Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Immunogenetics of new onset diabetes after transplantation in Kuwait
Authors Jahromi M , Al-Otaibi T, Othman N, Gheith O , Mahmoud T, Nair P, Halim MA , Nampoory N
Received 24 November 2018
Accepted for publication 7 March 2019
Published 20 May 2019 Volume 2019:12 Pages 731—742
DOI https://doi.org/10.2147/DMSO.S195859
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Mohamed Jahromi,1 Torki Al-Otaibi,2 Nashwa Othman,3 Osama Gheith,2 Tarek Mahmoud,2 Prasad Nair,2 Medhat A Halim,2 Narayanam Nampoory1,2
1Clinical Research, Medical Division, Dasman Diabetes Institute, Kuwait City, Kuwait; 2Nephrology Department, Hamid Al-Essa Organ Transplant Center, Kuwait City, Kuwait; 3Education, Clinical Services Division, Dasman Diabetes Institute, Kuwait City, Kuwait
Introduction and aim: New onset diabetes after transplantation (NODAT) is a serious metabolic complication following kidney transplantation. Although beta-cell dysfunction is considered the main contributing factor in the development of this complication, its exact etiology is yet to be identified. We aimed to investigate NODAT among kidney transplant cohort in Kuwait with special stress on correlation between its risk factors and interferon gamma genotyping.
Materials and methods: We surveyed 309 kidney transplant recipients from Hamed Al Essa Transplantation Centre, Kuwait. The participants were categorized into cohorts according to the development of NODAT diagnosed based on the American Diabetes Association guidelines. Statistical analyses were performed using SPSS software. We genotyped interferon gamma as the leading immunosignature for T lymphocyte.
Results: No relationship between ethnicity and the development of NODAT was identified. However, there was a significant difference in age between cohorts. Younger patients demonstrated a lower rate of NODAT while, NODAT reached its maximum in 40–60-year age group. IFNG TT genotype was significantly associated with NODAT (p=0.005), while IFNG AA was considerably higher in the non-NODAT group.
Conclusion: Beside the conventional contributing factors of NODAT, our results might represent a suitable platform for a larger cytokine and chemokine spectrum genotyping.
Keywords: ethnicity, NODAT, transplantation, immunosuppression
Introduction
New onset diabetes after transplantation (NODAT) occurs in a substantial number of patients following renal transplantation. NODAT is associated with increased mortality and morbidity rates of cardiovascular disease and infection, which are some of the leading causes of death in kidney transplant recipients (KTR). The incidence of NODAT varies according to duration of the transplant, the study population and the immunosuppressive agents used. The diagnosis of NODAT is made when individuals are on a maintenance dose of immunosuppressants and are clear of infection with stable graft function. Generally, these conditions occur three months post-transplant.
Diabetes and impaired glucose tolerance occurring as a complication of organ transplantation has been recognized for many years. Different studies have reported incidence rates of 2–53%.2 NODAT has been reported to occur in 4–25% of KTR.3–5 It is more common in African Americans and Hispanics than Caucasians and Asians.6 Currently, aside from a few epidemiological studies, there is a dearth of information about NODAT in Arab populations.7–12 These studies suggest that around 25–30% of KTR develop NODAT in Arab countries. More specifically, the incidence of NODAT after kidney transplantation is 27% in Saudi Arabia,7,8 30% in Bahrain,9 22.2% in Egypt10 and recently it was reported as 25.6% in Kuwait.11,12
One-third of non-diabetic KTR develop impaired glucose metabolism six months post-transplant and those with transient hyperglycemia may be at higher risk of NODAT later.13 Therefore, healthcare professionals must remain vigilant when managing this high-risk group. The need to screen for diabetes is crucial, alongside patient education, prior to transplant, on the risk of developing NODAT within 12 months of transplantation.
Risk factors for the development of NODAT are categorized as non-modifiable and modifiable. Non-modifiable factors include patient age, ethnicity, race or gender of organ recipient or donor and human leukocyte antigen (HLA)-match. Modifiable agents include patient weight and immunosuppressive therapies (eg, tacrolimus, cyclosporine and corticosteroid), infectious agents (eg, BK virus, hepatitis C virus [HCV] and cytomegalovirus [CMV]), fasting plasma glucose (FPG) level and glucose tolerance.2,14,15 Modifiable risk factors exert their effects through the body’s immune system.16
The evidence suggests that immunosuppressant medication (particularly tacrolimus) is responsible for 74% of NODAT diagnoses.15 Calcineurin inhibitors (CNI) might contribute to NODAT via islet cell toxicity and inhibition of insulin secretion or expression; the pro-diabetic effects of CNIs have differed between cyclosporine (CSA) and tacrolimus (Tac)17 with lower risk of NODAT in CSA than Tac-based regimens. The lower diabetogenicity of CSA than Tac has been further supported by literature showing that switching from Tac to CSA in kidney transplants resulted in resolution of NODAT.18,19 Moreover, the Santos group concluded that the combination of sirolimus and tacrolimus was the most diabetogenic, followed by sirolimus and mycophenolate; however, sirolimus and cyclosporine were the least diabetogenic.20
Interferon-gamma (IFN-γ), also known as type II interferon or macrophage-activating factor,21 is a multipotent cytokine with a molecular weight of around 17 kDa that is secreted by activated T cells and natural killer cells and modulates many facets of the immune response.22
It is well established that the IFN-γ gene polymorphism at position +874*T/A (IFNG) of the first intron is correlated with the serum level of IFN-γ production and mRNA expression in vitro and in vivo; the TT genotype is correlated with high production, and TA and AA are correlated with intermediate and low production, respectively.22–26 This polymorphism coincides with a putative NF-κB binding site that may mediate high production of IFN-γ.27,28 Immunogenetic genotyping was not assessed fully among renal transplants with NODAT.
