Back to Journals » Journal of Inflammation Research » Volume 15
Imbalanced T-Cell Subsets May Facilitate the Occurrence of Osteonecrosis of the Femoral Head
Authors Chen C , Zhao X, Luo Y, Li B, Li Q, Zhao C, Huang Y, Kang P
Received 20 March 2022
Accepted for publication 10 July 2022
Published 22 July 2022 Volume 2022:15 Pages 4159—4169
DOI https://doi.org/10.2147/JIR.S367214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Monika Sharma
Changjun Chen,1 Xin Zhao,1,2 Yue Luo,1 Bohua Li,1 Qianhao Li,1 Chengcheng Zhao,1 Yan Huang,3 Pengde Kang1
1Department of Orthopedics, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Department of Orthopedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China; 3Health Management Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Yan Huang, Health Management Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China, Email [email protected] Pengde Kang, Department of Orthopedics, West China Hospital, Sichuan University, Chengdu, People’s Republic of China, Email [email protected]
Background: Osteonecrosis of the femoral head (ONFH) is a complex disease resulting in degeneration of the hip joint. The pathogenesis of ONFH is largely unknown, but alterations in immunological factors have been proposed to play a role.
Methods: We included 109 patients with ONFH and 109 age-, sex-, and body mass index-matched healthy controls in this study. The percentage of circulating CD3+, CD4+, and CD8+ lymphocytes among the total lymphocytes was identified by flow cytometry and compared between the cases and controls. Subgroup analysis within each etiological group and correlation analysis of T-cell subset levels with disease duration were performed. Furthermore, we compared the expression patterns of CD4, RANKL, and FoxP3 in the femoral head of healthy and glucocorticoid (GC)-treated ONFH rats.
Results: The results showed that CD3+ and CD4+ T-cell counts and the CD4+/CD8+ ratio were significantly higher in patients with ONFH and that CD3+ lymphocyte levels were negatively correlated with disease duration. The CD4+ T-cell levels and CD4+/CD8+ ratios in the GC-ONFH etiological group were lower than those in the idiopathic-, traumatic-, and alcoholic-ONFH groups, while the CD8+ T-cell levels were higher. Furthermore, the CD3+, CD4+, and CD8+ T-cell counts and the CD4+/CD8+ ratio were higher in the GC-ONFH group than in the control group. Finally, we observed diminished levels of FoxP3/CD4 double-positive T regulatory cells and increased RANKL+ T-cell levels in the bone marrow of the femoral head in GC-ONFH rats.
Conclusion: The imbalance of T-cell subsets might be involved in the pathophysiological process of ONFH, and diminished CD4+/FoxP3+ T regulatory cells may be associated with increased RANKL+ T cells in the bone marrow of the femoral head in GC-ONFH, which may facilitate bone resorption and collapse of the femoral head.
Trial Registration: This study was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR2100042642).
Keywords: CD3+, CD4+, lymphocytes, ONFH, osteoimmunology
Introduction
Osteonecrosis of the femoral head (ONFH) is a progressive and refractory disease with a complex etiology and unclear pathogenesis that results in femoral head collapse and degenerative changes in the hip joint1 The exact worldwide incidence of ONFH is unknown, and few data related to each country have been reported. In China, an estimated 8.12 million patients aged ≥ 15 years have been diagnosed with nontraumatic osteonecrosis, and the costs associated with this disorder are considerable.2 Furthermore, compared with patients undergoing total hip arthroplasty for osteoarthritis, those undergoing total hip arthroplasty for osteonecrosis incur higher readmission-related costs and complication rates.3 Therefore, there is a need to better identify the potential mechanisms and biomarkers for patients with a higher risk of developing ONFH to reduce the huge financial burden.
