Back to Journals » Cancer Management and Research » Volume 12
Impact of Amrubicin Monotherapy as Second-Line Chemotherapy on Outcomes in Elderly Patients with Relapsed Extensive-Disease Small-Cell Lung Cancer
Authors Igawa S, Ono T, Kasajima M, Manabe H, Fukui T, Mitsufuji H, Yokoba M, Kubota M , Katagiri M , Sasaki J, Naoki K
Received 26 March 2020
Accepted for publication 9 June 2020
Published 23 June 2020 Volume 2020:12 Pages 4911—4921
DOI https://doi.org/10.2147/CMAR.S255552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Satoshi Igawa,1 Taihei Ono,1 Masashi Kasajima,1 Hideaki Manabe,1 Tomoya Fukui,1 Hisashi Mitsufuji,2 Masanori Yokoba,3 Masaru Kubota,3 Masato Katagiri,3 Jiichiro Sasaki,4 Katsuhiko Naoki1
1Department of Respiratory Medicine, Kitasato University School of Medicine, Sagamihara-City, Kanagawa 252-0374, Japan; 2Kitasato University School of Nursing, Sagamihara-City, Kanagawa 252-0329, Japan; 3School of Allied Health Sciences, Kitasato University, Sagamihara-City, Kanagawa 252-0373, Japan; 4Research and Development Center for New Medical Frontiers, Kitasato University School of Medicine, Sagamihara-City, Kanagawa 252-0374, Japan
Correspondence: Satoshi Igawa
Department of Respiratory Medicine, Kitasato University School of Medicine, 1-15-1, Kitasato, Minami-Ku, Sagamihara-City, Kanagawa 252-0374, Japan
Tel +81 42 778 8506
Fax +81 42 778 6412
Email [email protected]
Purpose: Amrubicin (AMR) is an anticancer drug for patients with relapsed small-cell lung cancer (SCLC). However, the efficacy of AMR in elderly patients with relapsed SCLC after chemotherapy by carboplatin plus etoposide (CE) has not been sufficiently evaluated.
Patients and Methods: The medical records of patients with relapsed SCLC who received AMR as second-line chemotherapy were retrospectively reviewed, and their treatment outcomes were evaluated.
Results: Forty-one patients with a median age of 76 years were analyzed. The overall response rate was 26.8%. Median progression-free survival (PFS) and overall survival (OS) were 3.5 and 8.1 months, respectively. While the median PFS of 4.7 and 2.8 months in the sensitive relapse and the refractory relapse group differed significantly (P=0.043), respectively, the median OS of 10.7 and 6.8 months in the respective relapse groups did not indicate a statistically significant difference (P=0.24). The median PFS in a group with a modified Glasgow prognostic score (mGPS) of 0 and a group with a mGPS 1 or 2 were 4.5 and 1.6 months (P=0.052), respectively, and the median OS in the respective mGPS groups were 10.7 and 4.4 months (P=0.034). Multivariate analysis identified good performance status, limited disease, and mGPS 0 as favorable independent predictors of PFS and OS of AMR monotherapy. Grade 3 or higher neutropenia was observed in 23 patients (56%), and febrile neutropenia was observed in nine patients (22%). Non-hematological toxic effects were relatively mild, and pneumonitis and treatment-related deaths were not observed.
Conclusion: AMR is an effective and feasible regimen for elderly patients with relapsed SCLC after CE therapy.