In this study, we aimed to investigate new onset diabetes after transplant among kidney transplant cohort in Kuwait with special stress on correlation of conventional risk factors and interferon gamma genotyping.
Materials and methods
Study population
In this cross-sectional study, all patients were enrolled from Dasman Diabetes Institute (DDI) Diabetes Education Department and outpatient clinics of the Hamad Al Essa Organ Transplant Centre (OTC) between May 2015 and December 2016. Recruited patients were categorized into two groups according to the presence or absence of NODAT: NODAT group comprised 154 kidney transplant patients who developed NODAT within 6 months post-transplant and non-NODAT group comprised 155 kidney transplant patients without NODAT post-transplantation. All patients received their kidney grafts between 2000 and 2015, had no history of diabetes before transplantation and had stable kidney function at the time of enrolment. Table 1 summarizes the demographic data of the studied patients.
 | Table 1 Demographic characterization of our study subjects |
Signed informed consent forms were obtained from all the patients in this study. The research was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines and adhered to local and national regulatory requirements and laws. We excluded pediatric patients, pregnant women and mentally retarded patients. Patients with estimated glomerular filtration rate (eGFR) below 30 ml/min/m2 were not considered in this study due to well-being issue throughout the study period.
Diagnosis of NODAT and interferon genotyping
We screened all KTRs for FPG and HbA1c, according to KDIGO guidelines.29 If an abnormal result was obtained, we performed an oral glucose tolerance test to confirm the diagnosis. In these cases, diabetes management was introduced immediately, including diet, exercise, oral agents and/or insulin, in addition to regular monitoring of blood sugar at home.
Genomic DNA was extracted from 5 mL of fresh peripheral blood samples using the QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer’s instructions. Extracted DNA samples had a final concentration of 55–365 ng/mL. Samples were stored at −20°C prior to use. Moreover, genotyping was performed using triprimer amplification-refractory mutation system (ARMS) polymerase chain reaction (PCR) analysis, and patient identification was anonymized to conceal the case/control status.
Immunosuppression protocol
Our immunosuppression protocol consisted of five doses of anti-thymocyte globulin (Sanofi US, Bridgewater, NJ, USA) for high-risk patients (re-transplants, prior pregnancy, blood transfusion, HLA-antibody positive and/or more than four HLA mismatches), or two doses of IL-2 receptor blocker (Basiliximab, Novartis, Inc., Switzerland) for low-risk patients. Maintenance therapy consisted of prednisolone, mycophenolic mofetil (MMF) and a CNI. We gradually decreased the dose of CNI over 12 months, guided by 12 hrs trough level.
Acute cellular rejection (ACR) was treated with intravenous methylprednisolone sodium succinate (solumedrol, 1 g daily for 3 days) or thymoglobulin (1 mg/kg for 7–10 days) for steroid-resistant rejection. Any patient with an episode of acute antibody-mediated rejection (AMR) was treated with 10 sessions of volume plasma exchange, intravenous immunoglobulin (IVIG) (2 g/kg) and a single dose of rituximab (375 mg/m2). All rejection episodes were biopsy-proven according to Banff criteria. Patients who received thymoglobulin as anti-rejection therapy were managed by universal chemoprophylaxis for both CMV and Pneumocystis Jirovecii Pneumonia (PJP). Valganciclovir (VGC) was used as CMV secondary prophylaxis for one month, while those who developed CMV viremia during this period were managed with a therapeutic dose for three weeks, followed by three months prophylaxis. Trimethoprim was used for one month as a prophylaxis for PJP. Patients were monitored daily during their hospital stay and, then, at each outpatient visit for complete blood picture, serum creatinine, creatinine clearance, liver function tests (bilirubin, liver enzymes and albumin) and drug levels. CMV DNA was tested by PCR at the time of transplant, and at 1, 2, 3, 6, 9- and 12 months post-transplantation. Patients with a significant CMV quantitative PCR titre were treated with VGC or intravenous ganciclovir (GCV), according to the clinical situation. Treatment was given for three weeks, followed by secondary VGC prophylaxis of 900 mg/day for three months. Associated infections were recorded if they necessitated hospital admission. Details of patients who developed CMV disease or rejection episodes during the study period were recorded.
Patient follow-up
Before enrolment of patients in this study, they were followed up in OTC clinics according to the following schedule: twice-weekly for the first month after transplantation, once a week in months 2 and 3, every 2 weeks for months 4–6, every 4 weeks for months 6–12 and every 1.5–2 months thereafter, if no complications occurred.30 Demographic data of the enrolled patients were collected in both the OTC and DDI with special interest on patient and donor age and sex, donor type, immunosuppressive therapy, dialysis type and duration, primary renal disease, rejection or infection episodes and graft and its outcome. Laboratory work regarding interferon genotyping was performed in DDI.
