Back to Journals » Drug Design, Development and Therapy » Volume 15
Immunomodulatory Effect of a New Ingredients Group Extracted from Astragalus Through Membrane Separation Technique
Authors Zhang D, Guo Y, Wang Y
Received 3 March 2021
Accepted for publication 31 March 2021
Published 15 April 2021 Volume 2021:15 Pages 1595—1607
DOI https://doi.org/10.2147/DDDT.S309422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Di Zhang,1,* Yafei Guo,2,* Yingli Wang2
1Department of Basic Medicine, Shanxi Medical University, Taiyuan, People’s Republic of China; 2Department of Traditional Chinese Medicine and Food Engineering, Shanxi University of Traditional Chinese Medicine, Jinzhong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Di Zhang Email [email protected]
Introduction: Astragalus is a commonly used traditional Chinese medicine in China, which has been widely applied to enhance the immunomodulatory function of the body. The main bioactive components are complicated. To explore the role of the components, various techniques have been applied in Astragalus extraction. Membrane separation technique featured with green processing condition and high efficiency is of signification interest in the application of Astragalus treatment.
Methods: In this study, a new ingredients group A4 was separated from Astragalus using membrane separation technique. The quantification and identification of A4 were achieved by UV-vis spectrometry and UPLC-MS measurements. Pathological approaches along with serum metabolomics were utilized to study the immunoprotective effects of the extracts and explore the underlying mechanisms on metabolic activity.
Results: It was observed that A4 could promote the secretion of IL-2 and IFN-γ, stimulate the activated CD4+CD25+ and CD8+ CD25+ T lymphocytes in splenocytes and protect rat spleen to some extent. Seven crucial biomarkers that related to immunity regulations were screened out and identified through serum metabonomic analysis coupled with nuclear magnetic resonance. The enrichment analysis revealed that A4 alleviated the immune dysfunction by modulating amino acid metabolism and energy metabolism for the first time.
Conclusion: The new ingredients group A4 isolated from the Astragalus membrane can reduce the immune dysfunction by regulating the amino acid metabolism and energy metabolism of rats.
Keywords: Astragalus, membrane separation, immunomodulatory, serum metabolomics
Introduction
Astragalus (AR) is the dried roots of leguminous plant Astragalus membranaceus (Fisch.) Bge. Var. Mongholicus (Bge.) Hsiao and Astragalus membranaceus (Fisch.) Bge. It is one of the largest flowering plants in Leguminosae and widely distributed in temperate and arid areas.1 As a commonly used traditional Chinese medicine in China, AR is known for its therapeutic benefits on immune-related diseases, such as inflammation, cancer, diabetes and hyperglycemia.2 It has been proven that AR exhibits its immunomodulatory effects through improving the immune organ developments,3 regulating the secretion of mucus, enhancing the quantity and activity of innate immunity,4 promoting the proliferation and differentiation of acquired immunity cells, and increasing the concentration of serum antibodies.5
In AR, there are over 100 ingredients and the main bioactive components are saponins, flavonoids and polysaccharides.5 To have a better understanding of the underlying mechanism, various techniques have been applied to extract and purify AR, including organic solvent extraction,6 macro-porous resin chromatography, enzyme-assisted extraction,7 and ultrafine pulverization.8 However, these processes either are time-consuming with low-yield, or involve organic solvents, or create a great deal of secondary waste. In comparison, membrane separation with the characteristics of mild conditions and high efficiency, makes the process green.9 Recently, membrane separation technology has been widely used in processing food9,10 and traditional Chinese medicine.11,12 Negin et al concentrated the polyphenolic compounds from pistachio hull extract through a polysulfone membrane assembled in a batch dead-end filtration plastic cell and evaluated the antioxidant activity.9 Avram et al extracted polyphenols from blueberry pomace through crossflow filtration technology with nanofiltration membranes.10 Pei et al successfully isolated the antimicrobial peptides from the mucus of A. davidianus using the magnetic cell membranes.11 Tang et al employed sequential ultrafiltration membranes to purify polysaccharide fractions from the water extract of Lentinus edodes.12 However, limited researches have been focused on AR membrane filtration. Thus, it is of great significance to explore the application of membrane separation in AR ingredients.
Metabolomics is a useful tool aiming to identify the interactions between the endogenous metabolites and external stimulation, and determine its influences on the pathobiology of disease.13 Since the metabolites are sensitive to the disturbance of physiological homeostasis, their changes dynamically reflect the physiological and pathological state in the body.14 Recently, metabolomics has been extensively applied to the discovery and therapeutic evaluation for the physiological functions of AR. Jia et al explored AR mechanism responding to progressive drought stress through analyzing its transcriptome and metabolome.15 Liu et al applied urine metabolomics to discuss the benefits of drug efficacy when adding honey in AR.16 Wang et al proved that AR polysaccharide could ameliorate the digestive and absorptive functions by serum metabolomics.17 Zeng et al reported that AR polysaccharide regulated the energy metabolism of heat stressed dairy cows through glucose metabolism and amino acid metabolism.18 Metabolomics is therefore a desirable technique to study the immunomodulatory mechanism of AR ingredients.