Keywords: small-cell lung cancer, amrubicin, elderly, second-line chemotherapy, modified Glasgow prognostic score
Introduction
Although small-cell lung cancer (SCLC) is one of the most chemo-sensitive solid tumor types, its prognosis is extremely poor.1 Most patients with SCLC experience relapse owing to the emergence of drug-resistant tumor cells even after successful induction therapy.2–4 Approximately 50% of all SCLC patients in Japan are 70 years of age and older.5 Until 2019, chemotherapy with carboplatin plus etoposide (CE) was the standard treatment modality for elderly patients with SCLC, as recommended by the Japan Lung Cancer Society.6 Recently, a Phase III randomized trial (IMpower-133) demonstrated that adding an immune checkpoint inhibitor (atezolizumab) to CE improved both progression-free survival (PFS) and overall survival (OS).7
The synthetic prodrug amrubicin (AMR) hydrochloride is a 9-amino-anthracycline derivative that is metabolized in the liver to its active form, amrubicinol. It blocks DNA topoisomerase II, which generates a cytotoxic effect by stabilizing a cleavable DNA-topoisomerase II complex. Amrubicinol has an approximately 10-fold lower DNA-intercalating potency than the representative anthracycline drug doxorubicin.8,9 The in vitro cell-growth inhibitory activity of amrubicinol is 18- to 220-fold higher than that of its prodrug.10 AMR has almost no cardiotoxicity, and its antitumor activity against several human tumor xenografts implanted in nude mice is more potent than that of doxorubicin.11,12 In a previous study using AMR against chemo-naïve SCLC,13 the patients had a response rate of 79% and a median survival time of 11.0 months. These results indicated that the treatment of SCLC with AMR monotherapy is very beneficial. Previous clinical trials revealed that compared with topotecan, AMR significantly improved the response and survival rates, particularly in patients with SCLC with refractory relapse.14–17 Thus, AMR monotherapy has become the standard second-line chemotherapy for extensive-disease (ED)-SCLC in Japan.
However, the efficacy of AMR in elderly patients with relapsed ED-SCLC after CE therapy has not been sufficiently evaluated. Therefore, in this study, we focused on evaluating the efficacy and safety of AMR in relapsed elderly patients with ED-SCLC.
Patients and Methods
Patient Selection and Data Collection
The WHO classification for lung cancer was revised as the 4th edition in 2015.18 SCLC, large cell neuroendocrine carcinoma and carcinoid tumors were classified as neuroendocrine tumors in the revised classification. Among the subtypes of neuroendocrine tumors, we focused elderly patients with SCLC in this study. The eligibility criteria for this retrospective study were as follows: histologically or cytologically proven SCLC; age ≥70 years during the administration of AMR as second-line treatment of CE therapy at Kitasato University Hospital between March 2010 and December 2019; and measurable target lesions on imaging examination via chest radiography, computed tomography (CT) of the chest and abdomen, or other procedures, such as magnetic resonance imaging (MRI) of the head, positron emission tomography (PET), or combined PET/CT imaging. The clinical stage at the initial diagnosis of SCLC was determined using the World Health Organization classification, version 8. Patients with clinical stage IIIC, IVA, and IVB of the TNM classification were included to have ED-SCLC. In this study, we evaluated a representative marker of systemic inflammatory responses, such as the modified Glasgow prognostic score (mGPS).18 Briefly, patients with albumin (Alb) ≥3.5 g/dL and C-reactive protein (CRP) ≤1 mg/dL were defined as mGPS 0, patients with Alb ≥3.5 g/dL and CRP > 1 mg/dL or Alb < 3.5 g/dL and CRP ≤1 mg/dL were defined as mGPS 1, and patients with Alb <3.5 g/dL and CRP >1 mg/dL were defined as mGPS 2.19
Amrubicin Regimen
AMR dissolved in 20 mL normal saline was administered intravenously as a 5-minute infusion once daily on days 1 to 3 every 3 weeks. The AMR dose was 40 mg/m2/day. The treatment regimen was repeated at the attending oncologist’s discretion (after four cycles, the oncologist decided whether a fifth and sixth cycle was appropriate) and was continued until disease progression, unacceptable adverse events, or at the patient’s request.
Response Evaluation
Lesions were evaluated using plain chest radiography, CT of the chest and abdomen, PET or bone scintigraphy, and CT or MRI of the cranium. To evaluate the tumors, CT imaging of the chest and abdomen was performed at least every 2 cycles. PET or bone scintigraphy and CT or MRI of the cranium were performed at 6-month intervals or earlier if patients had significant tumor-associated symptoms. Tumor control was assessed according to the Response Evaluation Criteria in Solid Tumors guidelines (version 1.1). The best overall response and maximum tumor control were recorded as the tumor response.