Statistical analysis
Statistical analyses were performed with SPSS software (SPSS, version 20.0, IBM Corporation, Armonk, NY, USA). Sample size was calculated to accept a marginal error of 6.6% (95% CI) in a normally distributed population. Variables and means were compared using paired-sample t-test, independent sample t-test, chi-squared test, Fisher exact test and ANOVA, as appropriate. Results are expressed as means ± standard deviation, and differences were considered significant at P≤0.05.
Ethical Approval
This research was performed upon receipt of written approval from our institutional International Scientific Advisory Board (ISAB) under RA 2015-013 reference.
Results
In our cohorts, most of the patients (56%) were Kuwaiti, vs (43.8%) non-Kuwaiti (Table 1). Moreover, the two groups were comparable regarding their original kidney disease, dialysis type, donor type and the type of both induction and maintenance immunosuppression (p>0.05). Also, pre-transplant co-morbidities were comparable in both groups, including hypertension, history of exposure to tuberculosis bacilli, ischemic heart disease, bone disease, anemia and hyperlipidemia (p>0.05). We noticed that younger patients, up to 40 years old, were significantly prevalent in non-NODAT cohort (p<0.0001). Although most of our patients were in the middle age group (41–60 years old), patients over 60 were more prevalent in the NODAT cohort group (p<0.0001) (Figure 1). They had all received grafts from 30- to 40-year-old donors (p>0.05). We observed that patients with chronic hepatitis C were significantly more prevalent in the NODAT group (12 cases in NODAT vs 2 cases in non-NODAT, p=0.006), as shown in Table 1. Moreover, the number of patients with positive CMV IgG was significantly higher in the NODAT group (153 vs 148, p=0.024); however, the two groups were comparable regarding pre-transplant CMV IgM (p>0.05). Post-transplant graft function was assessed, and we found better graft function (as represented by immediate and slow graft function) among patients in the control group (p=0.031). We observed that the mean body mass index (BMI), at the time of transplant, was significantly higher in the NODAT group (28.07±5.5 vs 26.11±7; p=0.014), while mean BMI, at the time of enrolment, was comparable in both groups (p=0.21).
From Table 2, we found that KTRs with BK viremia or BK viral-associated nephropathy were comparable in the studied groups (p>0.05), while cases with CMV viremia were significantly more prevalent in the control group (p=0.012).
 | Table 2 Post-transplant complications in the study group |
Regarding post-transplant immunological complications, we found that the mean number of acute rejection episodes was comparable between the two groups (p=0.33). Moreover, we did not find any significant difference between the two groups regarding both graft and patient outcome (p>0.05) (Table 2). We found a significant association between IFNG TT genotype and NODAT (p=0.005), while the IFNG AA genotype was considerably more prevalent in the non-NODAT group (both Kuwaiti and non-Kuwaiti) (p=0.004) (Table 4). The allelic distribution of the IFNG T allele in NODAT and the IFNG A allele in the non-NODAT group were significantly higher (p<0.0001) (Table 4); however, this effect was only observed when the alleles were paired by genotype. There was a significantly higher prevalence of patients with the TT genotype among the diabetic non-Kuwaiti cohort and Kuwaiti non-diabetics (p=0.02). Both AA and AT genotypes were significantly more prevalent in the NODAT group, especially in those with HCV infection (Table 4). Moreover, the TT genotype was significantly more prevalent in the non-NODAT group especially those with post-transplantation CMV viremia (Table 4).
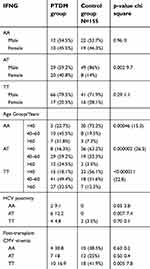 | Table 4 IFNG in relation to demographic variables |
We observed that there was a significantly higher prevalence of patients with chronic HCV infection in the PTDM group (12 cases in the PTDM group compared with two cases in the control group (p=0.006)). The AT genotype was significantly higher in non-Kuwaiti diabetic patients (p=0.006).
Discussion
Different reports showed variation in the prevalence of NODAT among KTR as it ranged from 4% to 25%.3–5 In Kuwait, Mahmoud T et al in their survey in 2017, that included nearly 1,400 renal transplant recipients, found that the prevalence of NODAT after a mean follow-up of 10.25±6.25 years was 25.6% (356 out of 1,392). Kasiske and co-workers reported a prevalence of 26% at 3-year follow-up in the United States.31
IFN- γ is a proinflammatory cytokine that plays a crucial role in the host defense by directing the immune response to proinflammatory mediators such as TNF-alpha, IL-2 and IL-6;32 therefore, IFN- γ is regarded as a key mediator in several models of inflammatory diseases.27,32–36 Depending on genotype and IFN- γ production rate in vitro, individuals are either categorized as low (AA), intermediate (AT) or high (TT) IFN- γ producers. However, there is considerable interindividual variation in the degree of systemic inflammatory activation as cytokine production is partly genetically determined.
In this study, we aimed to investigate new onset diabetes after transplant among kidney transplant cohort in Kuwait with special stress on correlation of conventional risk factors and interferon gamma genotyping.
PTDM has emerged as an increasingly important determinant of outcome and survival in RTRs. During our study period, 309 of the patients who visited the outpatient clinic at the Hamed Al-Essa Organ Transplant Center agreed to take part in the study. Before enrolment of patients in our study, graft function was assessed at 6 months and 12 months after transplant. We found no significant difference between the NODAT and non-NODAT patients regarding mean serum creatinine. In the current study, age-specificity was significantly different between NODAT versus non-NODAT cohorts (p=0.02, >21 years; p=<0.00001, 21–40 years) the difference was most extreme in the 41–60 group (p<0.00001) and was high in the over-60 group (p>0.00001), as shown in Table 1 and Figure 1.