Herein, we report a new ingredients group extracted from AR using polymer membranes. Identification and quantification of the extracts were analyzed by UV-vis spectrometry and UPLC-MS measurements. Cyclophosphamide (CTX) and hydrocortisone (HC) were utilized for the establishment of immunosuppressive rat models. Serum metabolomics along with pathological approaches were further conducted to further explore its immunomodulatory mechanism.
Materials and Methods
AR Separation and Purification
Dried AR 210 g was soaked in 10 times of distilled water for 30 min, brought to boil over high heat and then simmered for 40 min. The supernatant was collected by filtration over 30 °C. The precipitant was soaked again in 10 times of distilled water and extracted by repeating the above steps. The supernatants were centrifuged at 2500 rpm for 5 min and filtered again. The filtrates were subjected to multi-stage membrane separation and the process is shown in Figure 1.9–12 The fraction with molecular weight of 600–2500 Da, which mainly saponins and flavonoids, was collected and prepared as lyophilized powder. To obtain the optimal separation condition, the separation conditions were carefully examined and determined the temperature was 25 °C, solid-liquid was 1:25, pH was 4.85 and processing time was 9 min. The achieved new ingredients group was designated as A4.
 |
Figure 1 Flow chart of Astragalus membrane separation process. |
Quantification and Identification of A4
UV-vis spectrometry was used to measure the contents of saponins and flavonoids. A4 lyophilized powder was dissolved in 0.1 mL distilled water. 0.4 mL ethanol, 0.5 mL 8% vanillin in anhydrous ethanol and 5 mL H2SO4 were added subsequently. The mixture was warmed in water bath at 60 °C for 25 min and cooled to ambient temperature for the measurement of the saponins quantity. Similarly, the content of flavonoids was detected. 0.1 mL A4 dissolved sample was mixed with 0.5 mL sodium nitrite, 0.5 mL 10% aluminium nitrate and 4 mL NaOH successively. The mixture was then thoroughly stirred for 10 min and dilute to 10 mL for further analysis.
To identify the ingredients in A4 lyophilized powder, UPLC-MS was performed using Q-Exactive UPLC-MS/MS system. The Acquity UPLC HSS T3 column (100×2.1 mm, 1.8 μm) was used with column temperature maintained at 35°C. The mobile phases at a flow rate at 0.3 mL/min, were acetonitrile (A) and water (B). The injection volume was 5 μL.
Animal and Drug Administration
A total of 25 males and 25 females specific-pathogen free Sprague Dawley rats weighting 200±20 g were purchased from Beijing Changyang Xishan Farm and acclimated to the environment for 7 days before starting the experiments. All rats were housed in an animal room with 12-hr light/dark cycle and given ad libitum access to water and food.
After acclimated to the environment, rats were randomly assigned into as follows: (1) control group in which the rats were intraperitoneally injected physiological saline for a total of 14 days and intragastrically administered physiological saline for a total of 10 days from day 4 of the experiment; (2) Cyclophosphamide (CTX) and hydrocortisone (HC) groups, in which rats were intraperitoneally injected 40 mg·kg−1·d−1 CTX and HC for a total of 14 days and intragastrically administered equal amount of physiological saline for a total of 10 days from day 4 of the experiment, respectively; (3) CTX-A4 and HC-A4 groups in which the rats were intraperitoneally injected 40 mg·kg−1·d−1 CTX and HC for a total of 14 days and intragastrically administered 50 mg·kg−1·d−1 A4 aqueous solution for a total of 10 days from day 4 of the experiment, respectively. Each group has 5 female rats and 5 male rats.
All animal procedures were performed in accordance with the guidelines for Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1996), and the study protocol was reviewed and approved by the ethics committee of Shanxi University of Traditional Chinese Medicine.
Sample Collection and Pharmacological Index Testing
On the 14th day, rats were fasted overnight but allowed to drink water at ad libitum. On the next day, within 0.5 h after intragastric administration, they were weighed, and their blood samples were taken from the vein behind eyeballs. After stand for 30 min, these blood samples were centrifuged at 3500 rpm for 10 min and the serums were collected and frozen at −80°C. Serum IL-2 and IFN-γ levels were tested by ELISA (purchased from Shanghai Chuangshi Technology Co., Ltd.).