Osteoimmunology is a relatively new field of immunology that is based on evidence that the immune and bone systems share the same microenvironment and that the immune system can regulate osteocytes and affect the process of bone remodeling.4 Dysfunction of the immune system can destroy the balance between bone formation and resorption, resulting in an abnormal osteoimmunology status.5 Functional disorders of immune cells, such as B or T cells, have been implicated in the pathogenesis of some diseases, including cancers and some immune disorders.6–9 A recent study suggested that immune cells, such as cluster of differentiation 3 (CD3)+ T cells and Ts cells (CD3+CD8+), occurred in significantly higher numbers in patients with nontraumatic ONFH than in healthy controls.10 Furthermore, the T lymphocyte percentage was significantly lower in patients at the Association Research Circulation Osseous stage IV than in patients at stages II and III.10 In addition, Rabquer et al11 found that patients with ONFH exhibit significant synovial inflammation, in which the inflamed synovium consists primarily of infiltrated macrophages and CD4+ T cells. Similarly, Daltro et al12 found that patients with sickle cell disease with osteonecrosis had increased circulating CD4+ T cells and that an increased proportion of these cells produce a broad spectrum of proinflammatory cytokines to maintain the inflammatory state. These studies have shown that T-cell-mediated immunity may be involved in the pathogenesis of ONFH; however, studies with larger sample sizes and broad etiologies of ONFH are needed to better compare the changes in T-cell subsets between patients with ONFH and healthy individuals.
CD4+ and CD8+ T-cell populations are representative of T cells. Naive CD4+ T cells can differentiate into T-helper (Th) type 1, Th2, Th9, Th17, and Th22; regulatory T (Treg); and follicular helper T cells depending on the environmental stimulus,13 while CD8+ T cells are cytotoxic T lymphocytes that are responsible for regulating immune responses and targeted cell destruction.14 Evidence suggests that T cells secrete cytokines to regulate bone remodeling in an autocrine or paracrine manner.13,15 For example, T cells can increase the receptor activator of nuclear factor kappa-Β ligand (RANKL)/osteoprotegerin ratio and play an important role in osteoclast formation, which may facilitate bone resorption.16–18 Additionally, Th17 cells are a specialized inflammatory subset associated with bone resorption.19 Furthermore, Won20 found that CD4+ T cells can induce RANKL-mediated osteoclastogenesis by stimulating interleukin-17 production. Moreover, Tregs can suppress osteoclast differentiation and bone resorption via the secretion of soluble cytokines.21 Normal bone remodeling relies on a balance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. An imbalance between osteoblastogenesis and osteoclastogenesis is known to play important roles in the pathogenesis and progression of ONFH.22
Therefore, we aimed to further elucidate the role of T-cellcell subsets, especially CD4+ lymphocytes, in ONFH pathogenesis. We think that the imbalance of T-cell subsets might be involved in the pathophysiological process of ONFH. Then, we conducted experiments to verify our hypothesis. By comparing changes in T-cell subsets in patients with ONFH and in healthy controls and further investigating the expression patterns of T-cell-related proteins in healthy and glucocorticoid (GC)-induced ONFH rats, we provide novel insights into ONFH pathogenesis and preliminary targets for the treatment of the disease.
Materials and Methods
Clinical Experiments
Case and Control Groups
One hundred nine patients with ONFH and 109 healthy matched controls were enrolled in this study. Patients were diagnosed with ONFH after total hip arthroplasty in our orthopedic ward between October 2020 and August 2021. These patients were diagnosed by more than two senior orthopedic surgeons, whose diagnosis was combined with clinical history, physical examination, radiography, and magnetic resonance imaging evaluation. The individuals included in the control group were admitted to the health management center of our hospital between October 2020 and August 2021 and were matched (1:1) to the cases by age, sex, and body mass index (BMI). The controls were randomly selected and matched by a computer. Individuals who met the following criteria were excluded from our study: (i) individuals with a history of hip infection or tuberculosis and/or response to anti-tuberculosis therapy; (ii) individuals with severe cardiovascular disease, chronic obstructive pulmonary disease, kidney disease, or neoplastic diseases; and (iii) individuals with known diseases (such as diabetes, thyroiditis, HIV, or viral infection) or a history of drug use that might influence the status of T cells.