Interestingly, in both groups, male gender dominance was an apparent characteristic of our cohorts, which was in agreement with several studies that reported that recipients of organs were mainly males, possibly reflecting a gender bias in the incidence of transplant-related pathologies.37 NODAT was reported to be more common in female graft recipients.38 However, in the present study, we did not notice any significant gender specificity in our NODAT cohort (Table 1). Conversely, in a general Saudi population, NODAT was found to be slightly more prevalent in males.8
We found that the IFNG TT genotype was significantly associated with PTDM (p=0.005), while IFNG AA was considerably higher in the control group (p=0.004). This suggests that IFNG TT, which corresponds to higher IFN-γ levels, might be significantly associated with the progression of PTDM. On the other hand, IFNG AA, which is correlated with low IFN-γ, can be a protective genotype against PTDM progression. The allelic distribution of the IFNG T allele in PTDM and the IFNG A allele in the control group were significantly higher (p=0.0002) (Table 3); however, this effect was only noted when the alleles were paired according to genotype. This observation could be explained by the IFN-gamma receptor which might play a key role in CD4+ T cell-mediated β cell destruction and therefore, FN-γ receptor deficiency prevents diabetes induction by diabetogenic CD4+ T cells but not CD8+ T cells.39
 | Table 3 Ethnic distribution and interferon gamma alleles/genotypes in the studied groups |
Similarly, compared with the AA and AT genotypes, Yates et al in 2012 found a strong association between TT and IFNG in the PTDM group compared with the control group. In addition, there was a correlation between the IFNG TT genotype and high IFN-γ protein levels.17 Furthermore, the IFNG AA genotype was more prevalent in the control group; thus, the correlation between the AA genotype and low serum IFN-γ protein may serve to protect against PTDM. In our cohort, despite the strong association between the T and A alleles, a phenotypic effect was only obvious upon their combination as a genotype (Table 3).
TT genotype was significantly more prevalent among the diabetic non-Kuwaiti cohort and the Kuwaiti control group (p=0.02). Moreover, TT genotype was significantly more prevalent in the control group with post-transplantation CMV viremia (Table 4). Similarly, we found that most of the control patients were younger than 40 years, while most of the PTDM patients were older than 40 years (Table 4). Our results did not agree with the univariate analysis described by Babel et al, who reported that IFNG AA genotype was associated with PTDM.40 They reported an association between IFNG AA and type 2/steroid-induced diabetes, while our cohorts did not have a history of diabetes (Table 1).
Oxenkrug et al reported that aging was associated with chronic, low grade, TH-1 type inflammation. The hypothesis of the IFNG-inducible IDO/GTPCH inflammation cascade helps to explain the increased association between aging, inflammation and aging-associated psychiatric and other medical disorders such as insulin resistance and obesity.41 Previous studies have shown that aging is accompanied by an increased inflammatory status42,43 and is associated with remodeling of the immune system.44 Moreover, due to elevated levels of IFN-γ in nephropathy patients, it could be concluded that IFN-γ is involved in nephropathy complications of type 2 diabetes.45
RTRs often undergo severe immunotherapeutic regimens that affect the immune system. The present study aimed to identify the immunity status of our PTDM cohort by analyzing the mechanism of IFNG as a TH1 cytokine profile initiator. Our results revealed that both groups were homogeneous in terms of the number of patients with different immunosuppressive agents, which nullified the influence of the immunotherapeutic regimen.
Mahmoud et al reported that the homozygous genotypes AA and TT were more commonly associated with diabetes in rheumatoid arthritis (RA) patients, with a contribution of the A allele. In their study, IFN-γ serum levels were elevated in RA patients, with and without diabetes, and were significantly correlated with C-reactive protein (CRP) levels.46 RA patients with diabetes showed significantly elevated CRP and IFN-γ levels compared with RA patients without diabetes, indicating greater disease activation and immune stimulation in these patients. Moreover, RA patients have an increased risk of insulin resistance and type 2 diabetes due to the associated systemic inflammation.47 IFN-γ was found to be involved in the pathogenesis of DM, and the frequency of the low IFN-γ production allele (A allele) was significantly higher in patients with type 2 diabetes compared with the controls.48 IFN-γ was found to be associated with various diseases, including infectious diseases such as hepatitis B, Helicobacter pylori gastritis and tuberculosis,49,50 as well as autoimmune diseases such as systemic lupus erythematosus and scleroderma.51Our cohort patients were matched regarding H. pylori infection, exposure to TB and autoimmune disease as all patients in both groups had negative screen results pre-transplant.
HCV infection has been shown to be associated with IFNG and the development of type 2 DM in the general population.52 In the present study, we observed that there was a significantly higher prevalence of patients with chronic HCV infection in the PTDM group, especially among cases with the AT genotype (Tables 1 and 4). This finding was in accordance with previous studies that highlighted chronic HCV as a risk factor for PTDM.53 There was a significantly higher prevalence of AA and AT genotypes in the PTDM group (p=0.05 and 0.007, respectively), especially in those with HCV infection (Table 4). This might be due to sex-dependence of HCV as well as IFNG.54–57
Some previous studies suggested that both asymptomatic CMV infection and CMV disease were independently associated with the development of PTDM,58 while other studies reported that CMV was not a risk factor for PTDM.59–61 In the present study, while a significantly higher number of patients were positive for CMV IgG in the PTDM group (p=0.02), pre-transplantation CMV IgM was comparable between the two groups (p>0.05). However, there were significantly more patients with post-transplantation CMV viremia in the control group, especially with the TT genotype (Table 2). This could be explained by the routine chemoprophylaxis used for all patients after transplant which nullified CMV as a risk for PTDM in our cohort.