According to ethical requirements, we anesthetized the rats with 5% sodium pentobarbital, took blood samples, and then euthanized them. Afterwards, under sterile conditions the spleens of the rats were collected in a sterile environment. After removal of the surface blood with filter paper, spleen samples were weighed freshly. Spleen of 4 rats in each group were fixed with 4% paraformaldehyde fixation solution. The spleen was later sectioned, stained using hematoxylin–eosin (HE) staining and examined under a fluorescence microscopy imaging system (BX53, Olympus, Japan).
Spleen samples from the other rats in each group were used to isolate splenocytes after stimulated with concanavalin A (Sigma, Germany), and stained with rat CD4, CD8, and CD25 monoclonal antibodies (eBioscience ™, Thermo Fisher Scientific, USA). A total of about 50,000 cells were subjected to flow cytometry analysis (FAC Scanto II, BD, USA) to detect CD4+CD25+ T cells and CD8+CD25+ T cells.
Study on Serum Metabolomics in Rats
A total of 450 μL serum samples were thoroughly mixed with 1500 μL methanol by vortexing for 1 min. After incubated at −20°C for 20 min, precipitated proteins were removed by centrifugation at 13,000 rpm at 4°C for 20 min. One milliliter of supernatant was transferred into an EP tube, dried under a stream of nitrogen and reconstituted in 650 μL of 100 mM sodium phosphate buffer, pH 7.4, containing 10% D2O and 0.01% TSP. After centrifugation again at 13,000 rpm at 4°C for 20 min, 600 μL supernatant was transferred to a 5 mm NMR tube for 1H NMR analysis on a Bruker Advance III 600 MHz spectrometer (Bruker, Germany) at 25°C.
Each 1H NMR spectrum was processed by Mest ReNova software (V6.1.0-6624). After phase and baseline calibration using TSP (δ0.00) as the standard, the spectrum was integrated in the range of δ0.0–10.0 ppm with integration interval of 0.02 ppm, except δ4.70–5.05 (residual water peaks) and δ3.30–3.40 (residual methanol peaks). The sum of the integral areas of the spectrum was normalized to generate the data matrix of all segments and corresponding areas. The data matrix was then imported into Metabo Analyst online analysis platform (V4.0, https://www.metaboanalyst.ca/faces/home.xhtml) for multivariate statistical analysis.
The principal component analysis (PCA) and Orthogonal partial least squares-discriminant analysis (OPLS-DA) analysis were subsequently used to identify the differential metabolites using a variable importance factor (VIP) >1.0 and P<0.01. The online analysis platform Metabo Analyst was selected to analyze the metabolic pathways related to the differential metabolites, and the KEGG database was used to conduct a preliminary discussion on the related metabolic pathways.
Data Processing
The experimental data was statistically analyzed using SPSS 19.0 software, and the results were expressed as mean ± standard deviation. Differences between groups were compared using the one-way analysis of variance, and p<0.05 was considered statistically significant.
Results
Characterization of A4 Lyophilized Powder
To avoid the introduction of organic solvent, membrane separation was selected for AR treatment and a new ingredients group A4 with high concentrations of saponins and flavonoids was obtained. Considering that the temperature, solid–liquid ratio, pH and processing time had significant impacts on the separation efficiency, these factors were carefully investigated and optimized. UPLC-MS measurements were further applied to identify the constituents in A4 (Figures S1 and S2). It was detected that the bioactive powder mainly contains 14 types of flavonoids and 8 types saponins (Table 1).
 |
Table 1 Identification of A4 by UPLC-MS Measurements |
UV-vis spectrometry was further applied to quantify the ingredient in A4. It was found that the contents of saponins in A4 was 207.6 mg/g, which increased by 2.67 times compared that in AR stock solution (Figure 2A). The content of flavonoids in AR stock solution and A4 were 5.8 mg/g and 2.2 mg/g, respectively (Figure 2B). Moreover, the contents of typical substances, Astragaloside IV (Figure 2A) and calycosin-7-O-β-D-glucoside (Figure 2B), were determined UPLC measurements, which were 1185.7 μg/g and 598.6 μg/g, respectively.
 |
Figure 2 The content of (A) saponins and (B) flavonoids in AR stock and A4. |
Pathological Analysis of Rat Spleens
Microscopic examination of HE stained spleen specimens was conducted and found that the spleen nodules in the control rats were uniform in size without obvious abnormalities (Figure 3). Compare to the control group (Figure 3A), the administration of CTX led to significant red pulp admixed with inflammatory cells infiltration, moderate membrane shrinkage, moderate white pulp atrophy and mild fibrosis (Figure 3B). But these changes were alleviated to some extent after applying A4 (Figure 3C). Similarly, the intake of HC moderately widened the spleen margin and induced slight white pulp atrophy (Figure 3D), but further administration of A4 reduced spleen margin widening (Figure 3E). All these results indicated that A4 has a certain protective effect on spleen pathological changes caused by CTX and HC.
