Back to Journals » OncoTargets and Therapy » Volume 13
Immunotherapy Combined with Chemotherapy as a Promising Therapy for a EGFR Exon 19 Deletion with MET Amplification Patient with Non-Small-Cell Lung Cancer: A Case Report
Authors Ni Q , Pan C, Dai S, Wang P
Received 28 December 2019
Accepted for publication 24 March 2020
Published 9 April 2020 Volume 2020:13 Pages 3039—3044
DOI https://doi.org/10.2147/OTT.S243988
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
QingTao Ni,1 Chi Pan,2 ShengBin Dai,1 Peng Wang1
1Department of Oncology, Jiangsu Taizhou People’s Hospital, Taizhou 225300, People’s Republic of China; 2Department of General Surgery, Jiangsu Taizhou People’s Hospital, Taizhou 225300, People’s Republic of China
Correspondence: Peng Wang
Department of Oncology, Jiangsu Taizhou People’s Hospital, Hailing South Road 399, Taizhou 225300, People’s Republic of China
Tel +86 134 0552 4908
Email [email protected]
Abstract: Advanced non-small-cell lung cancer (NSCLC) patients with EGFR exon 19 deletion often get benefits from the treatment of tyrosine kinase inhibitors (TKI). In the same way, the NSCLC patients with mesenchymal-to-epithelial transition (MET) amplification get benefits from crizotinib. The treatment becomes extremely difficult for the patients with both EGFR exon 19 deletion and MET amplification, after failure of first-line TKI. An advanced NSCLC patient with EGFR exon 19 deletion was treated with TKI. However, the disease recurred after four months. MET amplification was found after biopsy again. The patient was treated with the combination of crizotinib, while the disease recurred after eight months. The patient was treated by pembrolizumab and pemetrexed + carboplatin chemotherapy as salvage therapy. The therapeutic effect has been remarkable up to present. In conclusion, immunotherapy combined with chemotherapy could be a promising therapy for the NSCLC patients with both EGFR exon 19 deletion and MET amplification after the failure of first-line TKI treatment. Thus, further insights into the variant genes contribute to NSCLC treatment.
Keywords: non-small-cell lung cancer, immunotherapy, chemotherapy, targeted therapy, PD-L1
Introduction
For patients with advanced non-small-cell lung cancer (NSCLC) patients with a mutant epidermal growth factor receptor (EGFR), EGFR tyrosine kinase inhibitors (TKIs) are the standard first-line therapy.1 EGFR-TKIs have superior survival in terms of the objective response rate (ORR) (67.0%) and median progression-free survival (PFS) (10.9 months).2 However, acquired resistance is almost inevitable within 9–14 months.3 NSCLC harboring EGFR exon 19 deletion mutations is sensitive to 1st-generation TKI including gefitinib and erlotinib.4 Mesenchymal-to-epithelial transition (MET) amplification has been shown to develop as a resistance mechanism to treatment with first-line EGFR-TKIs in NSCLC.5
Case Report
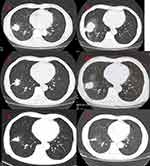
The current study presents the case of a 61-year-old male who developed irritable cough without medical history of interest presented to our hospital in October 2017. A positron emission tomography computed tomography (PET-CT) was obtained one month later, which demonstrated abnormal increase of glucose metabolism in the lower lobe of the right lung, considered as malignant tumor; abnormal high glucose metabolism in the liver, abnormal increase of glucose metabolism and soft tissue density in both adrenal regions, considered as metastasis (Figure 1). Pathology of masses in lung and liver indicated tumor cells, NapsinA+, thyroid transcription factor-1 (+), alpha-fetoprotein (AFP) (+), Hepa-1 (−), Prostate-specific antigen (PSA) (–), CD10 (−), CD34 showed vessels, cytokeratin 7 (CK7) (+), carcinoembryonic antigen CEA (+), Ki-67 positive index about 70%. The two tumors were similar in morphology. They conformed to primary lung adenocarcinoma. The results of EGFR mutations showed EGFR exon 19 deletion mutation. Therefore, the targeted therapy was given to the patient with gefitinib 250mg per os QD in December 12, 2017. Re-examination of chest CT showed the lung focus was obviously shrinked (Figure 2A). It was regarded that pulmonary lesions as partial remission (PR) by clinical evaluation.