Our data suggest that inflammation of islet β-cells may play a crucial role in the pathogenesis of PTDM in RTRs. The significant variations in IFNG to pancreatic β-cell inflammation and loss could be due to a deviation of SOCS/Treg Fox P3 cross-regulation toward T cell-mediated immunity. Our results support a previously reported association of a deviation in SOCS/Treg Fox P3 cross-regulation causing immune dysregulation by IFN-γ. While RTRs are maintained under an immunosuppressive maintenance dose control, it may be advisable to tailor drug administration according to the genetic makeup of the patient. To date, there is no study in such detail is performed. This might represent a suitable platform for a large multicenter study. Further studies are required to evaluate the role of cytokine genes in PTDM to confirm our findings.
Other studies have found lower kidney function in patients with NODAT. However, in those reports, the follow-up was longer. A 12-year graft survival of 48% was reported in patients who developed NODAT, compared with 70% in non-NODAT, with type 2 diabetes mellitus being an independent and strong predictor of graft loss (relative risk of 3.72%).62 In the same study, serum creatinine levels at 5 years were noted to be significantly lower in non-NODAT cases.62
NODAT has been found to be associated with a significantly higher incidence of major cardiac events in comparison with post-transplant patients who do not develop diabetes (25% and 7%) and with lower survival rates (63% and 80%) over eight successive years.63 In our study, NODAT patients were, on average, 10 years older than the non-NODAT which was in accordance with what has been reported previously.31 Body physiology might be an important factor in this regard.59,64
Sumrani et al (1991)24 reported that NODAT is more frequently observed in patients who receive a deceased-donor kidney transplant than in those who receive a living-donor kidney transplant; however, our finding did not reflect this.
Prediction of NODAT would be useful as an effective preventive measure. Several reports have recently suggested that a daily post-transplant oral glucose tolerance test could be useful in predicting later development of NODAT.65 Others have claimed that oral glucose tolerance tests performed at 10–12 weeks post-transplant have a better predictive value.38 An FPG level of ≥126 mg/100 mL on day 5 post-transplant has also been suggested as a useful predictor of later development of NODAT.66 A risk-prediction model was used in the general population for the development of type 2 diabetes mellitus to see their efficacy in predicting the development of NODAT. The model used in this study was the San Antonio Diabetes Prediction Model.67 The area under the curve-receiver operator characteristic curve of the San Antonio Diabetes Prediction Model score to predict NODAT was 0.807 (95% CI, 0.728–0.885; p< 0.001), with positive and negative predictive values of the San Antonio Diabetes Prediction Model score over the 75th percentile of 31.2% and 93.7%.67 We think that cytokine profile could help in predicting those who will develop NODAT.
NODAT has emerged as an increasingly important determinant of outcomes and survival in transplant recipients. Patient education and self-management are crucial for ensuring successful outcome post-transplantation.68 Many risk factors for developing NODAT were reported as: older, heavier, higher BMI and glucose levels, family history of diabetes mellitus and higher FBG 24 hrs after the transplant procedure. In our study, we found that the conventional risk factors for NODAT as neither age nor ethnicity showed any association towards NODAT progression. Moreover, young age transplant recipients were found to be less likely to develop NODAT, which may be due to active immunity and general body physiology. The above findings identified our cohorts as suitable for studying immunogenetic backgrounds for an immune-derived complication of renal transplantation. In this direction, we found that the IFNG TT genotype was significantly associated with NODAT (p=0.005), while IFNG AA was considerably higher in the control group (p=0.004). An observation which suggests that IFNG TT, which corresponds to higher IFN-γ levels, is significantly associated with the progression of NODAT while IFNG AA, which is correlated with low IFN-γ, is a protective genotype against PTDM progression.
Our results did not agree with the univariate analysis described by Babel et al, who reported that IFNG AA genotype was associated with NODAT.40 The difference could be due to difference in ethnicity and associated risk factors.
Moreover, chronic HCV infection was significantly more prevalent among our KTRs with NODAT (7.7% vs 1.3%, p=0.006) (Table 1) which supports many other reports.10,23,34,36,37 The relatively high incidence of NODAT among HCV positive patients might be explained by a direct or immune-mediated effect on β-cells of pancreatic islets.69
In our cohort, we found that patients with positive CMV IgG were significantly higher in NODAT (p<0.05). Further, lower prevalence of cases with post-transplant CMV viremia could be due to our adopted policy of mass CMV chemoprophylaxis.
Further, many reports indicated that CNI might contribute to NODAT by β-cell toxicity and inhibition of insulin secretion-4.18,19 No significant difference was noted between NODAT and non-NODAT cohorts regarding the type of immunosuppressive regimen despite (p>0.05).
Conclusion
Beside the conventional contributing factors – as older the age, high BMI at the time of transplant and HCV infection – of NODAT in our cohort, our results might represent a suitable platform for a T lymphocyte initiated multi-cytokine and chemokine SNP association to study the role of these immune-signatures in pathogenesis of NODAT.