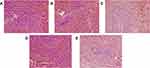 |
Figure 3 Pathological analysis of rat spleens (HE, ×200) in (A) control, (B) CTX, (C) CTX-A4, (D) HC, (E) HC-A4 groups. |
Effects of A4 on Serum IL-2 and IFN-γ in Rats
The levels of interleukin-2 (IL-2) and Interferon-γ (IFN-γ) in rat serum were measured by ELISA. It was found that IL-2 and IFN-γ levels in the CTX-rats were significantly reduced (P<0.01) as compared with those in the control group, which indicating the successful establishment of CTX-induced immunosuppression rat model (Figure 4A). After the administration of A4, the serum levels of IL-2 and IFN-γ of rats in the CTX-A4 group were obviously increased (P<0.05), proving that A4 was effective on the immunity of CTX-induced rats. Similar observations were achieved in HC-induced groups (Figure 4B). Compared with those in the control group, serum IL-2 and IFN-γ levels of rats in the HC group were also reduced (P<0.01), demonstrating the successful establishment of HC-induced immunosuppression rat model. Furthermore, compared with those of the HC group, serum IL-2 level in the HC-A4 group were significantly increased (P<0.05), illustrating that A4 is beneficial for the immunity recovery of HC-induced immunosuppression rats.
 |
Figure 4 Effects of A4 on the levels of IFN-γ (A) and IL-2 (B)in rats ( |
Effects of A4 on CD4+CD25+ and CD8+CD25+ in Splenocytes
The levels of CD4+CD25+ and CD8+CD25+ were characterized by flow cytometry (Figure 5). Compared with the control group, rats in CTX group had the reduced proportion of activated CD4+CD25+ (Figure 5A) and CD8+CD25+ (Figure 5B) T lymphocytes, as well as a decreased ratio of CD4+CD25+/CD8+CD25+ (Figure 5C), which illustrating that the administration of CTX inhibited rat immunity. Moreover, the decrease was reversed after A4 administration, indicating that A4 could boost immune activity and alleviate immunosuppression caused by CTX.
Similarly, compared with the control group, the proportions of activated CD4+CD25+ and CD8+CD25+ T cells, as well as the ratio of activated CD4+/CD8+ were significantly decreased in HC-induced immunosuppression rats, indicating that administration of HC suppressed rat immunity seriously (Figure 5). After A4 treatment, these decreases were reversed again, demonstrating that A4 could effectively modulate the immune conditions in rats.
Effects of A4 on Metabolites in Immunosuppression Rats
The metabolic profiling of the serum samples was performed using NMR spectrometry (Figures S3 and S4). Moreover, the metabolites were identified according to their chemical shifts, coupling constants and peak splits (Table S1). PCA analysis was further conducted to evaluate the alterations in the metabolome in each group, which were at the marked 95% confidence interval and showed clearly separations in both CTX (Figure 6A) and HC (Figure 6B) models.
 |
Figure 6 PCA analysis from rat serum in CTX model (A) and HC model (B). |
To eliminate the errors caused by individual difference and biodiversity, OPLS-DA was adopted for sample discrimination, where the predicted score of the first principal component, t[1] score, as the abscissa, and the predicted score of the orthogonal principal component, t0[1] score as the ordinate. It was observed that the data collected was well separated and without overfitting phenomenon in CTX (Figure 7A and B) and HC (Figure 7C and D) models, indicating that the immunosuppression models were successfully established, and A4 had obvious influences on the two models.
 |
Figure 7 OPLS-DA analysis from rat serum in CTX model (A and B) and HC model (C and D). |
Permutations substitution test was also used to evaluate each model predicted by OPLS-DA at the number of repetitions setting to 1000. The results are shown in Figure 8, in which R2 represents the interpretation rate of the built model to the data matrix, and Q2 represents the model’s predictive ability. It can be seen from Figure 8A that the parameters R2 and Q2 between control and CTX group are 0.945 and 0.138, respectively. The value suggested that the model was well-created and reliable. Similar speculations were achieved in the other three comparable groups (Figure 8B–D).
 |
Figure 8 Evaluation of each model using permutations substitution test. (A) Control vs CTX groups, (B) CTX vs CTX-A4 groups, (C) control vs HC groups, and (D) HC vs HC-A4 groups. |
Analysis of Potential Biomarker Metabolites
VIP in the projection from OPLS-DA model was selected to evaluate the potential biomarkers and variables with P<0.01 and VIP>1 were regarded as differential metabolites (Figure S5). According to the order of influencing factors from high to low, the metabolites with significant differences between control and CTX groups, as well as between CTX and CTX-A4 groups, were screened out (Figures S6 and S7). It was illustrated that in CTX-induced immunosuppression model, A4 might modulate the immune functions via regulating lactate, creatine, glycerol, lysine, glycine, and ketoisocaproic acid (KA). The analysis of related metabolic pathway was then performed on Metabo Analyst and present in the bubble chart by taking the pathway influence factor as the abscissa and the P value as the ordinate. As shown in Figure 9A, the main metabolic pathways affected between CTX and CTX-A4 groups are serine and threonine metabolism, glycerol metabolism, glyoxylic acid and dicarboxylic acid metabolism, glutathione metabolism pathway and pyruvate metabolism.
