Back to Journals » OncoTargets and Therapy » Volume 13
Immune Checkpoint Inhibitor Therapy Achieved Complete Response for Drug-Sensitive EGFR/ALK Mutation-Negative Metastatic Pulmonary Large-Cell Neuroendocrine Carcinoma with High Tumor Mutation Burden: A Case Report
Authors Zhang X, Sun Y , Miao Y , Xu S
Received 25 April 2020
Accepted for publication 3 August 2020
Published 19 August 2020 Volume 2020:13 Pages 8245—8250
DOI https://doi.org/10.2147/OTT.S259893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Xin Zhang,1 Yanbin Sun,1 Yuan Miao,2 Shun Xu1
1The Department of Thoracic Surgery, The First Hospital of China Medical University, Shenyang 110001, Liaoning Province, People’s Republic of China; 2The Department of Pathology, The First Hospital of China Medical University, Shenyang 110001, Liaoning Province, People’s Republic of China
Correspondence: Shun Xu
The Department of Thoracic Surgery, The First Hospital of China Medical University, 155# Nanjing North Street, Shenyang 110001, People’s Republic of China
Tel +86 02483283170
Email [email protected]
Abstract: Large-cell neuroendocrine lung carcinoma (LCNELC) is classified into lung neuroendocrine tumors according to WHO 2015 classification guidelines and represents approximately 3% of all lung cancer. Because of the rarity of LCNELC, there is a lack of prospective studies guiding treatment. Here, we report a case of a patient with pT2aN2M0 stage IIIA LCNELC (drug-sensitive EGFR/ALK mutation-negative, PD-L1-negative but tumor mutation burden (TMB) high), who progressed rapidly after surgery but achieved a complete response to subsequent immune checkpoint inhibitor (ICI) therapy. The concentration of circulating tumor DNA (ctDNA) following the treatment course strongly reflects the response to ICI therapy. This report highlights the efficacy of ICI treatment in metastatic LCNELC patients with a high TMB and suggests that ctDNA analysis in detecting molecular residual disease may facilitate the personalization of ICI therapy.
Keywords: metastatic large-cell neuroendocrine lung carcinoma, adrenal gland metastasis, next generation sequencing, immune checkpoint inhibitor, circular tumor DNA
Introduction
Large-cell neuroendocrine lung carcinoma (LCNELC) is a poorly differentiated tumor that exhibits large cells with neuroendocrine features and a low nuclear-to-cytoplasmic ratio, representing approximately 3% of all lung cancer.1 Because of the rarity of LCNELC, there is a lack of prospective studies or strong evidence to guide treatment. The primary treatment strategy for early-stage LCNELC patients is complete resection with mediastinal lymph node dissection, but the disease often rapidly recurs.2–4 Therefore, efficient adjuvant therapy after surgery is necessary for LCNELC patients.5 However, a multi-center retrospective study from China showed that patients with LCNELC had a progression-free survival (PFS) of only 11.5 and 7.2 months, respectively, when receiving small-cell lung cancer (SCLC)-based regimens and non-small-cell lung cancer (NSCLC)-based regimens.6 The inhibition of the antigen-specific T-cell responses by the immune checkpoint leads to tumor immune escape,7 which can be corrected by immune checkpoint inhibitors (ICIs) though reversing the tumor-derived inhibitory immune reactions or activating anti-tumor immune activities.8 Here, we report a metastatic LCNELC patient with a high tumor mutation burden (TMB) level who benefited from ICI treatment and achieved a PFS of 20 months.
Case Report
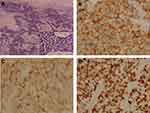
We report a 54-year-old man with a 40 pack-year history of smoking, who underwent a routine examination in July 2018. The patient gave written informed consent for publication of his case details and images. The publication of the case details was approved by the ethics committee of The First Hospital of China Medical University (AF-SOP-07-1). Computed tomography (CT) of the chest revealed a lung lesion measuring 4 cm in the right upper lobe with No.4R lymph node enlargement (Figure 1A and B). No distant metastases were found through brain magnetic resonance imaging (MRI), full-body bone scan, and ultrasonography of the abdomen and adrenal glands. Because of bullae in the lung around the tumor and the risk of pneumothorax after biopsy, CT-guided needle biopsy was rejected by the patient. A lobectomy and lymph node dissection were performed in August 2018, and pT2aN2M0 stage IIIA LCNELC with No.4R lymph node metastasis was confirmed via pathological diagnosis. Immunohistochemical analysis showed positive staining for CK, TTF-1, synaptophysin, PAS, chromogranin A and Ki-67 (80%), focal positive staining for CK7, CD56 and CD117, but negative staining for CK5/6, P40, P63, CD45 (LCA) and PSA-AB, consistent with a LCNELC origin (Figure 2). Adjuvant chemotherapy was rejected by the patient.