Acknowledgments
Authors would like to acknowledge Dr Adel Ahmad, previous head of Clinical Research in Dasman Diabetes Institute, for his contribution in initiating this study. We would like to extend our thanks to Ms Asma Al Hubail, head of clinical laboratory and phlebotomy services, and her team for their cooperation. We would also like to thank DDI Biobank team members for their quality of work and processing our samples. This research has been funded generously by DDI which an entity of Kuwait Foundation for the Advancement of Science (KFAS). We received no external funding for the current work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ghisdal L, Van Laecke S, Abramowicz MJ, Vanholder R, Abramowicz D. New-onset diabetes after renal transplantation: risk assessment and management. Diabetes Care. 2012;35(1):181–188. doi:10.2337/dc11-1230
2. Davidson J, Wilkinson A, Dantal J, et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75(10Suppl):SS3–SS24. doi:10.1097/01.TP.0000069952.49242.3E
3. Chadban S. New-onset diabetes after transplantation – should it be a factor in choosing an immunosuppressant regimen for kidney transplant recipients? Nephrol Dial Transplant. 2008;23(6):1816–1818. doi:10.1093/ndt/gfn052
4. Chadban S, Morris R, Hirsch HH, Bunnapradist S, Arns W, Budde K. Immunosuppression in renal transplantation: some aspects for the modern era. Transplant Rev. 2008;22(4):241–251. doi:10.1016/j.trre.2008.05.003
5. Marcen R, Morales JM, Arias M, et al. Ischemic heart disease after renal transplantation in patients on cyclosporine in Spain. J Am Soc Nephrol. 2006;17(12 Suppl 3):S286–S290. doi:10.1681/ASN.2006080928
6. Sulanc E, Lane JT, Puumala SE, Groggel GC, Wrenshall LE, Stevens RB. New-onset diabetes after kidney transplantation: an application of 2003 International Guidelines. Transplantation. 2005;80(7):945–952.
7. Aleid H, Alhuraiji A, Alqaraawi A, et al. New-onset diabetes after kidney transplantation: incidence, risk factors, and outcomes. Saudi J Kidney Dis Transpl. 2016;27(6):1155–1161.
8. Al-Daghri NM, Al-Attas OS, Alokail MS, et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med. 2011;9:76. doi:10.1186/1741-7015-9-76
9. Al-Ghareeb SM, El-Agroudy AE, Al Arrayed SM, Al Arrayed A, Alhellow HA. Risk factors and outcomes of new-onset diabetes after transplant: single-centre experience. Exp Clin Transplant. 2012;10(5):458–465. doi:10.6002/ect.2012.0063
10. Nagib AM, Refaie AF, Akl AI, et al. New onset diabetes mellitus after living donor renal transplantation: a unique pattern in the Egyptian population. J Diabetes Metab. 2015;6(4):1–5.
11. Johny KV, Nampoory MR, Costandi JN, et al. High incidence of post-transplant diabetes mellitus in Kuwait. Diabetes Res Clin Pract. 2002;55(2):123–130.
12. Tarek M, Yagan J, Gheith O, et al. Post transplant diabetes mellitus in Kuwait. Nephrol Dial Transplant. 2017;32(Issue suppl_3):iii275. doi:10.1093/ndt/gfx149.SP451
13. Hecking M, Kainz A, Werzowa J, et al. Glucose metabolism after renal transplantation. Diabetes Care. 2013;36(9):2763–2771. doi:10.2337/dc12-2441
14. Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant. 2005;5(10):2433–2440. doi:10.1111/j.1600-6143.2005.01040.x
15. Kaposztas Z, Gyurus E, Kahan BD. New-onset diabetes after renal transplantation: diagnosis, incidence, risk factors, impact on outcomes, and novel implications. Transplant Proc. 2011;43(5):1375–1394. doi:10.1016/j.transproceed.2011.04.008
16. Schachtner T, Stein M, Reinke P. Diabetic kidney transplant recipients: impaired infection control and increased alloreactivity. Clin Transplant. 2017;31(7). doi:10.1111/ctr.12986
17. Yates CJ, Fourlanos S, Hjelmesaeth J, Colman PG, Cohney SJ. New-onset diabetes after kidney transplantation-changes and challenges. Am J Transplant. 2012;12(4):820–828. doi:10.1111/j.1600-6143.2011.03855.x
18. Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7(6):1506–1514. doi:10.1111/j.1600-6143.2007.01749.x
19. Ghisdal L, Bouchta NB, Broeders N, et al. Conversion from tacrolimus to cyclosporine A for new-onset diabetes after transplantation: a single-centre experience in renal transplanted patients and review of the literature. Transpl Int. 2008;21(2):146–151. doi:10.1111/j.1432-2277.2007.00589.x
20. Santos AH
21. Cartwright N, Demaine A, Jahromi M, Sanders H, Kaminski ER. A study of cytokine protein secretion, frequencies of cytokine expressing cells and IFN-G gene polymorphisms in normal individuals. Transplantation. 1999;68(10):1546–1552.