Similarly, in HC-induced immunosuppression model, A4 improving the immune responses by regulating lactate, glucose, and KA (Figures S6 and S7). Besides, the five influential metabolism pathways are sweet starch and sucrose metabolism, pyruvate metabolism, galactose metabolism, valine, leucine and isoleucine degradation, valine, leucine and isoleucine biosynthesis (Figure 9B).
Discussion
CTX is a DNA alkylating agent with striking immunity suppression ability. It can inhibit the humoral and cellular immune responses of animals by destroying the DNA structure, interfering DNA synthesis and blocking cell replication.19 HC also plays important roles in immune process, which not only inhibiting the phagocytosis and clearance of macrophages to antigens, but also decreasing lymphocytes and antibody production.20 CTX and HC both are commonly used immunosuppressive chemical modeling drugs, but targeted to different objectives and metabolized through different pathways. Therefore, it is necessary to design two pathogenic models to evaluate comprehensively the immunomodulatory effects of A4.
Spleen, containing numerous T and B lymphocytes, is the largest immune organ in the body. It plays a key role in humoral immunity and cellular immunity, so spleen index is objective to reflect immune reactivity. When immunosuppression took place in vivo, the spleen was obviously atrophied. In contrast, the treatment of A4 can relief the shrinkage and restore spleen structure to some extent. Therefore, A4 is a protective agent for immune organs.
Both IL-2 and IFN-γ are lymphokines secreted by helper T lymphocytes and have positive correlations with the body’s immune state. IL-2, known as T cell growth factor, has a variety of immune regulatory functions. It adjusts the proliferation and differentiation of immune cells, which thereby reflecting the body’s cellular immune level and specific immune response ability. IFN-γ is a small polypeptide that stimulating immunoregulation by activation of monocytes/macrophages, induction the increase of major histocompatibility complex (MHC) molecule expression, and promotion of T cell differentiation.21 Our results showed that the expression of IL-2 and IFN-γ inhibited significantly in the immunosuppression rats, and was partially reversed after A4 intake. It was suggested that A4 is effective for enhancing immunologic functions.
T lymphocyte, mediated cellular immunity, is the most important part of immune system. It consists of T helper cells with surface antigen CD4+ and T suppressor cells carrying surface antigen CD8+. CD4+ T cells are critical for immunologic functions, which regulate immune activity, assist B cells to produce specific antibody, participate in delayed inflammatory response mediated by cytotoxic and local inflammatory response, transmit antigen information, and secret a variety of cytokines.22 CD8+ T lymphocytes are involved in immune responses related to immunosuppression and cytotoxicity, such as attacking tumor cells and virus-infected cells. In normal physiological condition, the ratio of CD4+/CD8+ in vivo maintains a homeostasis, which indicating the stability of immune system. However, when the ratio is less than 1, it was associated with biomarkers of activation and inflammation.23 Therefore, the CD4+CD25+/CD8+CD25+ ratio was considered as a frontier marker for immune dysfunction. In the study, it was detected that the contents of CD4+CD25+ and CD8+CD25+ decreased in immunosuppression rat models and partially normalized after the treatment of A4. Besides, the ratio of CD4+CD25+/CD8+CD25+ was remarkably elevated after A4 injection. It was demonstrated that A4 is helpful to maintain immune balance.
Metabolites are important in various human diseases and the exploration of the relationship between metabolites and diseases contributes to in-depth understanding of the pathogenesis. In present study, the metabolites in rat serum were carefully identified by NMR and the changes were analysed by PCA and OPLS-DA methods, where seven differential metabolites were figure out that close related to immunity regulations (Figure 10). It was discovered that CTX and HC mainly affected amino acid metabolism and energy metabolism, which is consistent with previous reports.15,24,25 Besides, the enrichment analysis revealed that A4 was more inclined to regulate body’s amino acid metabolism in CTX-rats and energy metabolism in HC-rats.
Amino acids are the basic substances of immune system, which are involved in the immune system formation, organ development and function implementation. It was known that amino acids regulate the immune response by activating T lymphocytes, B lymphocytes, natural killer cells and macrophages; controlling the redox state of cells, gene expression and lymphocyte proliferation; and adjusting the production of antibodies, cytokines and other cytotoxic substances.26 In our work, metabolomics analysis hinted that A4 relieved the immune dysfunction in CTX and HC rats by regulating amino acid metabolism (Table S2).