Toxicity Assessment and Treatment Modification
Toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0. At our institution, the criteria for dose reduction were grade 4 neutropenia lasting ≥4 days, febrile neutropenia, and grade 4 thrombocytopenia. If any of these events occurred, the AMR dose was reduced by 5 mg/m2/day in subsequent cycles. Patients received supportive care as required. The treatment protocol specified that 50 μg/m2/day or 2 μg/kg/day recombinant human granulocyte colony-stimulating factor (G-CSF) should be used in accordance with the national health insurance coverage of Japan. The indications for G-CSF administration were as follows: (a) fever (in principle, body temperature above 37.5 °C) with a neutrophil count of ≤1000/mm3; (b) a neutrophil count of 500/mm3; and (c) fever with a neutrophil count of ≤1000/mm3 or a neutrophil count of 500/mm3 during the previous course followed by a neutrophil count of ≤1000/mm3 after completing the same chemotherapy regimen. G-CSF is a prophylactic agent against leukopenia or neutropenia that was administered at the physician’s discretion.
Statistical Analyses
All data were analyzed with a cut-off date of March 1, 2020. PFS was measured from the start of AMR monotherapy to treatment failure (death, documentation of disease progression) or date of censoring at the last follow-up examination. OS was defined as the interval between the start of AMR monotherapy and death from any cause or date of censoring at the last follow-up. The survival curves were plotted using the Kaplan–Meier method, and differences based on relevant parameters, including performance status (PS), type of relapse to prior chemotherapy, and mGPS, were analyzed by the Log rank test. Variables, including gender, PS, stage, brain metastasis status, type of relapse to prior chemotherapy, and mGPS, were used for fitting Cox’s proportional hazard models to predict the hazard rates for PFS and OS of AMR monotherapy. The differences in the response rates according to the type of relapse were compared using Fisher’s exact test. P value <0.05 was used as the criterion for statistical significance. All statistical analyses were performed using the SPSS software program, version 23 (SPSS Inc., Chicago, Illinois) for Windows.
Results
Patient Characteristics
Forty-one patients who were treated between March 2010 and December 2019 were identified in this retrospective cohort study; all patients were included in the efficacy and safety analyses. The patients’ demographic data are shown in Table 1. There were 35 men and six women, and the median patient age was 76 (range, 70–85) years. Among the 41 patients, 11 SCLC patients had LD, and 30 SCLC patients had ED at the initial diagnosis of SCLC.
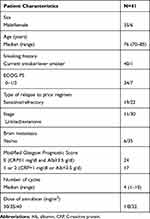 |
Table 1 Patient Characteristics |
When the AMR monotherapy was administered, 19 had a sensitive relapse and 22 a refractory relapse to an earlier CE therapy. The number of AMR treatment cycles per patient ranged from 1 to 10 (median, four cycles). According to mGPS, 24 patients had mGPS 0, and 17 patients had mGPS 1 or 2.
Response
A partial response was observed in 11 out of 41 patients, indicating an overall response rate of 26.8% (95% confidence interval [CI]: 12.7–40.9%, Table 2). The tumor response was not evaluable in two patients owing to early termination of the treatment protocol triggered by their hospital transfer. Among eight patients receiving 35mg2 of AMR, partial response was observed in two patients indicating 25% of response rate. SD was observed in one patient receiving 30mg2 of AMR. The response rate was 31.6% (95% CI: 11.7–51.5%) in patients with sensitive relapse and 22.7% (95% CI: 4.8–40.7%) in patients with refractory relapse, indicating no statistically significant differences (P=0.52).
 |
Table 2 Response to Amrubicin Monotherapy |
Survival
The median PFS and OS for all patients were 3.5 (95% CI: 2.4–4.6) and 8.1 (95% CI: 5.0–11.2) months, respectively (Figure 1). The median follow-up time was 8.2 months. The median PFS according to the type of relapse to the prior regimen was significantly higher in patients with sensitive relapse than in those with refractory relapse (4.7 [95% CI: 2.3–7.1] months vs 2.8 [95% CI: 0.5–5.1] months, P=0.043, Figure 2A). However, the median OS did not significantly differ between the two groups (10.7 [95% CI: 8.3–13.1] months in the sensitive group vs 6.8 [95% CI: 4.2–9.4] months in the refractory group, P=0.24, Figure 2B). Patients with a PS of 0–1 had a significantly higher median PFS than those with a PS of 2 (4.4 [95% CI: 3.8–5.0] months vs 1.1 [95% CI: 0.2–2.0] months, P=0.0001, Figure 3A). Similarly, the median OS did significantly differ between these two patient groups (10.7 [95% CI: 8.4–13.0] months in the sensitive group vs 5.6 [95% CI: 1.7–9.5] months in the refractory group, P=0.0001, Figure 3B). Moreover, the median PFS according to the mGPS tended to be higher in patients with an mGPS of 0 than in those with an mGPS of 1 or 2 (4.5 [95% CI: 3.7–5.3] months vs 1.6 [95% CI: 0.9–2.3] months, P=0.052, Figure 4A). Moreover, the median OS did significantly varied between these two groups (10.7 [95% CI: 8.1–13.3] months in patients with mPFS 0 vs 4.4 [95% CI: 1.7–7.3] months in patients with mPFS 1 or 2, P=0.034, Figure 4B). Multivariate analysis identified good PS, limited disease (LD), and mGPS 0 as favorable independent predictors of PFS and OS in AMR monotherapy for elderly patients with relapsed SCLC (Tables 3 and 4).
