consent obtained from parents or legal guardians. Data collected from each study participant and results of laboratory tests were kept confidential. Children with positive stools for any helminth infections were treated according to Ethiopian Medicines Formulary recommendations30 in collaboration with the Gurage Zone Health Department.
Results
Characteristics of children under study
Overall, 597 children at baseline and 310 after annual treatment were involved in the present study. The mean age of enrolled children at baseline was 10.4 (5–15) years. Those enrolled after treatment were aged 10–15 years with a mean of 10.7 years. There were more boys (58.7%) than girls (41.3%) at baseline.
Impact on prevalence and intensity of STH and S. mansoni infections
Overall reductions in prevalence and geometric mean infection intensity were 41.1% (from 23.6 to 13.9) and 22.3% (from 112 to 87), respectively. S. mansoni prevalence at baseline was 12.9%, while prevalence was 11.2% after annual mass chemotherapy, representing a 13.2% reduction rate. At baseline, 25.9% of the children had heavy S. mansoni infections (≥400 EPG), and the geometric mean infection intensity of all infected study participants was 158 EPG. Only 4.5% of S. mansoni-infected children were categorized as having heavy infection intensity after annual mass chemotherapy (Table 1).
 |
Table 1 Proportions of children infected with STHs and Schistosoma mansoni and average infection intensity at baseline and after annual treatment |
The prevalence of hookworm infections was 4.3% at baseline and 3.6% after annual treatment, with a reduction rate of 15.3%. Similarly, prevalence reduction for A. lumbricoides and T. trichiura were 94.4% and 80%, respectively. All hookworm, A. lumbricoides, and T. trichiura infections were categorized as light infection intensity at baseline, with estimated geometric mean infection intensity of 115 EPG, 301 EPG, and 31 EPG, respectively. Mean geometric infection intensity–reduction rates for hookworms, A. lumbricoides, and T. trichiura were 80.8%, 20.2%, and 96.7%, respectively (Table 1).
Factors associated with STH and S. mansoni infections
Factors associated with STH and S. mansoni infections were investigated using logistic regression. Infection patterns and children’s characteristics were generally comparable between baseline and annual follow-up. STH and S. mansoni infections were positively associated with frequency of playing on soil and not washing hands after defecation. Children aged 5–9 years (COR 1.2, 95% CI 1.9–2.1; Table 2), whose mothers used river water for washing raw food and vegetables (COR 2.1, 95% CI 1.3–3.3), and with a habit of bathing in rivers (COR 1.9, 95% CI 1.1–2.2) had significantly higher prevalence of STH and S. mansoni infections at baseline (Table 3).
 |
Table 2 Sociodemographic characteristics of SAC infected with STHs and Schistoma mansoni at recruitment and after annual treatment |
 |
Table 3 Factors associated with STHs and Schistosoma mansoni infections at recruitment and after annual treatment among SAC |
Multivariate logistic regression at baseline estimated that children aged 5–9 years were 1.43 times (AOR 1.43, 95% CI 0.4–0.9) as likely to get infected with STHs and S. mansoni than those aged 10–15 years (Table 2). Children whose mothers used river water for washing raw food and vegetables were 2.4 times (AOR 2.4, 95% CI 0.16–0.75) more likely to be infected with STHs and S. mansoni than children whose mothers used tap water for washing raw food and vegetables. The odds of STH and S. mansoni infections were 2.1 times higher (AOR 2.1, 95% CI 0.3–0.9) for children who bathed in rivers than those who bathed at home. In addition, children who had irregular handwashing habits after defecation were 3.1 times (AOR 3.1, 95% CI 1.2–3.1) more likely to get infected with STHs and S. mansoni than those who had regular handwashing habits after defecation. Children who played on soil irregularly were 2.1 times (AOR 2.1, 95% CI 1.3–6.8) as likely to be infected with STHs and S. mansoniafter annual treatment than children who did not play on soil (Table 3).
Discussion
Currently, preventive mass-chemotherapy campaigns are taking place in various parts of Africa, Asia, and South America, particularly targeting SAC for STH- and S. mansoni-infection control.17 Although the WHO recommends a parasitological check every few years, these are being conducted infrequently without a coherent long-term strategy and without any standardized monitoring protocol.18 However, timely impact evaluation is crucial to monitor progress and initiate corrective measures.