Lysine and glycine are essential amino acids for human health. They are the basic structure of many important substances such as methionine, glutathione, heme, purine nucleotide and some hormones, and play protective effects on anti-inflammatory, immunomodulatory and direct cytoprotective actions.27–32 Many studies have confirmed that the lack of lysine and glycine could limit the protein synthesis, the proliferation of lymphocytes and the antibody response.28,33 Besides, glycine expresses immune activity by stimulating the glycine-gated chloride channel on Kupffer, macrophages and leukocytes cells, as well as inhibiting tumor necrosis factor-alpha (TNF-α) and IL-1β production.33,34 Our results showed that CTX decreased the content of serum lysine and glycine, which were subsequently reversed by A4. It was speculated that CTX led to the excessive consumption of lysine and insufficient glycine synthesis, and A4 effectively prevent the metabolic disorders, which further modify the immune reactivity.
KA is the transamination product of L-leucine and has the functions of promoting the synthesis of skeletal muscle, regulating the nitrogen balance in the body, inhibiting the secretion of glucagon, stimulating the secretion of insulin, maintaining the balance of blood glucose and enhancing immunity.35,36 Our results showed KA in rat metabolites decreased after the intake of CTX and HC, indicating the decomposition of leucine was disturbed. Moreover, the elevation of KA confirming the efficacy of A4 on adjusting the leucine metabolism pathway, which was compatible with the enrichment analysis.
From the molecular point of view, the levels of metabolites related to energy metabolism, including lactate, glycerol, glucose and creatine, were significantly different, when comparing the metabolites of CTX and HC model group with those of the A4 groups (Table S2). ATP is important for energy conversion and supply, which is mainly formed through glucose metabolism and lipid metabolism. In glucose metabolism pathway, glucose under the catalysis of a variety of enzymes, produces pyruvate during glycolysis. Through anaerobic respiration, pyruvate transforms to lactate, while under aerobic conditions, pyruvate enters mitochondria and releases ATP through the tricarboxylic acid cycle. Lipid metabolism is also the main source of ATP, where triglycerides decomposed into glycerol and fatty acids for energy metabolism, and glycerol converted into glycerol-3-phosphate and releases energy. Under normal physiological conditions, the contents of lactate, glycerol and glucose should be balance and stable. However, our results showed a decrease of lactate and an increase of serum glucose and glycerol in rats treated by CTX and HC. It was hypothesized that the synthesis of the key enzymes in energy metabolism was interfered by the shortage of amino acids. In addition, the administration of A4 reversed the levels of energy-related metabolites, which is consistent with our detection that A4 treatment could normalize amino acid contents, which are sufficient for enzymes utilizations.
Creatine, composed of arginine, glycine and methionine, is distributed in the brain, skeletal muscle and myocardium. Creatine can be obtained not only from food, but also from the synthesis of glycine, arginine and methionine in liver. Previous studies have shown that creatine is beneficial for immune modulation by inhibiting IFN–γ, enhancing IL-4 activity and stimulating the immune activation function of macrophages.37 In the experiment, the serum creatine was increased in the CTX group. It might be attributed to the decreased exercise intensity of immunosuppressive rats, which affected the transport of creatine from exercise-related tissues. It is also possible due to the lack of amino acids, which are the raw material for creatine synthesis. The application of A4, however, improved anabolism and catabolism of protein, strengthened the body movement system, which thereby reducing the creatine contents and enhancing the immunoprotective functions.
Although membrane separation has been widely used in the pretreatment of natural drugs and bio-products. However, its application in industry is sometimes restricted by organic membrane fouling. Membrane fouling can result in the degradation of the membrane performance, especially in the permeate parameter, which may adversely affect the membrane’s attraction and filtration. Therefore, it is preferable to adopt assisted control method, such as vibration, gas injection, recoil, pulsating flow and electric field.
Conclusion
In this study, the bioactive components were extracted from AR by green membrane separation. Serum metabolomics coupled with histopathological analyses were subsequently used to reveal the effect and mechanism of A4 in the immunosuppression rat models. It was demonstrated that A4 could effectively alleviate the reduction of immune functions by modulating amino acid metabolism and energy metabolism. This work is expected to provide potential application of membrane separation for AR treatment and beneficial evidence for the clinical application of Astragalus.
Abbreviations
AR, Astragalus; CTX, Cyclophosphamide; ELISA, enzyme linked immunosorbent assay; HC, hydrocortisone; HE, hematoxylin-eosin; IL-2, Interleukin-2; IFN-γ, interferon-γ; KA, ketoisocaproic acid; MHC, major histocompatibility complex; NMR, nuclear magnetic resonance; OPLS-DA, orthogonal partial least squares-discriminant analysis; PCA, principal component analysis; TSP, tetramethylsilane; UPLC-MS/MS, Ultra high-performance liquid chromatography-tandem mass spectrometry; VIP, variable importance factor.