The study was governed under the 2007–2008 version of the World Medical Association’s Declaration of Helsinki. This study was approved by the Institutional Review Board of the West China Hospital of Sichuan University and was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2100042642), and written informed consent was obtained from all study participants.
Measurements of T Lymphocyte Subsets
Peripheral venous blood samples were collected from the study participants using ethylenediaminetetraacetic acid between 7:00 and 10:00 a.m. after fasting from midnight. The percentages of CD3+, CD4+, and CD8+ lymphocytes among the total lymphocytes were identified and determined by flow cytometry using a BD FACSCantoTM II Flow Cytometer (BD Biosciences, Heidelberg, Germany), and the CD4/CD8 ratio was calculated. For flow cytometry analyses, a reagent cocktail (10 μL) containing CD4-fluorescein isothiocyanate, CD8-phycoerythrin, and CD3 peridinin chlorophyll protein was added to 50 μL heparin-anticoagulated whole blood. Lymphocytes were gated using forward-scattered light and side-scattered light. For each sample, at least 10,000 cells were analyzed, and the percentage of cells expressing the CD3+, CD4+, and CD8+ markers was evaluated. All tests were performed by board-certified laboratory technicians who were blinded to the clinical data, according to the manufacturer’s instructions. All experiments were conducted in the clinical laboratory of our hospital.
Animal Experiments
Establishment of an ONFH Rat Model
All animal experiments were performed in a specific pathogen-free grade animal laboratory. Ten male Sprague Dawley rats, weighing 350–420 g, were used in this study. Six rats were induced with GC-ONFH by intramuscular injection with two doses of lipopolysaccharide (20 μg/kg) and three high doses of methylprednisolone (20 mg/kg).23 All injections had a time interval of 24 h. After 2 weeks, the rats were euthanized under anesthesia to obtain the femoral head. Normal femoral head was also sampled from the four healthy rats.
All experimental and animal care procedures were approved by the Animal Research Ethics Committee of the West China Hospital of Sichuan University and performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Immunofluorescence Assay
The tissue sections were treated with 1:50 dilutions of mouse anti‐CD4 (cat. no. Sc-20079; Santa Cruz Biotechnology Inc., Dallas, TX, USA), mouse anti-RANKL (cat. no. sc-377079; Santa Cruz Biotechnology Inc., Dallas, TX, USA), and rabbit anti-forkhead box P3 (FoxP3; cat. no. bs-10211R; Bioss Antibodies Inc., Beijing, China) primary antibodies for 15 h at 4 °C, followed by staining with 1:100 dilutions of Cy3‐labeled goat anti-mouse immunoglobulin G (cat. no. K1207; ApexBio Technology, Houston, TX, USA) and Alexa488‐labeled goat anti-rabbit immunoglobulin G (cat. no. K1206; ApexBio Technology, Houston, TX, USA) secondary antibodies for 1 h at 20 °C. To stain the nuclei, the sections were treated with 4,6‐diamino‐ 2‐phenylindole (AR1176; Boster Biological Technology Co. Ltd., Pleasanton, CA, USA) for 5 min at 25 °C. Protein expression was evaluated using ImageJ analysis software to determine the mean optical density.
Statistical Analysis
Data from the clinical experiments are presented as the mean ± standard deviation and were analyzed using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA). For clinical data, variables were assessed for normality using the Kolmogorov–Smirnov test. For variables with a nonnormal data distribution, the Mann–Whitney U-test was used for comparisons of quantitative variables between two etiological groups, while the Kruskal–Wallis test followed by Dunn’s test was used to compare quantitative variables between ≥ 3 etiological groups. The Spearman correlation test was used to study the correlation between quantitative parameters. For the data from the animal experiments, GraphPad Prism 8.4 (GraphPad Software Inc, San Diego, CA, USA) was used to conduct paired Student’s t-tests of variance. The significance level was set at P < 0.05.