 |
Table 3 Univariate and Multivariate Analyses for Progression-Free Survival |
 |
Table 4 Univariate and Multivariate Analyses for Overall Survival |
 |
Figure 1 Kaplan–Meier plots of survival. (A) Progression-free survival (PFS) and (B) overall survival (OS) of all patients. CI, confidence interval. |
 |
Figure 2 Kaplan–Meier plots of survival according to the type of relapse. (A) PFS and (B) OS. |
 |
Figure 3 Kaplan–Meier plots of survival according to performance status (PS). (A) PFS and (B) OS. |
 |
Figure 4 Kaplan–Meier plots of survival according to the modified Glasgow prognostic score (mGPS). (A) PFS and (B) OS. |
Toxicity Assessment and Dose Modification
The patients’ toxicity profiles are summarized in Table 5. The most common adverse events were hematological toxicities, such as neutropenia and leukopenia. Grade 3 or higher neutropenia and leukopenia occurred in 19 (46%) and 23 (56%) of the patients, respectively. Grade 3 or higher thrombocytopenia occurred in 11 (27%). Febrile neutropenia occurred in 9 patients (22%). A total of 152 cycles were administered. Among patients receiving 40 mg/m2/day as a starting dose of AMR, a dose reduction to 35 mg/m2/day was required in 10 patients (31%) since such as febrile neutropenia, grade 4 neutropenia lasted ≥4 days and grade 4 thrombocytopenia, but none of these patients required a subsequent dose reduction among them. Among patients receiving 35 mg/m2/day of AMR, a dose reduction to 30 mg/m2/day was required in one patient (13%) since grade 4 neutropenia lasted ≥4 days, but the patient did not require a subsequent dose reduction. A dose reduction was not required in one patient receiving an AMR starting dose of 30 mg/m2/day. Non-hematological toxic effects were relatively mild, and pneumonitis and treatment-related deaths did not occur.
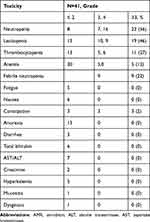 |
Table 5 Toxicities During AMR Chemotherapy |
Discussion
This retrospective study assessed the efficacy of AMR for the treatment of relapsed SCLC in elderly patients who had been previously treated with CE. Remarkably, our analysis revealed that AMR monotherapy was associated with a clinical response rate of 26.8%, a median PFS of 3.5 months, and a median OS of 8.1 months in the second line setting for elderly patients with ED-SCLC. In refractory cases, we observed a response rate of 22.7%, a PFS of 2.8 months, and an OS of 6.8 months. Considering that ED-SCLC patients typically have an OS of approximately about six weeks by a best supportive care,20 it is a critical piece of information that the findings of our study support the significance of AMR for refractory relapsed cases in elderly patients.
Among Euro-American cases with relapsed SCLC, topotecan has been the most widely used chemotherapy regimen for relapsed or refractory SCLC.21,22 However, it is known that TOP is not so effective for refractory cases based on a finding that an objective response rate by topotecan for the cases was only 5%.23 Horita reported a valuable systematic review and meta-analysis to evaluate clinical benefit and adverse events of AMR for patients with relapsed SCLC.24 The study revealed that AMR provides a better objective response for both types of relapse, a similar OS for sensitive-relapsed cases, better OS for refractory-relapsed cases, and a similar AE profile except for a higher risk febrile neutropenia compared to topotecan, and thus concluded that AMR is much more beneficial for Japanese patients with relapsed SCLC.24 We showed a list of clinical studies regarding AMR for relapsed SCLC patients including our present study in Table 6, indicating that the response rate of our study is comparable to those of the previous studies performed for mainly non-elderly patients.
 |
Table 6 Clinical Studies Regarding Amrubicin Monotherapy for Relapsed SCLC Patients |
Notably, Imai et al showed that AMR was effective and safe for elderly patients with relapsed ED-SCLC, reporting 3.4 months of PFS and 6.1 months of OS.26 In their study, the PFS and OS among the refractory cases were 2.7 and 5.5 months, respectively. Thus, the findings of our study in refractory cases are consistent with the observations of the previous study, indicating that AMR is a beneficial treatment option for elderly patients with either refractory or sensitive relapsed SCLC.