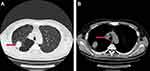 |
Figure 1 The CT scan of the patients before surgery. (A) The 4 cm tumor on the right upper lobe (Red arrow); (B) Metastatic right No.4 lymph node (Red arrow). |
To seek a more effective treatment strategy, tumor and plasma biopsies were subjected to genetic testing using targeted next generation sequencing (NGS) for 425 cancer-relevant genes (Gene seqPrime) and numerous tumor-specific mutations (Table 1) were observed with a high TMB at 25.8 mutations per MB (Method in Supplement 1). However, no drug-sensitive EGFR mutation or ALK rearrangement was identified.
 |
Table 1 Mutations Detected by NGS |
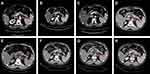
After surgery (2 months), chest and abdomen CT with contrast enhancement was conducted as the baseline before adjuvant therapy. The scans revealed a 2-cm-sized metastatic tumor in the left adrenal gland, which indicated progressive disease (Figure 3A). Although immunohistochemical staining of the primary tumor for the programmed death-ligand 1 (PD-L1) was negative (Figure 4), ICI therapy was recommended to the patient because of the high level of TMB and negative mutation of EGFR/ALK. Then the patient began to receive nivolumab treatment (240 mg) in October 2018.
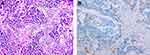 |
Figure 4 Immunohistochemical staining of primary tumor in lung for PD-L1. (A) Hematoxylin-eosin staining of the tumor tissue (200X). (B) Immunohistochemical staining for PD-L1 (Negative, 200X). |
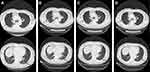
The efficiency of the treatment was evaluated after every 4 cycles of treatment through abdomen CT and neuron-specific enolase (NSE) detection. During 15 cycles of treatment, the patient achieved a complete response (CR), with significant regression of the metastatic tumor in the left adrenal gland (Figure 3B–E). Before the 16th cycle of treatment, the patient suffered from cough with mild dyspnea and a chest CT showed interlobular septal thickening and subpleural ground-glass opacities (Figure 5A). Nivolumab therapy was discontinued for 3 months but the symptoms and conditions revealed by the CT images became worse (Figure 5B). By treating the patient with acetylcysteine and corticosteroids for 3 months, the symptoms disappeared and the CT imaging results recovered (Figure 5C and D). By 4, 8 and 12 months after drug withdrawal, no progression occurred (Figure 3F–H) and the level of NSE remained in the normal range (Figure 6A). In addition, the concentration of circulating tumor DNA (ctDNA) from three sequential plasma samples collected before, and two and four cycles after nivolumab treatment showed a continued decrease during the treatment course, which indicated a durable response to nivolumab (Figure 6B). During 20 months of post-treatment follow-up (until May 2020), no recurrence or metastasis was observed in the patient.
Discussion
According to the Surveillance, Epidemiology and End Results (SEER) database, LCNELC is a rare disease that is more commonly detected in elderly and male patients, and in the upper lobe.9 LCNELC shares similarities with SCLC on the basis of clinical and pathological features,10 while LCNELC patients who have previously undergone complete resection and received adjuvant chemotherapy with the standard combination regimen of SCLC (cisplatin plus etoposide) show prolonged survival.6,11 However, therapeutic approaches for metastatic and unresectable tumors remain to be further evaluated. Recently, the efficacy of ICIs has been confirmed in various treatment approaches for neuroendocrine tumors.12 A retrospective cohort study in France showed that patients with LCNELC had a significant response to immune checkpoint inhibitors and achieved a median PFS of 57 weeks.13 In the case we report here, the patient exhibited a CR and achieved a PFS of 20 months (until May 2020) upon ICI treatment.
Interstitial lung disease (ILD) is a rare immune-related adverse event, which can occur in patients treated with ICIs. A French multi-center retrospective study showed that 3.5% of all patients treated with ICIs developed ICI-related ILD (ICI-ILD).14 However, it is more common in patients with lung cancer who are treated with ICIs, with the prevalence reported as 36.8%,15 13.2%,16 and 12%17 in three recent studies. The occurrence of ICI-ILD is more common in males with a history of smoking14 and is highly associated with pre-existing interstitial lung disease.16,17 ICI-ILD often occurs earlier in lung cancer, with a median of 2.1 months after ICI treatments,1 and presents as several radiologic patterns.18 A total of 70–80% of ICI-ILD cases can be controlled through the prompt initiation of a high dose of steroids.19 In our case, the male patient had a long history of smoking and his chest CT showed severe pre-existing interstitial lung disease, which might lead to the occurrence of ILD after 15 cycles of nivolumab treatment.
High TMB is regarded as a predictive biomarker of response to ICIs.20,21 It has been reported that TMB in LCNELC is commonly higher compared with other NSCLC subtypes,22 which indicates that ICIs might be an option for LCNELC. Wang et al reported a LCNELC patient with a high TMB who benefited from ICI treatment.23 In our case, the patient was PD-L1 negative but had a high TMB, at 25.6 mutations per Mb, and achieved a CR after ICI treatment, suggesting that immunotherapy is effective in this class of disease.
