Back to Journals » Cancer Management and Research » Volume 10
Immunocompetence and mechanism of the DRibble-DCs vaccine for oral squamous cell carcinoma
Authors Dong H , Su H, Chen L, Liu K , Hu HM, Yang WD, Mou YB
Received 2 November 2017
Accepted for publication 26 January 2018
Published 16 March 2018 Volume 2018:10 Pages 493—501
DOI https://doi.org/10.2147/CMAR.S155914
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Heng Dong,1,2,* Hang Su,3,* Lin Chen,1 Kai Liu,1 Hong-ming Hu,2 Weidong Yang,1 Yongbin Mou1,2
1Central Laboratory, Nanjing Stomatological Hospital, Medical School of Nanjing University, Nanjing, People’s Republic of China; 2Laboratory of Cancer Immunobiology, Robert W Franz Cancer Research Center, Earle A Chiles Research Institute, Providence Cancer Center, Portland, OR, USA; 3Department of Implantology, The Affiliated Stomatology Hospital of Tongji University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Background: Due to the high-quality immunogenicity of tumor-derived autophagosomes (DRibbles), we aimed to explore the antitumor ability and mechanism of DRibble-loaded dendritic cells (DRibble-DCs).
Materials and methods: DRibbles extracted from the oral squamous cell carcinoma cell line SCC7 express specific LC3-II and ubiquitination marker. Immunization of mice with the DRibble-DCs vaccine led to the proliferation and differentiation of CD3+CD4+IFN-γ+ and CD3+CD8+IFN-γ+ T cells. The expression of proteins in endoplasmic reticulum stress (ERS) pathways was determined by Western blotting. Additionally, the functional properties of the DRibble-DCs were examined in mice, and regulatory T cells were measured by flow cytometry.
Results: Excellent biocompatibility was observed in vitro when DCs were loaded with DRibbles. T cells of lymph nodes and spleens from mice immunized with DRibble-DCs had cytotoxic effects on SCC7 cells. DCs homeostasis and ERS-related proteins were affected by DRibbles. Moreover, the DRibble-DCs vaccine achieved significantly better antitumor efficacy than DRibbles and tumor cell lysate-loaded DCs.
Conclusion: The results validated the antitumor immune responses to the DRibble-DCs vaccine in vivo and in vitro. The ERS pathway can be affected by DRibbles.
Keywords: dendritic cells, DRibbles, oral squamous cell carcinoma, endoplasmic reticulum stress, antigen cross-presentation, vaccine, autophagosome
Introduction
Oral squamous cell carcinoma (OSCC) is a highly malignant tumor type.1 In addition to traditional treatments, such as surgery, radiotherapy, and chemotherapy, immunotherapy and tumor vaccines are receiving greater attention as promising strategies for the treatment of OSCC.2 Despite recent advances in cancer immunotherapy,3 effective responses are rarely achieved due to multiple factors, including defective antigen cross-presentation, ineffective vaccine delivery, infiltration-suppressing immune cells such as regulatory T cells (Tregs) and myeloid-derived suppressor cells, and immunosuppressive tumor microenvironments.4 Dendritic cells (DCs) are the most important professional antigen-presenting cells (APCs) and are typically present in low numbers in the blood.5 DC-based vaccines play an important role in antitumor biotherapy.6 Cytotoxic T lymphocytes activated by DCs loaded with tumor antigens or antitumor vaccines can effectively elicit antitumor immunity via the cross-presentation pathway.7
Based on our previous work, we found that autophagy in tumor cells played a critical role in cross-presentation of tumor antigens and identified induced autophagosomes as the novel, efficient carriers for cross-presentation of tumor-associated antigens (TAAs).8 TAAs are usually degraded by two major proteolysis pathways in the tumor cells, in which the long-lived proteins (LLiPs) are degraded by the lysosomes through the autophagy pathway, whereas the short-lived proteins (SLiPs) including defective ribosomal products (DRiPs) are ubiqutinated and degraded by proteasomes.9 A nanometer-level autophagosome-enriched vaccine named DRibbles was derived from tumor cells after inhibiting their proteasomal and lysosomal functions during autophagy induction.10 DRibbles sequester both LLiPs and SLiPs, including DRiPs, and can delay the growth of cancer and improve survival of tumor-bearing mice by loading on DCs.11 Our previous research showed DCs could be activated by DRibbles and induced effective antitumor efficacy in vitro and in vivo,12 but the immunologic mechanism, such as the influence of organelles in DCs and the degradation and presentation pathway of TAAs, is not clear after DRibbles are swallowed by DCs. Forthmore, the abilities of DRibbles to prevent tumor growth also need to be explored besides reducing the volumes of the established tumor in mice.