Author Contributions
Conceptualization, Di Zhang and Yingli Wang; Data curation, Yafei Guo; Formal analysis, Yafei Guo and Di Zhang; Funding acquisition, Yingli Wang; Methodology, Yafei Guo; Project administration, Yingli Wang; Resources, Yingli Wang; Software, Di Zhang and Yafei Guo; Supervision, Yingli Wang; Writing – original draft, Di Zhang, Yafei Guo; Writing– review & editing, Yingli Wang. All authors made substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
Astragalus Membranaceus Resources Industrialization and Industry International Cooperative Innovation Center Project in Shanxi Province (No. HQXTCXZX 2016-019); TCM discipline construction project of SXTCM (Direction 3) (1008Z3) and Foundation of Applied Basic Research Program of Science and Technology of Shanxi Province, 201801D221071.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Li X, Lu Q, Dong Y, et al. A review of recent research progress on the astragalus genus. Molecules. 2014;19:18850–18880. doi:10.3390/molecules191118850
2. Shan C, Sun B, Dalloul RA, et al. Effect of the oral administration of astragalus polysaccharides on jejunum mucosal immunity in chickens vaccinated against new castle disease. Microb Pathog. 2019;135:103621. doi:10.1016/j.micpath.2019.103621
3. Chen Z, Liu L, Gao C, et al. Astragali Radix (Huangqi): a promising edible immunomodulatory herbal medicine. J Ethnopharmacol. 2020;258:112895. doi:10.1016/j.jep.2020.112895
4. Qin S, Lin J, Huang K. Immune regulation effects of astragali radix. Chin Arch Traditional Chin Med. 2017;3:699–702.
5. Abuelsaad AS. Supplementation with astragalus polysaccharides alters aeromonas-induced tissue-specific cellular immune response. Microb Pathog. 2014;66:48–56. doi:10.1016/j.micpath.2013.12.005
6. Luo H, Li Q, Flower A, Lewith G, Liu J. Comparison of effectiveness and safety between granules and decoction of Chinese herbal medicine: a systematic review of randomized clinical trials. J Ethnopharmacol. 2012;140:555–567. doi:10.1016/j.jep.2012.01.031
7. Chen H, Zhou X, Zhang J. Optimization of enzyme assisted extraction of polysaccharides from Astragalus membranaceus. Carbohydr Polym. 2014;111:567–575. doi:10.1016/j.carbpol.2014.05.033
8. Chikari F, Han J, Wang Y, et al. Dual-frequency ultrasound-assisted alcohol/salt aqueous two phase extraction and purification of Astragalus polysaccharides. J Food Process Eng. 2020;43:e13366. doi:10.1111/jfpe.13366
9. Negin S, Ali SM, Mohsen B, Hasan AG. Concentration of pistachio hull extract antioxidants using membrane separation and reduction of membrane fouling during process. Food Sci Nutr. 2018;6:1–10.
10. Avram AM, Morin P, Brownmiller C, Howard LR, Sengupta A, Wickramasinghe S. Concentrations of polyphenols from blueberry pomace extract using nanofiltration. Food Bioprod Process. 2017;106:91–101. doi:10.1016/j.fbp.2017.07.006
11. Pei J, Jiang L. Antimicrobial peptide from mucus of Andrias davidianus: screening and purification by magnetic cell membrane separation technique. Int J Antimicrob Ag. 2017;50:41–46. doi:10.1016/j.ijantimicag.2017.02.013
12. Tang W, Liu C, Liu J, et al. Purification of polysaccharide from Lentinus edodes water extract by membrane separation and its chemical composition and structure characterization. Food Hydrocoll. 2020;105:105851. doi:10.1016/j.foodhyd.2020.105851
13. Chang H, Liu Q, Bai WF, et al. Protective effects of Amygdalus Mongolica on rats with renal fibrosis based on serum metabolomics. J Ethnopharmacol. 2020;257:112858. doi:10.1016/j.jep.2020.112858
14. Cai H, Su S, Li Y, et al. Protective effects of salvia miltiorrhiza on adenine-induced chronic renal failure by regulating the metabolic profiling and modulating the NADPH oxidase/ROS/ERK and TGF-β/Smad signaling pathways. J Ethnopharmacol. 2018;212:153–165. doi:10.1016/j.jep.2017.09.021
15. Jia X, Sun C, Zuo Y, et al. Integrating transcriptomics and metabolomics to characterise the response of Astragalus membranaceus Bge. var. mongolicus (Bge.) to progressive drought stress. BMC Genom. 2016;17:188. doi:10.1186/s12864-016-2554-0
16. Liu W, Li C, Huang J, et al. Application of pathways activity profiling to urine metabolomics for screening Qi-tonifying biomarkers and metabolic pathways of honey-processed Astragalus. J Sep Sci. 2018;41:2661–2671. doi:10.1002/jssc.201701371
17. Wang H, Liu A, Zhao W, et al. Metabolomics research reveals the mechanism of action of astragalus polysaccharide in rats with digestive system disorders. Molecules. 2018;23:3333. doi:10.3390/molecules23123333
18. Zeng H, Xi Y, Li Y, Wang Z, Zhang L, Han Z. Analysis of astragalus polysaccharide intervention in heat-stressed dairy cows’ serum metabolomics. Animals (Basel). 2020;10:574. doi:10.3390/ani10040574
19. Peláez B, Campillo JA, López-Asenjo JA, Subiza JL. Cyclophosphamide induces the development of early myeloid cells suppressing tumor cell growth by a nitric oxide-dependent mechanism. J Immunol. 2001;166:6608–6615. doi:10.4049/jimmunol.166.11.6608
20. Lv Y, Huang J, Cai M, et al. Comparison of experimental murine immunodeficiency models induced by cyclophosphamide and hydrocortisone. Wei Sheng Yan Jiu. 2012;41:951–955.