22. Jahromi M, Millward A, Demaine A. A CA repeat polymorphism of the IFN-gamma gene is associated with susceptibility to type 1 diabetes. J Interferon Cytokine Res. 2000;20(2):187–190. doi:10.1089/107999000312595
23. Hayase H, Ishizu A, Ikeda H, et al. Aberrant gene expression by CD25+CD4+ immunoregulatory T cells in autoimmune-prone rats carrying the human T cell leukemia virus type-I gene. Int Immunol. 2005;17(6):677–684. doi:10.1093/intimm/dxh238
24. Kang K, Park SH, Chen J, et al. Interferon-gamma represses M2 gene expression in human macrophages by disassembling enhancers bound by the transcription factor MAF. Immunity. 2017;47(2):235–250.e234. doi:10.1016/j.immuni.2017.07.017
25. Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnott PJ, Hutchinson IV. In vitro production of IFN-gamma correlates with CA repeat polymorphism in the human IFN-gamma gene. Eur J Immunogenet. 1999;26(1):1–3.
26. Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV. A single nucleotide polymorphism in the first intron of the human IFN-gamma gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-gamma production. Hum Immunol. 2000;61(9):863–866.
27. Biolo G, Amoroso A, Savoldi S, et al. Association of interferon-gamma +874A polymorphism with reduced long-term inflammatory response in haemodialysis patients. Nephrol Dial Transplant. 2006;21(5):1317–1322. doi:10.1093/ndt/gfk033
28. Shiu KY, McLaughlin L, Rebollo-Mesa I, et al. Graft dysfunction in chronic antibody-mediated rejection correlates with B-cell-dependent indirect antidonor alloresponses and autocrine regulation of interferon-gamma production by Th1 cells. Kidney Int. 2017;91(2):477–492. doi:10.1016/j.kint.2016.10.009
29.
30. Baker RJ, Mark PB, Patel RK, Stevens KK, Palmer N. Renal association clinical practice guideline in post-operative care in the kidney transplant recipient. BMC Nephrol. 2017;18(1):174. doi:10.1186/s12882-017-0669-4
31. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178–185.
32. Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130.
33. Adamopoulos S, Kolokathis F, Gkouziouta A, et al. Cytokine gene polymorphisms are associated with markers of disease severity and prognosis in patients with idiopathic dilated cardiomyopathy. Cytokine. 2011;54(1):68–73. doi:10.1016/j.cyto.2011.01.004
34. Raisanen-Sokolowski A, Glysing-Jensen T, Koglin J, Russell ME. Reduced transplant arteriosclerosis in murine cardiac allografts placed in interferon-gamma knockout recipients. Am J Pathol. 1998;152(2):359–365.
35. Kim HJ, Kang SW, Chung JH, Kim SJ, Choe BK. Polymorphisms of the Interferon gamma gene and coronary artery disease in the Korean population. Mol Biol Rep. 2012;39(5):5425–5432. doi:10.1007/s11033-011-1342-9
36. Babu BM, Reddy BP, Priya VH, et al. Cytokine gene polymorphisms in the susceptibility to acute coronary syndrome. Genet Test Mol Biomarkers. 2012;16(5):359–365. doi:10.1089/gtmb.2011.0182
37. Puoti F, Ricci A, Nanni-Costa A, Ricciardi W, Malorni W, Ortona E. Organ transplantation and gender differences: a paradigmatic example of intertwining between biological and sociocultural determinants. Biol Sex Differ. 2016;7:35. doi:10.1186/s13293-016-0088-4
38. Pham PT, Pham PM, Pham SV, Pham PA, Pham PC. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes. 2011;4:175–186. doi:10.2147/DMSO.S19027
39. Yi Z, Li L, Garland A, et al. IFN-gamma receptor deficiency prevents diabetes induction by diabetogenic CD4+, but not CD8+, T cells. Eur J Immunol. 2012;42(8):2010–2018. doi:10.1002/eji.201142374
40. Babel N, Cherepnev G, Kowalenko A, Horstrup J, Volk HD, Reinke P. Nonimmunologic complications and gene polymorphisms of immunoregulatory cytokines in long-term renal transplants. Kidney Int. 2004;66(1):428–432. doi:10.1111/j.1523-1755.2004.00749.x
41. Oxenkrug G. Interferon-gamma – inducible inflammation: contribution to aging and aging-associated psychiatric disorders. Aging Dis. 2011;2(6):474–486.
42. Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254.
43. Shanley DP, Aw D, Manley NR, Palmer DB. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 2009;30(7):374–381. doi:10.1016/j.it.2009.05.001
44. Singh P, Goode T, Dean A, Awad SS, Darlington GJ. Elevated interferon gamma signaling contributes to impaired regeneration in the aged liver. J Gerontol A Biol Sci Med Sci. 2011;66(9):944–956. doi:10.1093/gerona/glr094
45. Nosratabadi R, Arababadi MK, Hassanshahi G, et al. Evaluation of IFN-gamma serum level in nephropatic type 2 diabetic patients. Pak J Biol Sci. 2009;12(9):746–749.
46. Mahmoud A, Sheneef A, Goda A, Ismail M, Abualfadl E. Association of interferon-cand its (+874 T/A)gene polymorphism with type 2 diabetes mellitus inrheumatoid arthritis patients. Egypt Rheumatol. 2015;38(4).