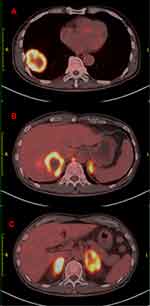 |
Figure 1 Whole-body PET-CT findings. PET-CT imagings showed abnormal increase of glucose metabolism in the lower lobe of the right lung (A), in the liver (B) and in both adrenal regions (C). |
However, the masses in lung were lager than before in April 23, 2018 (Figure 2B). Gene testing blood was performed again and the results showed EGFR E19 mutation and MET amplification. gefitinib (250mg per os QD) with crizotinib (250mg per os BID) was given to the patient as the targeted therapy. The lung CT showed the lung focus was obviously shrinked again in June 06, 2018 (Figure 2C). Moreover, Re-examination of chest CT showed recurrent progress of lesions in December 18, 2018 (Figure 2D). For further treatment, the patient underwent genetic of blood testing again. The results showed that programmed cell death 1 ligand 1 (PD-L1) positivity (50%), the tumor mutational burden (high), EGFR E19 mutation and MET amplification. The patient was treated by immunotherapy (pembrolizumab 100mg intravenous, every 21 days) combined with chemotherapy (pemetrexed 800 mg intravenous + carboplatin 0.4g d2 intravenous, every 28 days, six cycles, then, maintenance of pemetrexed 800 mg alone) as salvage therapy. CT scan revealed the clinical response with shrinkage of over 30% of the lung lesion on February 27, 2019 (Figure 2E). Examination of chest CT showed the patient was PR on June 26, 2019 (Figure 2F). The masses in liver were also significantly smaller than before (Figure 3). The diagnosis and administered treatments of this NSCLC patient were summarized (Figure 4).
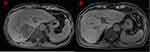 |
Figure 3 The masses in liver before and after treatment. The masses in liver of upper abdomen MRI before any treatment (A) and after immunotherapy combined with chemotherapy (B). |
 |
Figure 4 Timeline of events since the diagnosis and summary of administered treatments. |
At the time of submission of this manuscript, the patient maintains improved quality of life with no pulmonary symptoms, either secondary adverse events related to pembrolizumab, and responded optimally to the treatment with regular annual clinical and radiographic follow-ups.
Discussion
Lung cancer is the leading cause of cancer-related death worldwide. The prognosis of NSCLC still remains disappointing, with a 5-year survival rate was 19%.6 The discovery of lung cancer-driven genes and specific molecular targeted drugs has significantly improved the survival rate of patients.7 Current first-line treatment decisions for advanced NSCLC are guided by the presence of molecular driver. The mutation of EGFR is mainly located in exon 18–21, among which exon 19 deletion mutation and exon 21 L858R mutation are the most common EGFR gene sensitive mutations, accounting for 90% of all mutation types. A large number of clinical studies have shown that the objective response rate (ORR) and progression-free survival (PFS) of advanced NSCLC with EGFR mutation after first-line treatment with tyrosinase inhibitors are significantly better than the traditional platinum-containing two-drug chemotherapy regimen.8–10 EGFR-TKI has become the primary treatment for patients with EGFR mutation. The first generation of EGFR-TKI mainly includes gefitinib, erlotinib and icotinib. Lung cancer patients with EGFR mutation have obvious curative effect after treatment with EGFR-TKI, but drug resistance usually occurs in 9–14 months and disease deterioration occurs.11 Several mechanisms are believed to be responsible for acquired resistance to EGFR-TKI, including secondary EGFR T790M (50–65%), MET amplification (5%), activation of MET/HGF axis, and histological transformation.11,12 The gene detection results of the patient after the first drug resistance in our department showed MET amplification except EGFR E19 mutation. Crizotinib is a dual MET and ALK inhibitor and has shown response in NSCLC with MET amplification alone.13,14 Combining MET-TKI with EGFR-TKI had significant effects in EGFR-mutated and MET-dysregulated patients with NSCLC after resistance of EGFR-TKI treatment, with an ORR (objective response rate) of 47% in patients with a MET gene copy number greater than or equal to.15 Therefore, we chose gefitinib combined with crizotinib in this patient. Although the patients with EGFR exon 19 deletions had an average of 34 months median progression-free survival (PFS),16 while the patients with MET amplification could also obtain 6.5 months PFS from crizotinib.17 The patients relapsed unfortunately at four months and seven months, respectively, indicating that the multiple driving genes may play a role and high mutation complex of patients. This is consistent with the gene detection report.
Except targeted therapy, immune checkpoint inhibition, especially anti-PD-1, has drastically changed the treatment landscape of patients with NSCLC. Pembrolizumab is a kind of high-affinity anti-PD-1 humanized monoclonal antibody, which interacted with PD-L1 and PD-L2, and destroyed cancer cells by the autoimmune system.18 The results of KEYNOTE-042 showed that pembrolizumab alone is effective in patients a PD-L1 tumor-expression level ≥50% with 16.7 months OS and in patients a PD-L1 tumor-expression level <50% with 12.1 months OS.19 The results of KEYNOTE-189 showed that the median PFS was 8.8 months in the pembrolizumab-combination group and 4.9 months in the placebo-combination group.19 Moreover, the median PFS for patients with high (≥20 mutations/mb) was 12.8 months versus low to intermediate TMB was 3.3 months.21 On April 26, 2018, the National Comprehensive Cancer Network (NCCN) released the fourth edition of clinical practice guidelines for NSCLC in 2018. Based on the results of KEYNOTE-189 trial, the first-line treatment of advanced NSCLC with combination of pembrolizumab (Keytruda) and chemotherapy (cisplatin/carboplatin + pemetrexed) was included in the guidelines.20 However, the addition of immunotherapy has no benefit for patients with stages I to III NSCLC.22 In this patient, after the treatment of gefitinib combined with crizotinib, the disease was progressed again, unfortunately. The gene detections were performed again and found the PD-1>50%. Therefore, immunotherapy combined with chemotherapy was applied and the disease was controlled. In spite of some studies believe that NSCLC patients with EGFR mutations do not respond well to immunotherapy.3 Until now, the disease is no more progressed. Special attention should be paid to immune-related adverse events during the immunotherapy.23 KRAS, DDR2, and TP53 variants are also common mutations in lung cancer patients.24 Thus, deeper studying of these variants may help discover new therapeutic targets for NSCLC.