Results
Clinical Experiment Results
Clinical and Demographic Characteristics
The demographic characteristics of the study participants are presented in Table 1. In the case group, 20.3% of the patients were women, with a mean age of 49.46 ± 12.202 years and BMI of 23.54 ± 3.16 kg/m2 for the entire group. In the control group, 21.2% of the participants were women, with a mean age of 49.46 ± 12.202 years and BMI of 23.56 ± 3.44 kg/m2 for the entire group.
 |
Table 1 Basic Characteristics of Study Participants After (1:1) Matching by Age, Sex, and BMI |
Major Lymphocyte Populations Differ Between Cases and Controls
The flow cytometry results showed that CD3+ T-cell counts were significantly higher in the ONFH group than in the healthy group (74.21 ± 8.01% vs 68.05 ± 10.04%, respectively, P < 0.05). The same result was also found for the CD4+ T-cell counts; the CD4+ T-cell percentages were 45.47 ± 8.44% and 36.83 ± 8.11% in the ONFH and healthy groups, respectively. However, no significant difference in CD8+ T-cell counts was found between the two groups (cases: 25.13 ± 8.91%, controls: 25.38 ± 8.24%; P > 0.05; Table 2). The CD4+/CD8+ ratio was significantly higher in the case group than in the control group (2.12 ± 1.03 vs 1.65 ± 0.74, respectively, P < 0.05). Furthermore, no correlation was found between T-cell subsets and the total duration of ONFH, except for CD3+ lymphocytes, whose levels were found to be significantly increased when the total duration of ONFH decreased (Spearman correlation, ρ = –0.207, P = 0.030; Table 3).
 |
Table 2 Comparison of T Cell Subsets Percentage Within the Total Lymphocyte in Patients with ONFH and Healthy Controls |
 |
Table 3 Correlation Between T Cell Subsets Percentage Within the Total Lymphocyte and Total Disease Duration |
Lymphocyte Populations Differentially Represented in the GC-ONFH Etiological Group When Compared to Those of the Other Etiological Groups
Subsequently, subgroup analysis was conducted. We grouped the patients into idiopathic, GC-induced, traumatic, and alcoholic ONFH groups based on the Etiologic Classification Criteria of ARCO.24,25 The CD3+ T-cell percentage showed no significant difference among the etiological groups (P > 0.05). The CD4+ T-cell percentage (40.60 ± 9.39%) was lower in the GC-ONFH group than in the idiopathic- (46.70±8.84%), traumatic- (45.15±7.75%) and alcoholic- (47.37±7.14%) ONFH groups but was only significantly different between the GC-ONFH and alcoholic-ONFH groups (P < 0.05). Moreover, the CD8+ T-cell counts were higher in the GC-ONFH group than in the idiopathic-, traumatic-, and alcoholic-ONFH groups, but a significant difference was only found between the GC-ONFH and idiopathic-ONFH groups (P < 0.05). The CD4+/CD8+ ratio was also lower in the GC-ONFH group than in the idiopathic-, traumatic-, and alcoholic-ONFH groups. However, significant differences were only found between the GC-ONFH and alcoholic-ONFH (P < 0.05) and the GC-ONFH and idiopathic-ONFH (P < 0.05) groups. Details are presented in Table 4. Finally, all T-cell subsets and the CD4+/CD8+ ratio in the GC-ONFH group were higher than those in the corresponding healthy etiological groups; however, a significant difference was only found for CD4+ lymphocytes (Table 5).
 |
Table 4 Comparison of T Subsets Among Different Etiologies in the ONFH Group |
 |
Table 5 Comparison of T Cell Subsets in Patients with GC Induced ONFH and Age, Sex, and BMI Matched Healthy Controls |
Animal Experiment Results
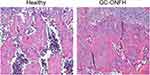
First, we verified the success of establishing a rat model of GC-ONFH by histological examination. Images of H&E‑staining showed that GC-ONFH induction resulted in evident signs of necrosis of the femoral head, including karyolysis, karyorrhectic osteocytes, marrow necrosis and fibrous invasion (Figure 1). The overall rate of ONFH was 83.3% (5 of 6). Thus, we included 4 rats in the heathy group and 5 rats in the GC-ONFH group for the immunofluorescence assay.