Endoplasmic reticulum stress (ERS) relief plays a pivotal role in reactivating the cross-presentation functions of tumor-associated DCs.13 Cancer antigens degraded by the proteasome are transported from the cytosol to the endoplasmic reticulum (ER) for binding to major histocompatibility complex class I molecules and antigen presentation to T-cell surfaces.14 ER transmembrane receptors detect the onset of ERS and induce the unfolded protein response to restart normal ER function. BiP, the relevant ER transmembrane protein,15 can promote eIF2α phosphorylation and thereby increase CHOP expression, resulting in extensive upregulation of the mRNA translation rate and efficient remission of the stress in ER.
In this study, we investigated whether DRibble-loaded DCs (DRibble-DCs) could induce T-cell proliferation and activation and enhance immunologic memory to regress OSCC in murine models. We also explored the function of antigen cross-presentation influenced by ERS after DCs loaded with DRibbles.
Materials and methods
Mice
C3H/HeJ mice were purchased from the Model Animal Research Center of Nanjing University and housed under pathogen-free conditions. All experimental procedures, including the use of the gifted cell lines, were approved by the Animal Care and Use Committee of the Medical School of Nanjing University and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No 85-23, revised 1996).
Cell culture
Monocytes were isolated from mouse bone marrow and cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum, 10 ng/mL recombinant murine granulocyte macrophage colony-stimulating factor (Gibco), and 1 ng/mL recombinant murine IL-4 (PeproTech, Rocky Hill, NJ, USA). Half of the medium was gently replaced on days 3 and 5. Immature DCs were collected on day 5.
Preparation and identification of DRibbles
DRibbles were prepared as previously described. Briefly, SCC7 cells and SCC7-OVA cells (gifted by Prof Hong-Ming Hu, Providence Cancer Center, Providence Portland Medical Center, Portland, OR, USA) were treated with 100 nmol/mL rapamycin (Enzo Life Sciences, Farmingdale, NY, USA), 100 nmol/mL bortezomib (Millennium Pharmaceuticals, Cambridge, MA, USA), and 10 mmol/mL NH4Cl (Sigma-Aldrich, St. Louis, MO, USA) in complete DMEM for 24 hours. DRibbles were collected from cell supernatants following centrifugation at 12,000× g for 15 minutes. LC3-II, a specific marker of autophagy,16 was detected by Western blotting. Tumor cell lysates (Lysates) were prepared through three freeze–thaw cycles, and the supernatants were collected by centrifugation at 12,000× g for 10 minutes.
Transmission electron microscopy
The DRibbles were collected and stained with 2% phosphotungstic acid. Thereafter, the samples were examined with a transmission electron microscope (JEOL, Tokyo, Japan).
Tumor cell cytotoxicity assay
Cytotoxicity was assessed using a CytoTox 96® Non-Radioactive Cytotoxicity Assay Kit (Promega, Fitchburg, WI, USA) according to the manufacturer’s instructions. Briefly, 1 × 105/mL SCC7 and 1 × 106/mL T cells purified by immunomagnetic beads were incubated in 96-well assay plates. After incubation for 6 hours, the supernatants were aspirated and assessed using a CytoTox 96® assay. The plates were centrifuged for 5 minutes at 400× g, and 5 minutes later, the absorbance of the plates was measured at 490 nm to detect cytotoxicity, which was calculated using the following formula: tumor inhibition rate (%) = (optical density [OD] 490 nm experimental group – OD 490 nm effector cell spontaneous – OD 490 nm target cell spontaneous)/(OD 490 nm target cell max – OD 490 nm target cell spontaneous) × 100%.