47. Nicolau J, Lequerré T, Bacquet H, Vittecoq O. Rheumatoid arthritis, insulin resistance, and diabetes. Joint Bone Spine. 2017;84(4):411–416. doi:10.1016/j.jbspin.2016.09.001
48. Tsiavou A, Hatziagelaki E, Chaidaroglou A, Koniavitou K, Degiannis D, Raptis SA. Correlation between intracellular interferon-gamma (IFN-gamma) production by CD4+ and CD8+ lymphocytes and IFN-gamma gene polymorphism in patients with type 2 diabetes mellitus and latent autoimmune diabetes of adults (LADA). Cytokine. 2005;31(2):135–141. doi:10.1016/j.cyto.2005.02.011
49. Zhu QR, Ge YL, Gu SQ, et al. Relationship between cytokines gene polymorphism and susceptibility to hepatitis B virus intrauterine infection. Chin Med J (Engl). 2005;118(19):1604–1609.
50. Zambon CF, Basso D, Navaglia F, et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine. 2005;29(4):141–152. doi:10.1016/j.cyto.2004.10.013
51. Morrison BA, Ucisik-Akkaya E, Flores H, Alaez C, Gorodezky C, Dorak MT. Multiple sclerosis risk markers in HLA-DRA, HLA-C, and IFNG genes are associated with sex-specific childhood leukemia risk. Autoimmunity. 2010;43(8):690–697. doi:10.3109/08916930903567492
52. Mukhtar NA, Ayala C, Maher JJ, Khalili M. Assessment of factors associated with pre-diabetes in HCV infection including direct and dynamic measurements of insulin action. J Viral Hepat. 2012;19(7):480–487. doi:10.1111/j.1365-2893.2011.01568.x
53. Liang J, Lv C, Chen M, et al. Effects of preoperative hepatitis B virus infection, hepatitis C virus infection, and coinfection on the development of new-onset diabetes after kidney transplantation. J Diabetes. 2018. doi:10.1111/1753-0407.12853
54. Klein S. Host factors mediating sex differences in viral infection. Gend Med. 2005;2(4):197–207.
55. Esmaeili A, Mirzazadeh A, Carter GM, et al. Higher incidence of HCV in females compared to males who inject drugs: a systematic review and meta-analysis. J Viral Hepat. 2017;24(2):117–127. doi:10.1111/jvh.12628
56. Youssef SS, Abd El Aal AM, Nasr AS, El Zanaty T, Seif SM. Interleukin-12B gene polymorphism frequencies in Egyptians and sex-related susceptibility to hepatitis C infection. J Interferon Cytokine Res. 2013;33(8):415–419. doi:10.1089/jir.2012.0161
57. Ruggieri A, Gagliardi MC, Anticoli S. Sex-dependent outcome of hepatitis B and C viruses infections: synergy of sex hormones and immune responses? Front Immunol. 2018;9:2302. doi:10.3389/fimmu.2018.02302
58. Hjelmesaeth J, Sagedal S, Hartmann A, et al. Asymptomatic cytomegalovirus infection is associated with increased risk of new-onset diabetes mellitus and impaired insulin release after renal transplantation. Diabetologia. 2004;47(9):1550–1556. doi:10.1007/s00125-004-1499-z
59. Sinangil A, Celik V, Barlas S, et al. The incidence of new onset diabetes after transplantation and related factors: single center experience. Nefrologia. 2017;37(2):181–188. doi:10.1016/j.nefro.2016.11.022
60. Marin M, Renoult E, Bondor CI, Kessler M. Factors influencing the onset of diabetes mellitus after kidney transplantation: a single French center experience. Transplant Proc. 2005;37(4):1851–1856. doi:10.1016/j.transproceed.2005.03.140
61. Dedinska I, Laca L, Miklusica J, Kantarova D, Galajda P, Mokan M. Correlation between CMV infection and post-transplantation new-onset diabetes mellitus. Int J Organ Transplant Med. 2016;7(3):173–182.
62. Miles AM, Sumrani N, Horowitz R, et al. Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation. 1998;65(3):380–384.
63. Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69(3):588–595. doi:10.1038/sj.ki.5000116
64. Yu H, Kim H, Baek CH, et al. Risk factors for new-onset diabetes mellitus after living donor kidney transplantation in Korea – a retrospective single center study. BMC Nephrol. 2016;17(1):106. doi:10.1186/s12882-016-0321-8
65. Kuypers DR, Claes K, Bammens B, Evenepoel P, Vanrenterghem Y. Early clinical assessment of glucose metabolism in renal allograft recipients: diagnosis and prediction of post-transplant diabetes mellitus (PTDM). Nephrol Dial Transplant. 2008;23(6):2033–2042. doi:10.1093/ndt/gfm875
66. Rodrigo E, Santos L, Pinera C, et al. Early prediction of new-onset diabetes mellitus by fifth-day fasting plasma glucose, pulse pressure, and proteinuria. Transplant Proc. 2011;43(6):2208–2210. doi:10.1016/j.transproceed.2011.05.005
67. Rodrigo E, Santos L, Pinera C, et al. Prediction at first year of incident new-onset diabetes after kidney transplantation by risk prediction models. Diabetes Care. 2012;35(3):471–473. doi:10.2337/dc11-2071
68. Muhlhauser I, Berger M. Evidence-based patient information in diabetes. Diabetic Med. 2000;17(12):823–829.
69. Knobler H, Stagnaro-Green A, Wallenstein S, Schwartz M, Roman SH. Higher incidence of diabetes in liver transplant recipients with hepatitis C. J Clin Gastroenterol. 1998;26(1):30–33.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