Data from earlier publications27,28 and a phase III study29 did not identify significant differences in the objective response rate and OS between the CE and AMR. Moreover, we previously reported that chemo-naïve elderly patients with ED-SCLC who received CE achieved a significantly longer PFS than those receiving AMR,30 consequently indicating that CE is the appropriate standard therapy for this population as well. The treatment landscape of SCLC is rapidly evolving. The published results of the first-line randomized trial comparing CE with CE plus atezolizumab (IMpower-133) indicated a longer PFS and OS among patients receiving atezolizumab, including elderly patients.7,31 The results of Impower-133 have changed the standard of care for elderly ED-SCLC patients and, thus, it should be mentioned that CE containing regimen is still the preferred regimen in chemotherapy for ED-SCLC patients.
Turning now to second line therapy by PD-1 inhibitor, the CheckMate-032 trial reported that the ORR and PFS of single agent nivolumab were 11% and 1.4 months in previously treated SCLC patients.32 Moreover, there was a recent press release that the phase III randomized trial (CheckMate-331) comparing nivolumab with standard of care (topotecan or amrubicin) in second-line therapy of ED-SCLC did not meet the primary endpoint of OS.33 As a result, the FDA approved nivolumab monotherapy for third-line SCLC.34 Considering the few available regimens as second-line chemotherapy for ED-SCLC patients whereas CE plus atezolizumab regimen was established as a new first line standard of care, it is certain that AMR represents an essential treatment option for elderly patients with relapsed ED-SCLC. We would like to emphasize that our study generated critical results demonstrating the efficacy and safety profile of AMR for the elderly patient population in the second-line setting.
We demonstrated that the pretreatment mGPS in patients with ED-SCLC was an independent predictor of PFS and OS, as well as PS and stage. The mGPS for lung cancer, which is based on serum Alb and CRP, was first described in 2003.35 It is a useful marker reflecting the state of inflammation and nutrition that has been identified as a prognostic factor in meta-analyses for non-SCLC (NSCLC).36,37 Although the mGPS has a clear cut-off value,19 few studies have considered the prognostic value of the mGPS for SCLC in contrast to the large number of studies in NSCLC and various other cancers.38–40 Furthermore, the prognosis of cancer patients is correlated to the nutritional status, and one-third of patient deaths are caused by malnutrition rather than cancer, and Alb is a convenient marker indicating the nutritional status.41 A previous study of pretreatment prognostic factors for survival in SCLC showed that Alb is significantly correlated with survival.42 Moreover, another study reported that mGPS was useful as a prognostic factor for OS in ED-SCLC patients, including the non-elderly population.43 To our knowledge, this is the first study describing the mGPS as a predictor of PFS and OS of AMR monotherapy for elderly SCLC patients relapsed to prior CE therapy.
Considering a recommending dose of AMR for elderly cases, previous Japanese studies indicated that 35 mg/m2 dose could be selected to relapsed SCLC patients,15,44,45 besides, a 25% of response rate was observed in patients receiving 35mg2 of AMR in our study. Thus, it is sure that both of 35 mg/m2 and 40 mg/m2 of AMR is recommended for relapsed elderly SCLC patients.
We previously reported a retrospective observational study46 and a non-randomized Phase II study,47 indicating that 40 mg/m2 of AMR could be considered as an appropriate treatment option for chemotherapy-naive elderly or poor-risk patients with ED-SCLC. Thus, we choose 40 mg/m2 of AMR as a starting dose for elderly patients with relapsed SCLC in the clinical practice. Meanwhile, although an AMR dose of 45 mg/m2 was reportedly effective, it produced intolerable toxicities and even treatment-related deaths in other studies.48,49 Furthermore, a randomized phase III study previously reported by Sekine et al indicated that higher incidences of febrile neutropenia and interstitial lung disease of grade 3 or worse occurred with 45 mg/m2 AMR; the authors concluded that AMR at 45 mg/m2 is intolerable in chemo-naïve elderly Japanese patients with ED-SCLC.27 These findings demonstrate that the appropriate AMR dose is critical for avoiding fatal adverse events, such as severe neutropenia or febrile neutropenia.