The Bone Marrow of GC-ONFH Rats Had Diminished FoxP3/CD4 Double-Positive Treg Cell Levels
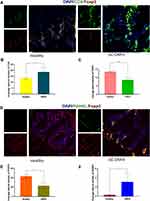
As depicted in Figure 2A–C, CD4+ T cells were found in the bone marrow of healthy femoral heads, and most of the CD4+ T cells were positive for FoxP3. The quantity of CD4+ T cells in the bone marrow was significantly increased in GC-ONFH rats compared to that in the controls (P < 0.05). However, the diseased bone marrow showed a decreased number of FoxP3+ T cells compared to that of the healthy bone marrow (Figure 2C), indicating that the increased CD4+ lymphocytes in the bone marrow of GC-ONFH rats showed a decreased positive staining pattern for FoxP3.
The Bone Marrow of GC-ONFH Rats Had Increased RANKL+ T-Cell Levels Along with Decreased FoxP3+ T-Cell Levels
A similar expression pattern for FoxP3 was found when the cells were stained with FoxP3/RANKL. The quantity of FoxP3+ cells in the bone marrow was decreased in the GC-ONFH group compared to that in the control group (P < 0.01). Nevertheless, along with a decrease in FoxP3+ cells, the staining pattern for RANKL increased considerably. In the bone marrow of GC-ONFH rats, a comparatively large number of RANKL+ cells were detected within the FoxP3+ lymphocytes compared to that of the healthy bone marrow (Figure 2D–F), which might be associated with local bone resorption and impaired bone repair.
Discussion
ONFH, a severe necrotic bone disease, is a multifactorial disease involving a combination of mechanisms that interact with each other to compromise the osseous healing mechanisms of the body. Although the precise etiology of ONFH is not known, evidence suggests that immunological factors play a vital role in the pathogenesis of osteonecrosis.26 Previous studies have indicated that T lymphocytes are the principal factors that regulate and coordinate immune responses, and imbalanced differentiation of T-cell subsets may contribute to the pathogenesis of immune diseases.13 However, a wide analysis comparing T-cell subsets in patients with a broad etiology of ONFH with those of healthy individuals has not been conducted thus far. Additionally, the specific role of CD4+ T cells in the pathogenesis of osteonecrosis has not yet been determined.
In this study, we found that the proportion of peripheral CD3+ and CD4+ T cells and the CD4+/CD8+ ratio were significantly elevated in patients with ONFH compared to healthy controls and that the increase in CD3+ T cells was not significantly different among the etiological groups. In addition, CD3+ lymphocyte levels were significantly negatively correlated with the total duration of ONFH. Together with the above observations, we found that the percentage of CD4+ T cells and the CD4+/CD8+ ratio in patients with steroid-induced ONFH were lower than those in patients with idiopathic-, traumatic-, and alcoholic-ONFH, while the CD8+ T-cell counts were higher. However, not all these changes were statistically significant. Additionally, the percentage of CD3+ and CD4+ T cells and the CD4+/CD8+ ratio in the GC-ONFH group were higher than those in the healthy controls; however, a significant difference was only found for CD4+ lymphocytes. Based on these findings, we conducted animal experiments to further investigate the role of T lymphocytes in the pathogenesis of GC-ONFH. We found that the bone marrow of the femoral heads of GC-ONFH rats had diminished FoxP3/CD4 double-positive Treg cell levels and increased RANKL+ T-cell levels. The increased RANKL+ T cells may be associated with decreased Treg cells. Therefore, these results indicate that imbalanced T-cell subsets may be involved in the pathogenesis of different ONFH etiologies and that dysfunction of Treg cells may facilitate the attack of GC-ONFH.