DRibble-DCs vaccine and T-cell activation
Immature DCs were stimulated with DRibbles and associated with 10 μg/mL TNF-α, 1 μg/mL PGE2, 10 ng/mL IL-6, and 10 ng/mL IL-1β for 12 hours. C3H/HeJ mice (same background with SCC7) were randomly divided into five groups with four mice in each group and received the DRibble-DCs vaccine. Briefly, on day 0, 10 μL of different concentrations of the vaccines was injected into the foot pads of the mice. The same DCs vaccines were also injected into the bilateral inguinal lymph nodes of the mice. The bilateral inguinal lymph nodes and spleens were isolated on day 14. T cells were then isolated by an immunomagnetic bead sorting kit (Miltenyi Biotech, Bergisch Gladbach, Germany). The peripheral blood, spleen, and lymph node cells from the immunized mice were also isolated. The cells were labeled with CD3, CD4, CD8, and IFN-γ (eBioscience, San Diego, CA, USA) antibodies, and then detected by flow cytometry (FCM). Data were acquired using a BD FACSCalibur machine and analyzed with FlowJo software (Tree Star Inc).
Apoptosis assay
The effects of different concentrations of DRibbles on DC apoptosis were determined using FCM. Briefly, immature DCs were incubated with DRibbles at a final concentration of 0, 2.5, 10, or 50 μg/mL for 12 hours. DRibble-DCs were stained with Annexin V and propidium iodide (PI; Biouniquer, Beijing, People’s Republic of China), and the expression levels of Annexin V and PI in DCs were then detected by FCM.
Detection of supernatant IFN-γ by ELISA
A mouse IFN-γ ELISA Ready-SET-Go kit (eBioscience) was used to detect the IFN-γ levels in the cell culture supernatant according to the manufacturer’s instructions. Briefly, an ELISA plate (Costar, Washington, DC, USA) was coated with 100 μL/well of IFN-γ capture antibody overnight at 4°C. After the plate was blocked with ELISA buffer, samples and standards were added to the wells and incubated for 2 hours at room temperature. Bound, biotinylated IFN-γ was detected. The value of OD was then read at 450 nm using a microplate reader (BioTek, Winooski, VT, USA).
Western blotting
To determine the ubiquitination, Lysates and DRibbles were stained with an anti-ubiquitin antibody. The ERS pathway-related proteins affected by DRibbles were also examined by Western blotting. Briefly, DCs were incubated with phosphate-buffered saline (PBS), DRibbles (2.5 μg/mL), or Lysates (2.5 μg/mL) for 12 hours. Tunicamycin (2 μmol/mL) and 4-phenylbutyric acid (4PBA; 1 mmol/mL) were used as positive and negative controls, respectively. Samples were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The nitrocellulose membranes were incubated with primary antibodies, including BiP, p-eIF2α, eIF2α, CHOP (Cell Signaling Technology, Danvers, MA, USA), and a secondary goat anti-mouse antibody at room temperature. The blots were visualized by enhanced chemiluminescence (Amersham, Little Chalfont, UK), and the density of β-actin served as an internal loading control. The mean fold induction of ERS pathway-related proteins and β-actin was calculated using ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA).
Antigen cross-presentation CPRG assay in vitro
In 96-well U-bottom plates, 50 µL of mutuDC cells (0.8 ¥ 106/mL) and 50 µL of different concentrations of the DRibbles-OVA from the SCC7-OVA cell line were plated; after 6 hours, the cells were co-cultured with 100 µL B3Z cells (2 ¥ 106/mL) overnight. The B3Z cell response was measured as the β-galactosidase activity induced upon ligand recognition. β-Galactosidase activity was measured by the sample’s absorbance at 595 nm and the absorbance of the cleavage product of chlorophenol red-β-D-galactopyranoside (CPRG; Sigma-Aldrich).
Animal experiments
Four groups (10 mice each) were immunized with PBS, DRibbles, DRibble-DCs, or Lysate-DCs in both foot pads on day 0, and another group without any intervention was regarded as the tumor-free control group. After 7 days, a second immunization was administered to the bilateral inguinal lymph nodes. At 14 days, 1 × 106 SCC7 cells in PBS were subcutaneously injected into the right abdomen. Thereafter, the tumor volume was measured every 3 days with vernier caliper and calculated using the formula V = 1/2 × a × b2. “V” represents tumor volume, “a” means the largest diameter (length), and “b” means the smallest diameter (width) of the tumor”. The mice were sacrificed on the 54th day, the peripheral blood and inguinal lymph nodes were collected, and the proportion of CD4+CD25+Foxp3+ Tregs in peripheral blood mononuclear cells (PBMCs) was determined by FCM.