This study has several limitations. First, the results cannot be considered definitive because of the study’s retrospective single-center design and the relatively small sample size. Second, although the individuals included in this study were elderly, data regarding their quality of life were not evaluated.
Conclusion
In our study, AMR was an effective and beneficial regimen for elderly patients with relapsed SCLC after CE therapy. We would like to emphasize that our new findings provide guidance on AMR monotherapy for pursuing a new direction in clinical research on the treatment of elderly patients with relapsed SCLC. We are currently conducting a prospective observational study evaluating the clinical outcomes of AMR monotherapy in SCLC patients relapsed to prior CE plus atezolizumab therapy.
Abbreviations
Alb, albumin; AMR, amrubicin; CE, carboplatin plus etoposide; CI, confidence interval; CRP, C-reactive protein; CT, computed tomography; ED, extensive-disease; G-CSF, granulocyte colony-stimulating factor; LD, limited disease; mGPS, modified Glasgow prognostic score; MRI, magnetic resonance imaging; NSCLC, non-small-cell lung cancer; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; PS, performance status; SCLC, small-cell lung cancer.
Ethics Approval and Informed Consent
The ethical review board committee of Kitasato University and its affiliated hospitals approved the present study, which received ethical approval for the use of an opt-out method.
Acknowledgments
We thank the staff members of the Department of Respiratory Medicine, Kitasato University School of Medicine for their assistance in data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Toyoda Y, Nakayama T, Ioka A, Tsukuma H. Trends in lung cancer incidence by histological type in Osaka, Japan. Jpn J Clin Oncol. 2008;38(8):534–539. doi:10.1093/jjco/hyn072
2. van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. doi:10.1016/S0140-6736(11)60165-7
3. Shi Y, Sun Y. Medical management of lung cancer: experience in China. Thorac Cancer. 2015;6(1):10–16. doi:10.1111/1759-7714.12168
4. Shi Y, Xing P, Fan Y, et al. Current small cell lung cancer treatment in China. Thorac Cancer. 2015;6(3):233–238. doi:10.1111/1759-7714.12218
5. Morita T. A statistical study of lung cancer in the annual of pathological autopsy cases in Japan, from 1958 to 1997, with reference to time trends of lung cancer in the world. Jpn J Cancer Res. 2002;93(1):15–23. doi:10.1111/j.1349-7006.2002.tb01195.x
6. Mitsudomi T, Akita H, Asamura H, et al. Medical Guideline of Lung Cancer of the Japan Lung Cancer Society. Vol. 3. Tokyo: Kanehara & Co. Ltd; 2016:188–198.
7. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small- cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
8. Ishizumi K, Ohashi N, Tanno N. Stereospecific total synthesis of 9-aminoanthracyclines: (+)-9-amino-9-deoxydaunomycin and related compounds. J Org Chem. 1987;52(20):4477–4485. doi:10.1021/jo00229a010
9. Hanada M, Mizuno S, Fukushima A, Saito Y, Noguchi T, Yamaoka T. A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complex. Jpn J Cancer Res. 1998;89(11):1229–1238. doi:10.1111/j.1349-7006.1998.tb00519.x
10. Yamaoka T, Hanada M, Ichii S, Morisada S, Noguchi T, Yanagi Y. Cytotoxicity of amrubicin, a novel 9-aminoanthracycline, and its active metabolite amrubicinol on human tumor cells. Jpn J Cancer Res. 1998;89(10):1067–1073. doi:10.1111/j.1349-7006.1998.tb00498.x
11. Morisada S, Yanagi Y, Noguchi T, Kashiwazaki Y, Fukui M. Antitumor activities of a novel 9-aminoanthracycline (SM-5887) against mouse experimental tumors and human tumor xenografts. Jpn J Cancer Res. 1989;80(1):69–76. doi:10.1111/j.1349-7006.1989.tb02247.x