Signal transduction induced by pre-T-cell receptor (pre-TCR)–CD3 and TCR–CD3 complexes is essential for thymocyte maturation during T-cell development.27 This complex, expressed on T cells, also determines the outcome of T-cell activation and differentiation, and the CD3 subunits play a pivotal role in intracellular assembly, surface expression, and signal transduction. As a T-cell coreceptor, CD3 activates both cytotoxic CD8+ T cells and CD4+ T helper cells.28 The role of CD3+ T cells in chronic immune diseases, such as perennial allergic rhinitis, has been previously highlighted.29 Evidence has also demonstrated that reconstituted CD3+ T cells can independently elevate serum RANKL and tumor necrosis factor (TNF) levels and induce bone loss, and this same phenomenon can be seen for CD8+ T-cell reconstitution.30 To the best of our knowledge, this study is the first to demonstrate higher CD3+ T-cell levels in patients with idiopathic, GC-induced, traumatic, and alcoholic ONFH, with no variability among the groups, and a negative correlation with the total duration of ONFH. Based on the function of CD3, we hypothesized that increased CD3+ T cells might facilitate the activation of cytotoxic CD8+ T cells or CD4+ T helper cells, which may accelerate bone loss in the femoral head and play a role in disease progression. However, additional studies are required to characterize the functional properties of CD3+ T cells in patients with ONFH.
Although there is some consensus regarding the role of CD3+ T cells in the pathogenesis of ONFH, the specific role of CD4+ T cells has not been widely investigated. It has been reported that CD4+ T cells can increase the levels of RANKL and TNF to promote bone resorption, affecting both trabecular and cortical compartments, whereas CD8+ T cells increase bone marrow TNF levels and induce trabecular bone loss only.31 Our results indicate that the percentage of CD4+ lymphocytes in patients with ONFH was significantly increased compared to that in healthy controls, of which the percentage in idiopathic-, traumatic-, and alcoholic-ONFH was higher than that in GC-ONFH. According to a systematic review conducted by Mont et al,32 the prevalence of collapse in corticosteroid-related ONFH was 26%, excessive alcohol consumption related to ONFH was 47%, and idiopathic-ONFH had a 38% prevalence. Here, the overall prevalence of collapse of alcoholic- and idiopathic-ONFH was higher than that of GC-ONFH; thus, we speculate that the occurrence of collapse of the femoral head might correlate with the change in CD4+ lymphocytes. Our study further found that, compared with that in healthy bone marrow, FoxP3+/CD4+ T-cell numbers in GC-ONFH bone marrow were decreased, and GC-ONFH bone marrow had abundant infiltration of RANKL+ lymphocytes along with decreased expression of FoxP3+ cells. Based on our findings, we speculate that increased peripheral CD4+ T-cell numbers and CD4+/CD8+ ratio levels represent an overall activation of the CD4+ T-cell-mediated immune response; naive CD4+ T cells may have abnormal differentiation and activation, resulting in reduced Treg cells and increased proinflammatory subsets, which are associated with enhanced bone resorption and compromised bone healing.
However, this study had some limitations. First, the control group was only matched for age, sex, and BMI, whereas other factors, such as the amount of alcohol intake, smoking, disease duration, or disease stage, may affect the composition of T cells. These confounding variables were difficult to address in our study. Second, our study was conducted in a single center with a limited number of patients, and some of the differences were not statistically significant; thus, a large-scale multicenter study is needed. Finally, we did not compare the levels of T cell subpopulations in human tissues, and there might be some inconsistencies. Therefore, studies comparing the levels of T cell subpopulations in human tissues are needed. Furthermore, studies are also needed to assess the role of cytokines in Th1, Th2, Th17, and Treg cells. Additionally, studies are required to ensure the reproducibility of our findings.
Conclusions
Overall, the present study suggested that the imbalance of T-cell subsets might be involved in the pathophysiological process of ONFH, and diminished CD4+/FoxP3+ T regulatory cells may be associated with increased RANKL+ T cells in the bone marrow of the femoral head in GC-ONFH, which may facilitate bone resorption and collapse of the femoral head. In addition, our data also indicated that any differences may mean that the different etiologies may have different pathways but that ultimately the same final result - osteonecrosis. Thus, our study contributes to a better understanding of the disordered osteoimmunology of ONFH, which may aid in the development of targeted therapeutics for better management of ONFH.