Conclusion
In conclusion, chemotherapy combined with PD-1 is a potential treatment for patients with multiple driving genes/high mutation load such as EGFR and MET who fail in TKI treatment. Thus, further insights into the variant genes contribute to NSCLC treatment.
Ethics and Consent Statement
The study was approved by the Human Ethics Review Committee of Jiangsu Taizhou People’s Hospital. No institutional approval was required to publish the case details.
Consent to Publish
Written informed consent to publish this report and the associated medical images were provided by the patient.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mok TS, Wu Y, Ahn M, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi:10.1056/NEJMoa1612674
2. Wu YL, Saijo N, Thongprasert S, et al. Efficacy according to blind independent central review: post-hoc analyses from the Phase III, randomized, multicenter, IPASS study of first-line gefitinib versus carboplatin/paclitaxel in Asian patients with EGFR mutation-positive advanced NSCLC. Lung Cancer. 2017;104:119–125. doi:10.1016/j.lungcan.2016.11.022
3. Lin A, Wei T, Meng H, et al. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer. 2019;18:139. doi:10.1186/s12943-019-1062-7
4. Hsu PC, Jablons DM, Yang CT, et al. Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC). Int J Mol Sci. 2019;20(15):3821. doi:10.3390/ijms20153821
5. Duncan DJ, Vandenberghe ME, Scott M, et al. Fast fluorescence in situ hybridisation for the enhanced detection of MET in non-small cell lung cancer. PLoS One. 2019;14:e223926. doi:10.1371/journal.pone.0223926
6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi:10.3322/caac.21551
7. Shea M, Costa DB, Rangachari D. Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches. Ther Adv Respir Dis. 2016;10:113–129. doi:10.1177/1753465815617871
8. Lee CK, Davies L, Wu YL, et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J Natl Cancer Inst. 2017;109. doi:10.1093/jnci/djw279
9. Xie Y, Liang J, Su N. [Gefitinib versus Erlotinib as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer]. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:446–449. Chinese.
10. Lin L, Zhao J, Hu J, et al. Impact of weight loss at presentation on survival in epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) sensitive mutant advanced non-small cell lung cancer (NSCLC) treated with first-line EGFR-TKI. J Cancer. 2018;9:528–534. doi:10.7150/jca.22378
11. Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–i19. doi:10.1093/annonc/mdx703
12. Morgillo F, Della CC, Fasano M, et al. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1:e60. doi:10.1136/esmoopen-2016-000060
13. Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6:942–946. doi:10.1097/JTO.0b013e31821528d3
14. Gainor JF, Niederst MJ, Lennerz JK, et al. Dramatic response to combination erlotinib and crizotinib in a patient with advanced, EGFR-mutant lung cancer harboring de novo MET amplification. J Thorac Oncol. 2016;11:e83–e85. doi:10.1016/j.jtho.2016.02.021
15. Wu YL, Zhang L, Kim DW, et al. Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J Clin Oncol. 2018;36:3101–3109. doi:10.1200/JCO.2018.77.7326
16. Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi:10.1158/1078-0432.CCR-05-1846
17. Song Z, Wang H, Yu Z, et al. De novo MET amplification in Chinese patients with non-small-cell lung cancer and treatment efficacy with crizotinib: a multicenter retrospective study. Clin Lung Cancer. 2019;20:e171–e176. doi:10.1016/j.cllc.2018.11.007
18. De Mattia E, Cecchin E, Guardascione M, et al. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J Gastroenterol. 2019;25(29):3870–3896. doi:10.3748/wjg.v25.i29.3870
19. Mok T, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, Phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi:10.1016/S0140-6736(18)32409-7
20. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078–2092. doi:10.1056/NEJMoa1801005
21. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi:10.1158/1535-7163.MCT-17-0386
22. Zhu J, Li R, Tiselius E, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2017;12:D11300.
23. Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;19(1):558. doi:10.1186/s12885-019-5701-6
24. Fathi Z, Mousavi S, Roudi R, et al. Distribution of KRAS, DDR2, and TP53 gene mutations in lung cancer: an analysis of Iranian patients. PLoS One. 2018;13:e200633. doi:10.1371/journal.pone.0200633
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.