12. Noda T, Watanabe T, Kohda A, Hosokawa S, Suzuki T. Chronic effects of a novel synthetic anthracycline derivative (SM-5887) on normal heart and doxorubicin-induced cardiomyopathy in beagle dogs. Invest New Drugs. 1998;16(2):121–128. doi:10.1023/A:1006088907271
13. Yana T, Negoro S, Takada M, et al. West Japan Thoracic Oncology Group: phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: west Japan Thoracic Oncology Group (WJTOG) study. Invest New Drugs. 2007;25(3):253–258. doi:10.1007/s10637-006-9012-9
14. Murakami H, Yamamoto N, Shibata T, et al. A single‐arm confirmatory study of amrubicin therapy in patients with refractory small‐cell lung cancer: Japan Clinical Oncology Group Study (JCOG0901). Lung Cancer. 2014;84(1):67–72. doi:10.1016/j.lungcan.2014.01.012
15. Kaira K, Sunaga N, Tomizawa Y, et al. A phase II study of amrubicin, a synthetic 9‐aminoanthracycline, in patients with previously treated lung cancer. Lung Cancer. 2010;69(1):99–104. doi:10.1016/j.lungcan.2009.09.012
16. Inoue A, Sugawara S, Yamazaki K, et al. Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: north Japan Lung Cancer Study Group Trial 0402. J Clin Oncol. 2008;26(33):5401–5406. doi:10.1200/JCO.2008.18.1974
17. Onoda S, Masuda N, Seto T, et al. Thoracic oncology research group study 0301: amrubicin for treatment of refractory or relapsed small-cell lung cancer: a phase II thoracic oncology research group study 0301. J Clin Oncol. 2006;24(34):5448–5453. doi:10.1200/JCO.2006.08.4145
18. Travis WD, Brambilla E, Nicholson AG, et al. WHO panel. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
19. Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment Glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol. 2012;7(4):655–662. doi:10.1097/JTO.0b013e318244ffe1
20. Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4(2):31–42.
21. Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer: ESMO clinical practice guidelines. Ann Oncol. 2013;24:vi99–vi105. doi:10.1093/annonc/mdt178
22. Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11:78–98. doi:10.6004/jnccn.2013.0011
23. Horita N, Yamamoto M, Sato T, et al. Topotecan for relapsed small-cell lung cancer: systematic review and meta-analysis of 1347 patients. Sci Rep. 2015;5(1):15437. doi:10.1038/srep15437
24. Horita N, Yamamoto M, Sato T, et al. Amrubicin for relapsed small-cell lung cancer: a systematic review and meta-analysis of 803 patients. Sci Rep. 2016;11(6):18999. doi:10.1038/srep18999
25. von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012–4019. doi:10.1200/JCO.2013.54.5392
26. Imai H, Sugiyama T, Tamura T, et al. Gunma-Ibaraki-Fukushima-Tochigi (GIFT) group: a retrospective study of amrubicin monotherapy for the treatment of relapsed small cell lung cancer in elderly patients. Cancer Chemother Pharmacol. 2017;80(3):615–622. doi:10.1007/s00280-017-3403-9
27. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer. 2007;97(2):162–169. doi:10.1038/sj.bjc.6603810
28. Quoix E, Breton JL, Daniel C, et al. Etoposide phosphate with carboplatin in the treatment of elderly patients with small cell lung cancer: a phase II study. Ann Oncol. 2001;12(7):957–962. doi:10.1023/A:1011171722175
29. Sekine I, Okamoto H, Horai T, et al. A randomized phase III study of single-agent amrubicin vs. carboplatin/etoposide in elderly patients with extensive-disease small-cell lung cancer. Clin Lung Cancer. 2014;15(2):96–102. doi:10.1016/j.cllc.2013.11.006
30. Igawa S, Shirasawa M, Ozawa T, et al. Comparison of carboplatin plus etoposide with amrubicin monotherapy for extensive-disease small cell lung cancer in the elderly and patients with poor performance status. Thorac Cancer. 2018;9(8):967–973. doi:10.1111/1759-7714.12772
31. Pacheco J, Bunn PA. Advancements in small-cell lung cancer: the changing landscape following IMpower-133. Clin Lung Cancer. 2019;20(3):148–160. doi:10.1016/j.cllc.2018.12.019