Abbreviations
ONFH, osteonecrosis of the femoral head; THA, total hip arthroplasty; OA, osteoarthritis; ON, osteonecrosis; RANKL, nuclear factor-κB ligand; IL, interleukin; Th17, IL-17-producing helper T; BMI, body mass index; EDTA, ethylenediaminetetraacetic acid.
Data Sharing Statement
All data reported in this study are available from the corresponding author (Yan Huang and Kang Pengde) upon reasonable request after institutional approval.
Ethics Approval
This study took place at Sichuan University West China Hospital, and the study protocol was approved by the local ethics committee.
Consent for Publication
Written informed consent was obtained from each patient to authorize the publication of their data, and the authors have seen the manuscript and approved its submission to your journal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
This work was supported by the National Natural Science Foundation of China (grant numbers 81974333) and the 1.3.5 Project for Disciplines of excellence, West China Hospital, Sichuan University (ZYJC18040).Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Changjun C, Donghai L, Xin Z, Liyile C, Qiuru W, Pengde K. Mid- to long-term results of modified non-vascularized allogeneic fibula grafting combined with core decompression and bone grafting for early femoral head necrosis. J Orthop Surg Res. 2020;15(1):116. doi:10.1186/s13018-020-1565-3
2. Zhao DW, Yu M, Hu K, et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese Population: results from a nationally representative survey. Chin Med J. 2015;128(21):2843–2850. doi:10.4103/0366-6999.168017
3. Sax OC, Pervaiz SS, Douglas SJ, Remily EA, Mont MA, Delanois RE. Osteoarthritis and osteonecrosis in total hip arthroplasty: 90-day postoperative costs and outcomes. J Arthroplasty. 2020;36(7):2343–2347. doi:10.1016/j.arth.2020.10.039
4. Chen X, Chen X, Lu L, Yu X. Alcoholism and osteoimmunology. Curr Med Chem. 2020;27(23):3924–3943. doi:10.2174/1567201816666190514101303
5. Wang T, He C. TNF-α and IL-6: the link between immune and bone system. Curr Drug Targets. 2020;21(3):213–227. doi:10.2174/1389450120666190821161259
6. Chen C, Si M, Gao X, Wang W, Wang S, Pan X. Primary cutaneous diffuse large B-cell lymphoma after total knee arthroplasty: a case study and a systematic review of its cutaneous manifestations and treatment options. journal article. Adv Dermatol Allergol. 2021. doi:10.5114/ada.2021.108444
7. Bhatia R, Thompson C, Ganguly K, Singh S, Batra SK, Kumar S. Alcohol and smoking mediated modulations in adaptive immunity in pancreatitis. Cells. 2020;9(8):1880. doi:10.3390/cells9081880
8. Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–688. doi:10.1016/j.immuni.2006.06.002
9. Wen T, Rothenberg ME. Cell-by-cell deciphering of T cells in allergic inflammation. J Allergy Clin Immunol. 2019;144(5):1143–1148. doi:10.1016/j.jaci.2019.10.001
10. Ma J, Ge J, Gao F, et al. The role of immune regulatory cells in nontraumatic osteonecrosis of the femoral head: a retrospective clinical study. Biomed Res Int. 2019;2019:1302015. doi:10.1155/2019/1302015
11. Rabquer BJ, Tan GJ, Shaheen PJ, Haines GK, Urquhart AG, Koch AE. Synovial inflammation in patients with osteonecrosis of the femoral head. Clin Transl Sci. 2009;2(4):273–278. doi:10.1111/j.1752-8062.2009.00133.x
12. Daltro PB, Ribeiro TO, Daltro GC, Meyer RJ, Fortuna V. CD4(+) T cell profile and activation response in sickle cell disease patients with osteonecrosis. Mediators Inflamm. 2020;2020:1747894. doi:10.1155/2020/1747894