Impact evaluation is necessary to ensure that programs are efficiently implemented and that recipients gain maximum benefit.19 In line with the WHO strategy for controlling STH and S. mansoni infections in endemic countries, the national Ethiopian school-based mass-chemotherapy program currently provides annual delivery of mebendazole to SAC.16
Consistently with our understanding of the dynamics of transmission and control of STHs and S. mansoni20 and previous studies,21,22 we found that both prevalence and infection intensity were substantially reduced after annual treatment with 500 mg mebendazole. However, even after mass chemotherapy, some children still had residual STH and/or S. mansoni infections. This may reflect the maintenance of transmission among untreated populations23,24 or a failure to clear infections after chemotherapy25 in a proportion of children.
Residual STH and S. mansoni infections provided an indication of either rapidly occurring re-infection or a lack of parasite clearance after treatment. Our study showed that reduction rates before and after annual treatment varied among species of helminths, which may have been due to mebendazole having variable efficacy against different helminths,26 though it had been found to be most appropriate for mass chemotherapy.25
Studies elsewhere27,28 have demonstrated that children with adequate access to improved sanitation in the household and at school were at reduced risk of acquiring STH and S. mansoni infections, and improved environmental hygiene was positively associated with a higher impact of antihelminthic treatment. Moreover, handwashing and access to soap were found to be associated with reduced odds of re-infection with STH and S. mansoni infections.29 In this study, handwashing after defecation and not playing on soil appeared to have a protective effect against residual STH and S. mansoni infections. Therefore, preventive mass chemotherapy should be accompanied by health education to ensure good health practices and avert transmission.
Conclusion
The WHO recommends early and regular administration of antihelminthic drugs to reduce the occurrence of and sustain reductions in helminth transmission. In support of this, annual mass chemotherapy in SAC in the present study showed a tremendous impact on the prevalence and infection intensity of STH and S. mansoni infections. However, despite preventive mass chemotherapy dramatically reducing the infection intensity of STHs and S. mansoni, it failed to clear the infections completely. This suggests a need to integrate annual mass chemotherapy with interventions that help to reduce infection exposure, such as access to improved personal hygiene and environmental hygiene at school and home to interrupt transmission.
Abbreviations list
EPG, eggs per gram; NTDs, neglected tropical diseases; NTPDs, neglected tropical parasitic diseases; SAC, school-age children; STHs, soil-transmitted helminths; PSAC, pre-SAC; RR, relative reduction; WHO, World Health Organization.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to sincerely thank heads of the zonal and district health offices, school directors, data collectors, and study participants. The study was sponsored by Wolkite University. The funder had no role in study design, data collection/analysis, decision to publish, or preparation of the manuscript.
Author contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373(9674):1570–1575. doi:10.1016/S0140-6736(09)60233-6
2. Fenwick A. The global burden of neglected tropical diseases. Public Health. 2012;126(3):233–236. doi:10.1016/j.puhe.2011.11.015
3. Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7(1):37. doi:10.1186/1756-3305-7-37
4. Helminthiases WS-t. Eliminating soil-transmitted helminthiases as a public health problem in children: progress report 2001–2010 and strategic plan 2011–2020. World Health Organization. 2012:19–29.
5. Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29(4):197–205. doi:10.1016/j.pt.2013.02.001
6. Negussu N, Mengistu B, Kebede B, et al. Ethiopia schistosomiasis and soil-transmitted helminthes control programme: progress and prospects. Emj. 2017;55(Suppl 1):75.
7. Deribew A, Kebede B, Tessema GA, et al. Mortality and disability-adjusted life-years (Dalys) for common neglected tropical diseases in Ethiopia, 1990-2015: evidence from the Global Burden of Disease Study 2015. EMJ. 2017;55(Suppl 1):3.
8. Bockarie MJ, Kelly-Hope LA, Rebollo M, Molyneux DH. Preventive chemotherapy as a strategy for elimination of neglected tropical parasitic diseases: endgame challenges. Phil Trans R Soc B. 2013;368(1623):20120144. doi:10.1098/rstb.2012.0163
9. Vercruysse J, Levecke B, Prichard R. Human soil-transmitted helminths: implications of mass drug administration. Curr Opin Infect Dis. 2012;25(6):703–708. doi:10.1097/QCO.0b013e328358993a
10. Farrell SH, Coffeng LE, Truscott JE, et al. Investigating the effectiveness of current and modified world health organization guidelines for the control of soil-transmitted helminth infections. Clin Infect Dis. 2018;66(suppl_4):S253–S9. doi:10.1093/cid/ciy002
11. Bundy DA, Appleby LJ, Bradley M. et al., 100 years of mass deworming programmes: a policy perspective from the World Bank’s Disease Control priorities analyses. In: Advances Parasitol. Vol. 100. Amsterdam: Elsevier; 2018:127–154.