Data analysis
All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Cumulative data are presented as the mean ± SD. Differences between the control group and experimental groups were assessed by performing Student’s two-tailed t-test or one-/two-way ANOVA and Bonferroni post hoc test, and statistical significance was set to p < 0.05.
Results
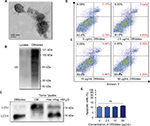
DRibbles were induced via the production of SCC7 autophagosomes
As shown in Figure 1A, the ultrastructure of DRibbles, as revealed by transmission electron microscopy (TEM), consisted of a double-membrane structure with dimensions ranging from 200 to 500 nm. Ubiquitination was clearly observed in DRibbles, whereas no ubiquitination was observed in the Lysates (Figure 1B). In addition, only LC3-II was observed in DRibbles obtained from the SCC7 cells with autophagy induced by bortezomib, rapamycin, and NH4Cl (Figure 1C). The expression levels of Annexin V and PI in DCs with different concentrations of DRibbles were detected by FCM, and as shown, treatment with DRibbles resulted in no obvious toxicity to DCs (Figure 1D and E).
Effects of DRibble-DCs on T-cell activation
The purity of the T cells, as effector cells, isolated from mouse spleen cells was determined, and these cells were incubated with SCC7 cells at a concentration of 10:1 for 6 hours. The tumor inhibition rate (%) of the spleen-derived T-cell and lymph node-derived T-cell groups showed that the cytotoxicity of SCC7 cells was the highest at a concentration of 2.5 μg/mL in the DRibble-DCs group. Tumor inhibition rates (%) were higher in all groups that received DRibble-DCs than in those that received only PBS, and the highest cytotoxicity of tumor cells incubated with T cells derived from either lymph nodes or spleens (Figure 2A) was obtained with DRibbles at a concentration of 2.5 μg/mL. An analysis of IFN-γ in supernatants by ELISA revealed that 2.5 μg/mL DRibbles could induce more IFN-γ secretion from T cells derived from both PBMCs and lymph nodes (Figure 2B). The highest level of peripheral blood CD3+CD4+IFN-γ+ T cells was found in the group immunized with 2.5 μg/mL DRibble-DCs. In addition, the proportion of CD3+CD4+IFN-γ+ T cells in the spleens and CD3+CD8+IFN-γ+ T cells in the lymph nodes of immunized mice (Figure 2C) was higher in the group immunized with 2.5 μg/mL DRibble-DCs than those in the other groups. However, the three graphs show that with an increased DRibbles concentration, the effect of the DCs vaccine was decreased. Thus, we aimed to explore what factors led to the decrease in DCs antigen-presentation function.
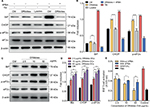
DRibbles alter the ERS pathway in DCs
After incubation with 2.5 μg/mL DRibbles for 12 hours, the expression of the ERS-related proteins BiP, CHOP, and p-eIF2α increased in DCs compared with those in DCs incubated with PBS and 2.5 μg/mL Lysates. There were significant differences in the expression of p-eIF2α between the groups. In the positive group, the expression levels of the proteins BiP, p-eIF2α, and CHOP were significantly increased. Interestingly, when the ERS inhibitor 4PBA was added to DRibble-DCs, the expression of the proteins BiP, CHOP, and p-eIF2α returned to their original levels (Figure 3A and B). Next, we incubated DCs with different concentrations of DRibbles for 12 hours. The expression levels of the ERS-related proteins BiP, CHOP, and p-eIF2α in DCs were increased accompanied by increased concentrations (Figure 3C and D). We previously found that with an increase in concentration, the effect of the DRibble-DCs vaccine worsens (Figure 2A). We speculated whether this change was due to an increase in ERS. We used a CPRG assay to detect DCs loading with DRibbles-OVA after stimulating B3Z T cells. Antigen presentation was the best at 2.5 μg/mL and was reduced as the concentration increased. Additionally, the effect of the DCs vaccine was restored to some extent when we treated DCs with 4PBA (Figure 3E). Thus, the 2.5 μg/mL concentration of DRibbles was used for further experiments.