21. Gosio MG, Guerassimov A. Chronic obstructive pulmonary disease. Inflammation of small airways and lung parenchyma. Am J Respir Crit Care Med. 1999;160:S21–S25. doi:10.1164/ajrccm.160.supplement_1.7
22. Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+ T Cells: differentiation and functions. Clin Dev Immunol. 2012;12:925135.
23. Davy-Mendez T, Napravnik S, Zakharova O, et al. Acute HIV infection and CD4/CD8 ratio normalization after antiretroviral therapy initiation. J Acquire Immune Deficiency Syndr. 2018;79:510–518. doi:10.1097/QAI.0000000000001843
24. Wang Y, Liu L, Ma Y, et al. Chemical Discrimination of astragalus mongholicus and astragalus membranaceus based on metabolomics using UHPLC-ESI-Q-TOF-MS/MS approach. Molecules. 2019;24:4064. doi:10.3390/molecules24224064
25. Wang EB, Liu T, Lu XL, et al. Comparison of aerial parts of astragalus membranaceus and astragali radix based on chemical constituents and pharmacological effects. Food Agr Immunol. 2019;30:1046–1066. doi:10.1080/09540105.2019.1663154
26. Li P, Yin YL, Li D, Kin SW, Wu G. Amino acids and immune function. Brit J Nutr. 2007;98:237–252. doi:10.1017/S000711450769936X
27. Chen C, Sander JE, Dale NM. The effect of dietary lysine deficiency on the immune response to Newcastle disease vaccination in chickens. Avian Dis. 2003;47:1346–1351. doi:10.1637/7008
28. Griffith RS, DeLong DC, Nelson JD. Relation of arginine–lysine antagonism to herpes-simplex growth in tissue culture. Chemotherapy. 1981;27:209–213. doi:10.1159/000237979
29. Konashi S, Takahashi K, Akiba Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Brit J Nutr. 2000;83:449–456.
30. Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G856–G863. doi:10.1152/ajpgi.00503.2001
31. Zhong Z, Wheeler MD, Li X, et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–240. doi:10.1097/00075197-200303000-00013
32. Wheeler MD, Thurman RG. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J Physiol. 1999;277:L952–L959. doi:10.1152/ajplung.1999.277.5.L952
33. Alarcon-Aguilar FJ, Almanza-Perez J, Blancas G, et al. Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur J Pharmacol. 2008;599:152–158. doi:10.1016/j.ejphar.2008.09.047
34. Stachlewitz RF, Li X, Smith S, Bunzendahl H, Graves LM, Thurman RG. Glycine inhibits growth of T lymphocytes by an IL-2-independent mechanism. J Immunol. 2000;164:176–182. doi:10.4049/jimmunol.164.1.176
35. Heissig H, Urban KA, Hastedt K, Zünkler BJ, Panten U. Mechanism of the insulin-releasing action of alpha-ketoisocaproate and related-keto acid anions. Mol Pharmacol. 2005;68:1097–1105. doi:10.1124/mol.105.015388
36. Cheng S, Zhang S, Yun J. Recent advances in microbial synthesis of α-ketoisocaproate. Chem Industry Eng Progress. 2016;37:4821–4829. doi:10.16085/j.issn.1000-6613
37. Ji L, Zhao X, Zhang B, et al. Slc6a8-mediated creatine uptake and accumulation reprogram macrophage polarization via regulating cytokine responses. Immunity. 2019;51:272–284. doi:10.1016/j.immuni.2019.06.007
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.