12. Worku K. Neglected tropical diseases program in ethiopia, progress and challenges. EMJ. 2017;55:4.
13. Mengitsu B, Shafi O, Kebede B, et al. Ethiopia and its steps to mobilize resources to achieve 2020 elimination and control goals for neglected tropical diseases: spider webs joined can tie a lion. Int Health. 2016;8(suppl_1):i34–i52. doi:10.1093/inthealth/ihw007
14. Shumbej T, Belay T, Mekonnen Z, Tefera T, Zemene E. Soil-transmitted helminths and associated factors among pre-school children in Butajira Town, South-Central Ethiopia: a community-based cross-sectional study. PLoS One. 2015;10(8):e0136342. doi:10.1371/journal.pone.0136342
15. Levecke B, Behnke JM, Ajjampur SS, et al. A comparison of the sensitivity and fecal egg counts of the McMaster egg counting and Kato-Katz thick smear methods for soil-transmitted helminths. Plos Neglect Trop D. 2011;5(6):e1201. doi:10.1371/journal.pntd.0001201
16. WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. World Health Organization; 2002.
17. Albonico M, Allen H, Chitsulo L, Engels D, Gabrielli A-F, Savioli L. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. Plos Neglect Trop D. 2008;2(3):e126. doi:10.1371/journal.pntd.0000126
18. Anderson R, Truscott J, Hollingsworth TD. The coverage and frequency of mass drug administration required to eliminate persistent transmission of soil-transmitted helminths. Phil Trans R Soc B. 2014;369(1645):20130435. doi:10.1098/rstb.2013.0435
19. Crompton DW. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006.
20. Humphries D, Nguyen S, Boakye D, Wilson M, Cappello M. The promise and pitfalls of mass drug administration to control intestinal helminth infections. Curr Opin Infect Dis. 2012;25(5):584–589. doi:10.1097/QCO.0b013e328357e4cf
21. Kirwan P, Asaolu SO, Molloy SF, Abiona TC, Jackson AL, Holland CV. Patterns of soil-transmitted helminth infection and impact of four-monthly albendazole treatments in preschool children from semi-urban communities in Nigeria: a double-blind placebo-controlled randomised trial. BMC Infect Dis. 2009;9(1):20. doi:10.1186/1471-2334-9-20
22. Wiria AE, Hamid F, Wammes LJ, et al. The effect of three-monthly albendazole treatment on malarial parasitemia and allergy: a household-based cluster-randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8(3):e57899. doi:10.1371/journal.pone.0057899
23. Korir H, Riner D, Kavere E, et al. Young adults in endemic areas: an untreated group in need of school-based preventive chemotherapy for schistosomiasis control and elimination. Trop Med Infect Dis. 2018;3(3):100. doi:10.3390/tropicalmed3030100
24. Anderson RM, Truscott JE, Pullan RL, Brooker SJ, Hollingsworth TD. How effective is school-based deworming for the community-wide control of soil-transmitted helminths? Plos Neglect Trop D. 2013;7(2):e2027. doi:10.1371/journal.pntd.0002027
25. Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299(16):1937–1948. doi:10.1001/jama.299.16.1937
26. Tchuenté LT. Control of soil-transmitted helminths in sub-Saharan Africa: diagnosis, drug efficacy concerns and challenges. Acta Trop. 2011;120:S4–S11. doi:10.1016/j.actatropica.2010.07.001
27. Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9(1):e1001162. doi:10.1371/journal.pmed.1001162
28. Jia T-W, Melville S, Utzinger J, King CH, Zhou X-N. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. Plos Neglect Trop D. 2012;6(5):e1621. doi:10.1371/journal.pntd.0001621
29. Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001620. doi:10.1371/journal.pmed.1001620
30. EFMHACA. Ethiopian Medicines Formulary.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.