13. Srivastava RK, Dar HY, Mishra PK. Immunoporosis: immunology of osteoporosis-role of T cells. Front Immunol. 2018;9:657. doi:10.3389/fimmu.2018.00657
14. Lieberman SM, Evans AM, Han B, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100(14):8384–8388. doi:10.1073/pnas.0932778100
15. Cerboni S, Gehrmann U, Preite S, Mitra S. Cytokine-regulated Th17 plasticity in human health and diseases. Immunology. 2020;163(1):3–18. doi:10.1111/imm.13280
16. Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265(1):144–150. doi:10.1006/bbrc.1999.1623
17. Neale Weitzmann M, Pacifici R. Parathyroid diseases and T cells. Curr Osteoporos Rep. 2017;15(3):135–141. doi:10.1007/s11914-017-0359-y
18. Chen C, Alqwbani M, Zhao J, Yang R, Wang S, Pan X. Effects of teriparatide versus salmon calcitonin therapy for the treatment of osteoporosis in Asia: a meta-analysis of randomized controlled trials. Endocr Metab Immune Disord Drug Targets. 2021;21(5):932–942. doi:10.2174/1871530320999200817114817
19. Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–2682. doi:10.1084/jem.20061775
20. Won HY, Lee JA, Park ZS, et al. Prominent bone loss mediated by RANKL and IL-17 produced by CD4+ T cells in TallyHo/JngJ mice. PLoS One. 2011;6(3):e18168. doi:10.1371/journal.pone.0018168
21. Zaiss MM, Axmann R, Zwerina J, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56(12):4104–4112. doi:10.1002/art.23138
22. Tian L, Sun S, Li W, Yuan L, Wang X. Down-regulated microRNA-141 facilitates osteoblast activity and inhibits osteoclast activity to ameliorate osteonecrosis of the femoral head via up-regulating TGF-β2. Cell Cycle. 2020;19(7):772–786. doi:10.1080/15384101.2020.1731053
23. Fan M, Peng J, Qin L, Lu S. Experimental animal models of osteonecrosis. Rheumatol Int. 2011;31(8):983–994. doi:10.1007/s00296-011-1819-9
24. Yoon BH, Jones LC, Chen CH, et al. Etiologic classification criteria of ARCO on femoral head osteonecrosis part 2: alcohol-associated osteonecrosis. J Arthroplasty. 2019;34(1):169–174.e1. doi:10.1016/j.arth.2018.09.006
25. Yoon BH, Jones LC, Chen CH, et al. Etiologic classification criteria of ARCO on femoral head osteonecrosis part 1: glucocorticoid-associated osteonecrosis. J Arthroplasty. 2019;34(1):163–168.e1. doi:10.1016/j.arth.2018.09.005
26. Tektonidou MG, Moutsopoulos HM. Immunologic factors in the pathogenesis of osteonecrosis. Orthop Clin North Am. 2004;35(3):259–63, vii. doi:10.1016/j.ocl.2004.02.003
27. Dave VP. Role of CD3ε-mediated signaling in T-cell development and function. Crit Rev Immunol. 2011;31(1):73–84. doi:10.1615/critrevimmunol.v31.i1.70
28. Ngoenkam J, Schamel WW, Pongcharoen S. Selected signalling proteins recruited to the T-cell receptor-CD3 complex. Immunology. 2018;153(1):42–50. doi:10.1111/imm.12809
29. Pawankar RU, Okuda M, Okubo K, Ra C. Lymphocyte subsets of the nasal mucosa in perennial allergic rhinitis. Am J Respir Crit Care Med. 1995;152(6 Pt 1):2049–2058. doi:10.1164/ajrccm.152.6.8520775
30. Ofotokun I, Titanji K, Vikulina T, et al. Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun. 2015;6:8282. doi:10.1038/ncomms9282
31. Weitzmann MN, Vikulina T, Roser-Page S, Yamaguchi M, Ofotokun I. Homeostatic expansion of CD4+ T cells promotes cortical and trabecular bone loss, whereas CD8+ T cells induce trabecular bone loss only. J Infect Dis. 2017;216(9):1070–1079. doi:10.1093/infdis/jix444
32. Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92(12):2165–2170. doi:10.2106/jbjs.I.00575
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.