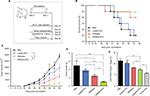
Antitumor immunity and inhibition of tumor growth by the DRibble-DCs vaccine
Tumor growth was observed for 54 days following the injection of different vaccines (Figure 4A). The differential impacts of the immunizations on mice were assessed by constructing survival curves (Figure 4B). The survival rates of the mice immunized with DRibble-DCs or DRibbles were significantly higher than those immunized with Lysate-DCs. The group of mice immunized with DRibble-DCs presented the smallest tumor size (Figure 4C) and tumor weight (Figure 4D). After the mice were sacrificed, the peripheral blood was collected, and the proportion of Tregs in PBMCs was determined. Moreover, the level of Tregs in PBMCs in the DRibble-DCs vaccine group was lower than those in the other groups (Figure 4E).
Discussion
With the incidence of OSCC increasing globally, the development of effective vaccines to prevent OSCC represents an urgent task.17 However, the traditional tumor antigens adopted to produce antitumor immunity in clinical studies are usually inefficient and ineffective.18
DRibbles are autophagic antigens produced by inhibiting the proteasomal and lysosomal pathways of tumor cells.19 Our studies showed that a large amount of LC3-II autophagy antigens were induced by proteasome and lysosome inhibitors. Ubiquitination, which involves protein conjugation, is used to tag proteins for autophagy.12 Compared with the Lysates, DRibbles expressed a large number of ubiquitinated proteins. TEM images revealed that DRibbles have a bilayer structure with a spherical appearance. These characteristics were consistent with the results obtained in our previous study.20
Due to the excellent immunogenicity of DRibbles, they are readily taken up by APCs and presented to effector cells to enhance antitumor immunity.16 DCs are the most potent professional APCs and have the most powerful antigen-presenting capacity based on their stimulation of T-cell activation and proliferation.21 We previously demonstrated the ability of DRibbles to induce antitumor immune effects.22 Another aspect to be determined is whether a DRibble-DCs vaccine can produce an effective immune response to inhibit tumor growth. Because the effectiveness of a vaccine is closely related to its intensity and concentration, DCs were loaded with serial dilutions of DRibbles. After optimizing the preparation and utilization methods, the optimal concentration for the antitumor vaccine was 2.5 μg/mL DRibbles, which is lower than that used in the original studies.12 DCs loaded with DRibbles were used to immunize mice to produce effector cells from vaccine-activated spleen and lymph node cells and to detect the killing effects on SCC7 target cells. The killing effects of the purified T cells in the mice vaccinated with DRibble-DCs were significantly higher than those in the mice immunized with PBS. The secretion of IFN-γ, an important tumor suppressor,23 in the T-cell culture supernatants incubated with SCC7 cells was consistent with the results obtained in the target-cell killing assay, which is a direct measure of the immune response in vaccine-immunized mice. IFN-γ is a recognized indicator of antitumor immunity; thus, the percentages of CD3+CD4+IFN-γ+ T cells (helper T cells) and CD3+CD8+IFN-γ+ T cells (cytotoxic T cells) in lymphoid organs and peripheral blood reflect the antitumor immunity of vaccines.24 DCs vaccines can activate naïve T cells to differentiate into CD4+ and CD8+ effector cells to elicit cytotoxic tumor killing.25,26 In the present study, the percentage of CD3+CD4+IFN-γ+ T cells in the peripheral blood and spleens and CD3+CD8+IFN-γ+ T cells in the lymph nodes of immunized mice was higher in the mice immunized with DRibble-DCs than in the mice in other groups.