The level of ctDNA in molecular residual disease detection during treatment strongly predicts recurrence for multiple tumor types.24 In our case, the concentration of ctDNA continued to decrease after two cycles of ICI treatment and was no longer detectable after four cycles. Follow-up imaging demonstrated consistent results, with no evidence of progressive disease 20 months after starting ICI treatment. Our findings suggest that ctDNA analysis could potentially guide the decision to distinguish responders at an early stage during ICI therapy in LCNELC patients.
Conclusion
In summary, we reported an advanced LCNELC patient with a high TMB level who achieved a CR on nivolumab treatment with a significant decrease of ctDNA concentration during treatment and achieved a PFS of 20 months. This report highlights the efficacy of ICI treatment in metastatic LCNELC patients with a high TMB and suggests that ctDNA analysis may facilitate the personalization of ICI therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fasano M, Della Corte CM, Papaccio F, et al. Pulmonary large-cell neuroendocrine carcinoma: from epidemiology to therapy. J Thorac Oncol. 2015;10(8):1133–1141. doi:10.1097/JTO.0000000000000589
2. Eichhorn F, Dienemann H, Muley T, et al. Predictors of survival after operation among patients with large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg. 2015;99(3):983–989. doi:10.1016/j.athoracsur.2014.10.015
3. Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol. 2005;23(34):8774–8785. doi:10.1200/JCO.2005.02.8233
4. Saji H, Tsuboi M, Matsubayashi J, et al. Clinical response of large cell neuroendocrine carcinoma of the lung to perioperative adjuvant chemotherapy. Anticancer Drugs. 2010;21(1):89–93. doi:10.1097/CAD.0b013e328330fd79
5. Filosso PL, Guerrera F, Evangelista A, et al. Adjuvant chemotherapy for large-cell neuroendocrine lung carcinoma: results from the European society for thoracic surgeons lung neuroendocrine tumours retrospective database. Eur J Cardiothorac Surg. 2017;52:339–345. doi:10.1093/ejcts/ezx101
6. Zhang JT, Li Y, Yan LX, et al. Disparity in clinical outcomes between pure and combined pulmonary large-cell neuroendocrine carcinoma: a multi-center retrospective study. Lung Cancer. 2020;139:118–123. doi:10.1016/j.lungcan.2019.11.004
7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
8. Su MY, Fisher DE. Immunotherapy in the precision medicine era: melanoma and beyond. PLoS Med. 2016;13(12):e1002196. doi:10.1371/journal.pmed.1002196
9. Cao L, Li ZW, Wang M, et al. Clinicopathological characteristics, treatment and survival of pulmonary large cell neuroendocrine carcinoma: a SEER population-based study. PeerJ. 2019;7:e6539. doi:10.7717/peerj.6539
10. Xu F, Chen K, Lu C, et al. Large cell neuroendocrine carcinoma shares similarity with small cell carcinoma on the basis of clinical and pathological features. Transl Oncol. 2019;12(4):646–655. doi:10.1016/j.tranon.2019.01.004
11. Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82(5):1802–1807. doi:10.1016/j.athoracsur.2006.05.109
12. Weber MM, Fottner C. Immune checkpoint inhibitors in the treatment of patients with neuroendocrine neoplasia. Oncol Res Treat. 2018;41(5):306–312. doi:10.1159/000488996
13. Levra MG, Mazieres J, Valette CA, et al. P1.07-012 efficacy of immune checkpoint inhibitors in large cell neuroendocrine lung cancer: results from a french retrospective cohort. J Thorac Oncol. 2017;12(1):S702–S703. doi:10.1016/j.jtho.2016.11.923
14. Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50(2):1700050. doi:10.1183/13993003.00050-2017
15. Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi:10.1016/j.lungcan.2017.11.019
16. Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–156. doi:10.1016/j.lungcan.2018.09.015
17. Sugano T, Seike M, Saito Y, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac Cancer. 2020;11(4):1052–1060. doi:10.1111/1759-7714.13364
18. Baba T, Sakai F, Kato T, et al. Radiologic features of pneumonitis associated with nivolumab in non-small-cell lung cancer and malignant melanoma. Future Oncol. 2019;15(16):1911–1920. doi:10.2217/fon-2019-0102
19. Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154(6):1416–1423. doi:10.1016/j.chest.2018.08.1048
20. Galuppini F, Dal Pozzo CA, Deckert J, et al. Tumor mutation burden: from comprehensive mutational screening to the clinic. Cancer Cell Int. 2019;19(1):202. doi:10.1186/s12935-019-0929-4
21. Cao D, Xu H, Xu X, et al. High tumor mutation burden predicts better efficacy of immunotherapy: a pooled analysis of 103078 cancer patients. OncoImmunology. 2019;8(9):1–12. doi:10.1080/2162402X.2019.1629258
22. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2
23. Wang VE, Urisman A, Albacker L, et al. Checkpoint inhibitor is active against large cell neuroendocrine carcinoma with high tumor mutation burden. J Immunother Cancer. 2017;5(1):75. doi:10.1186/s40425-017-0281-y
24. Gandara DR, Paul SM, Marcin K, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–1448. doi:10.1038/s41591-018-0134-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.