32. Ready NE, Ott PA, Hellmann MD, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the checkmate 032 randomized cohort. J Thorac Oncol. 2020;15(3):426–435. doi:10.1016/j.jtho.2019.10.004
33. Reck M, Vicente D, Ciuleanu T, et al. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann Oncol. 2018;29:abstr LBA5. doi:10.1093/annonc/mdy511.004
34. Ready N, Farago AF, de Braud F, et al. Third line nivolumab monotherapy in recurrent SCLC: checkMate 032. J Thorac Oncol. 2019;14(2):237–244. doi:10.1016/j.jtho.2018.10.003
35. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89(6):1028–1030. doi:10.1038/sj.bjc.6601242
36. Zhu L, Chen S, Ma S, Zhang S. Glasgow prognostic score predicts prognosis of non-small cell lung cancer: a meta-analysis. SpringerPlus. 2016;5:439. doi:10.1186/s40064-016-2093-9
37. Jin J, Hu K, Zhou Y, Li W. Prognostic value of the Glasgow prognostic score in lung cancer: evidence from 10 studies. Int J Biol Markers. 2018;33(2):201–207. doi:10.5301/ijbm.5000308
38. Zhou T, Hong S, Hu Z, et al. A systemic inflammation-based prognostic scores (mGPS) predicts overall survival of patients with small-cell lung cancer. Tumour Biol. 2015;36(1):337–343. doi:10.1007/s13277-014-2623-4
39. Kurishima K, Watanabe H, Ishikawa H, Satoh H, Hizawa N. Modified Glasgow prognostic score in patients with small-cell lung cancer. Mol Clin Oncol. 2017;7(1):121–124. doi:10.3892/mco.2017.1261
40. Minami S, Ogata Y, Ihara S, Yamamoto S, Komuta K. Pretreatment Glasgow prognostic score and prognostic nutritional index predict overall survival of patients with advanced small cell lung cancer. Lung Cancer (Auckl). 2017;8:249–257. doi:10.2147/LCTT.S142880
41. Garcia-Luna PP, Parejo Campos J, Pereira Cunill JL. [Causes and impact of hyponutrition and cachexia in the oncologic patient]. Nutricon Hospitalaria. 2006;21(Suppl3):10–16.
42. Maestu I, Pastor M, Gomez-Codina J, et al. Pretreatment prognostic factors for survival in small cell lung cancer: a new prognostic index and validation of three known prognostic indices on 341 patients. Ann Oncol. 1997;8(6):547–553. doi:10.1023/A:1008212826956
43. Sonehara K, Tateishi K, Komatsu M, et al. Modified Glasgow prognostic score as a prognostic factor in patients with extensive disease-small-cell lung cancer: a retrospective study in a single institute. Chemotherapy. 2019;64(3):129–137. doi:10.1159/000502681
44. Okamoto I, Hamada A, Matsunaga Y, et al. Phase I and pharmacokinetic study of amrubicin, a synthetic 9-aminoanthracycline, in patients with refractory or relapsed lung cancer. Cancer Chemother Pharmacol. 2006;57(3):282–288. doi:10.1007/s00280-005-0051-2
45. Igawa S, Yamamoto N, Ueda S, et al. Evaluation of the recommended dose and efficacy of amrubicin as second- and third-line chemotherapy for small cell lung cancer. J Thorac Oncol. 2007;2(8):741–744. doi:10.1097/JTO.0b013e31811f46f0
46. Igawa S, Ryuge S, Fukui T, et al. Amrubicin for treating elderly and poor-risk patients with small-cell lung cancer. Int J Clin Oncol. 2010;15(5):447–452. doi:10.1007/s10147-010-0085-2
47. Igawa S, Otani S, Ryuge S, et al. Phase II study of Amrubicin monotherapy in elderly or poor-risk patients with extensive disease of small cell lung cancer. Invest New Drugs. 2017;35(5):642–648. doi:10.1007/s10637-017-0482-8
48. Kato T, Nokihara H, Ohe Y, et al. Phase II trial of amrubicin in patients with previously treated small cell lung cancer (SCLC). J Clin Oncol. 2006;24(18 Suppl):7061. doi:10.1200/jco.2006.24.18_suppl.7061
49. Asao T, Nokihara H, Yoh K, et al. Phase II study of amrubicin at a dose of 45 mg/m2 in patients with previously treated small-cell lung cancer. Jpn J Clin Oncol. 2015;45(10):941–946. doi:10.1093/jjco/hyv107
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