The ER is the center of protein processing.27 Tumor antigens loaded on DCs are processed and released from the ER for T-cell presentation. The T-cell activation effects of DCs loaded with DRibbles were superior to those of Lysates, which may be related to ER function in DCs. However, a higher concentration of DRibbles resulted in a weaker DRibble-DCs effect. We suspect that DRibbles may cause changes in the DCs ERS that affect DCs function.28 Persistent or intense ERS can also lead to programmed cell death or apoptosis.29 ERS is necessary to increase the ability to collapse the ER, repair or remove damaged proteins, and reduce the number of proteins in the ER to lessen the burden on the organelle. These effects are primarily accomplished by the ERS pathways.30 We believe that tumor proteins in DRibbles that are phagocytosed and degraded by DCs will aggregate within the ER, which will induce the ERS and the chaperone protein from the ER protein cavity to dissociate from its complexes with unfolded proteins. The isolation of the chaperone protein activates the endoplasmic membrane protein and initiates ERS.31 We hypothesize that ERS will facilitate the processing of tumor antigens in DRibbles by ER while reducing the translation of its autologous protein by ERS. The present results showed that DCs resulted in a mild increase in BiP expression after incubation with DRibbles for 12 hours, indicating that BiP dissociation could be affected by DRibbles proteins. The increase in BiP protein was linked to activation and induction of eIF2α phosphorylation and induction of more CHOP expression in the cell nucleus.32 In contrast, at 2.5 μg/mL, the Lysates did not induce changes in the ERS of DCs. Due to their lack of necessary membrane structures, we consider that the concentration of 2.5 μg/mL Lysates is too low to be ingested and processed by DCs. ERS is inhibited by 4PBA,33 and after DRibbles were incubated with 4PBA, the protein expression of BiP, CHOP, and p-eIF2α was significantly restored. This tendency was similar to that of the two groups of complete medium and 2.5 μg/mL DRibbles with 4PBA. Based on our results, we concluded that high concentration of DRibbles can slightly activate the ERS of DCs during antigen presentation, and this finding might explain why low concentrations of DRibbles can improve DCs antigen presentation, but high concentrations cannot help DCs promote T-cell activation. We treated DCs with 4PBA and used DRibbles-OVA to load DCs to stimulate B3Z T cells;34 as a result, cross-presentation was restored. This result further validated our hypothesis that the effect of DRibbles is affected by ERS.
Based on the above mentioned results, the induced DRibbles were superior to Lysates and PBS in their antitumor immune responses. Immunization of mice with DRibble-DCs can significantly improve the survival rate and inhibit the tumor growth of tumor-bearing mice after challenge with tumor cells. The animal experiment indicated that the DRibble-DCs vaccine could effectively activate T cells, which secreted the cytokine IFN-γ and inhibited tumor growth. Notably, the results also revealed that the immune mice could be induced to show memory immunity after immunization with the DRibble-DCs, which inhibited tumor growth. Memory immunity generally occurs through antigen stimulation and produces a large number of immunosuppressive cells to suppress the immune response. Foxp3+ Tregs, one of the most important immunosuppressive cells, maintain immune homeostasis by limiting various types of inflammatory responses and can inhibit the antitumor immunity effect.35 In the present study, CD4+CD25+Foxp3+ Tregs in the PBMCs from the tumor-free mice were significantly lower than those from the mice in the other experimental groups. Furthermore, the ratio of Tregs in the DRibble-DCs-immunized mice was close to those in the tumor-free mice, indicating that immunization with DRibble-DCs could relieve immunosuppression by reducing the proliferation of Tregs.
Conclusion
The presented data indicate that the autophagic vaccine containing DRibbles has strong abilities to trigger antitumor effects, and the mechanism of this effect involves initiating an appropriate level of ERS, which activates cytotoxic T cells and decreases the ratio of Tregs.
Acknowledgments
The authors acknowledge the kind support of Dr Hao Xie, Yuxian Song, and Zhi-fa Wen for technical assistance, and Guohua Xia for language editing. They are grateful for the grants from the National Natural Science Foundation of China (81371680 and 81571800).
Disclosure
The authors report no conflicts of interest in this work.
References
Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. | ||
Chen TC, Wu CT, Wang CP, et al. Associations among pretreatment tumor necrosis and the expression of HIF-1α and PD-L1 in advanced oral squamous cell carcinoma and the prognostic impact thereof. Oral Oncol. 2015;51(11):1004–1010. | ||
Kapadia CH, Perry JL, Tian SM, Luft JC, DeSimone JM. Nanoparticulate immunotherapy for cancer. J Control Release. 2015;219:167–180. | ||
Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–220. | ||
Weng K, Zhang J, Mei X, et al. Lower number of plasmacytoid dendritic cells in peripheral blood of children with bronchiolitis following respiratory syncytial virus infection. Influenza Other Respir Viruses. 2014;8(4):469–473. | ||
Hoffmann TK. Systemic therapy strategies for head-neck carcinomas: current status. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2012;11:Doc03. | ||
Maschalidi S, Nunes-Hasler P, Nascimento CR, et al. UNC93B1 interacts with the calcium sensor STIM1 for efficient antigen cross-presentation in dendritic cells. Nat Commun. 2017;8(1):1640. | ||
Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68(17):6889–6895. | ||
Zhou M, Wen Z, Cheng F, et al. Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88-NF-kB signal pathway. Oncoimmunology. 2016;5(7):e1180485. | ||
Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol. 2011;6(10):645–650. | ||
Su S, Zhou H, Xue M, et al. Anti-tumor efficacy of a hepatocellular carcinoma vaccine based on dendritic cells combined with tumor-derived autophagosomes in murine models. Asian Pac J Cancer Prev. 2013;14(5):3109–3116. | ||
Su H, Luo Q, Xie H, et al. Therapeutic antitumor efficacy of tumor-derived autophagosome (DRibble) vaccine on head and neck cancer. Int J Nanomedicine. 2015;10:1921–1930. | ||
Garris CS, Pittet MJ. ER stress in dendritic cells promotes cancer. Cell. 2015;161(7):1492–1493. | ||
Graner MW, Lillehei KO, Katsanis E. Endoplasmic reticulum chaperones and their roles in the immunogenicity of cancer vaccines. Front Oncol. 2014;4:379. | ||
Hussien Y, Podojil JR, Robinson AP, Lee AS, Miller SD, Popko B. ER chaperone BiP/GRP78 is required for myelinating cell survival and provides protection during experimental autoimmune encephalomyelitis. J Neurosci. 2015;35(48):15921–15933. | ||
Li Y, Wang LX, Pang P, et al. Tumor-derived autophagosome vaccine: mechanism of cross-presentation and therapeutic efficacy. Clin Cancer Res. 2011;17(22):7047–7057. | ||
Huang R, Liu W. Identifying an essential role of nuclear LC3 for autophagy. Autophagy. 2015;11(5):852–853. | ||
Shamloo N, Lotfi A, Motazadian HR, Mortazavi H, Baharvand M. Squamous cell carcinoma as the most common lesion of the tongue in Iranians: a 22-year retrospective study. Asian Pac J Cancer Prev. 2016;17(3):1415–1419. | ||
Unger WW, Mayer CT, Engels S, et al. Antigen targeting to dendritic cells combined with transient regulatory T cell inhibition results in long-term tumor regression. Oncoimmunology. 2014;4(8):e970462. | ||
Shaid S, Brandts CH, Serve H, Dikic I. Ubiquitination and selective autophagy. Cell Death Differ. 2013;20(1):21–30. | ||
Martin K, Schreiner J, Zippelius A. Modulation of APC function and anti-tumor immunity by anti-cancer drugs. Front Immunol. 2015; 6:501. | ||
Baker K, Rath T, Lencer WI, Fiebiger E, Blumberg RS. Cross-presentation of IgG-containing immune complexes. Cell Mol Life Sci. 2013;70(8):1319–1334. | ||
Tian S, Jiang C, Liu X, et al. Hypermethylation of IFN-γ in oral cancer tissues. Clin Oral Investig. 2017;21(8):2535–2542. | ||
Tu SP, Quante M, Bhagat G, et al. IFN-γ inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res. 2011;71(12):4247–4259. | ||
Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16(2):142–152. | ||
Elemans M, Florins A, Willems L, Asquith B. Rates of CTL killing in persistent viral infection in vivo. PLoS Comput Biol. 2014;10(4): e1003534. | ||
Fossum E, Grødeland G, Terhorst D, et al. Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus. Eur J Immunol. 2015;45(2):624–635. | ||
Wang H, Yu X, Guo C, et al. Enhanced endoplasmic reticulum entry of tumor antigen is crucial for cross-presentation induced by dendritic cell-targeted vaccination. J Immunol. 2013;191(12):6010–6021. | ||
Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–1538. | ||
Zhou J, Gan X, Wang Y, et al. Toxoplasma gondii prevalent in China induce weaker apoptosis of neural stem cells C17.2 via endoplasmic reticulum stress (ERS) signaling pathways. Parasit Vectors. 2015; 8:73. | ||
Kim SR, Kim DI, Kang MR, et al. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor kB activation. J Allergy Clin Immunol. 2013;132(6):1397–1408. | ||
Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569(1–2):29–63. | ||
Lee WS, Sung MS, Lee EG, et al. A pathogenic role for ER stress-induced autophagy and ER chaperone GRP78/BiP in T lymphocyte systemic lupus erythematosus. J Leukoc Biol. 2015;97(2):425–433. | ||
Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 2015;61:45–52. | ||
Hsu PY, Wu CA, Shen SS, Yang YW. The role of tomatine adjuvant in antigen delivery for cross-presentation. Curr Drug Deliv. 2015;12(3):342–350. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.